Social Behavior and Welfare in Nile Tilapia
Abstract
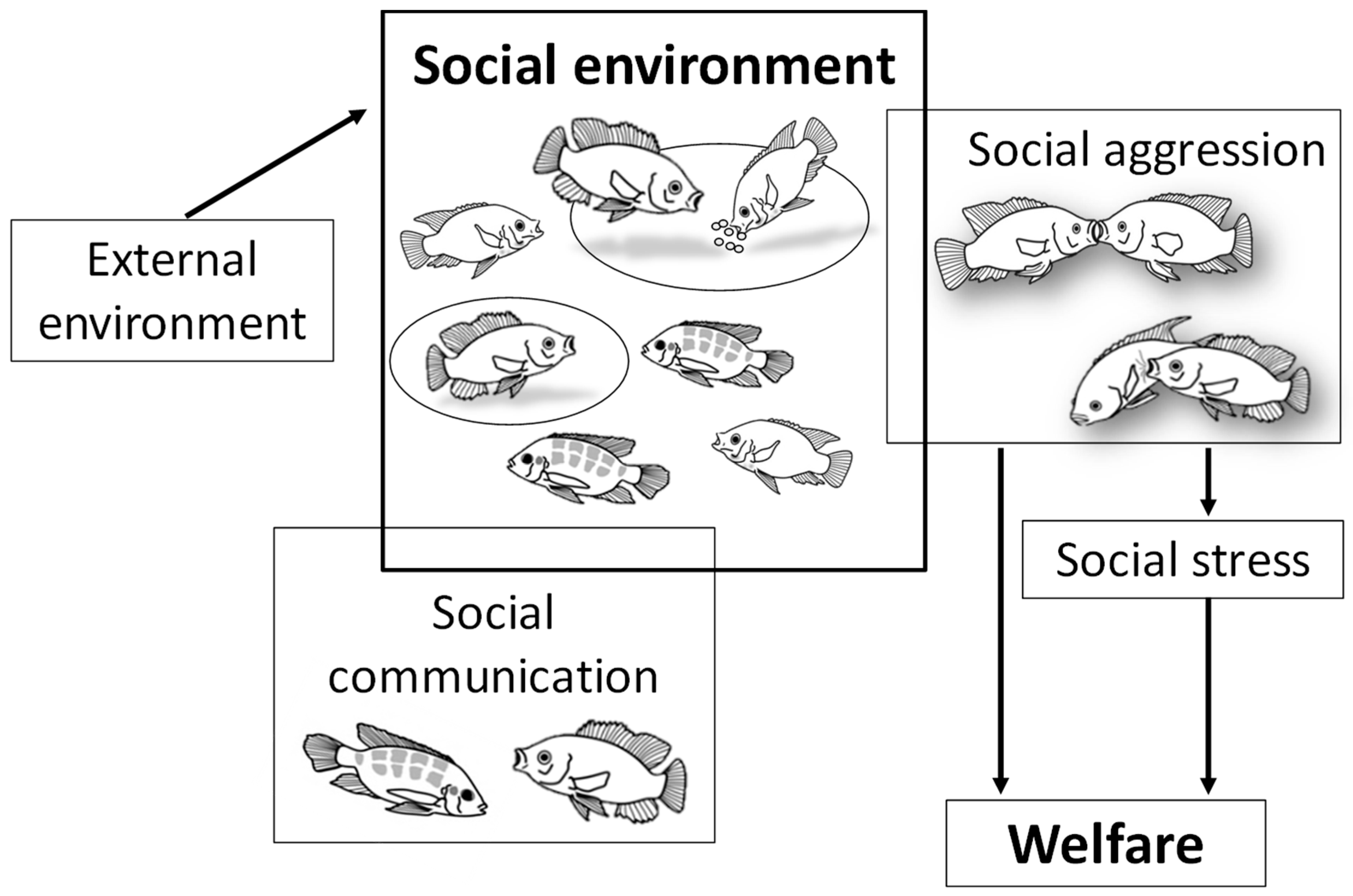
1. Introduction
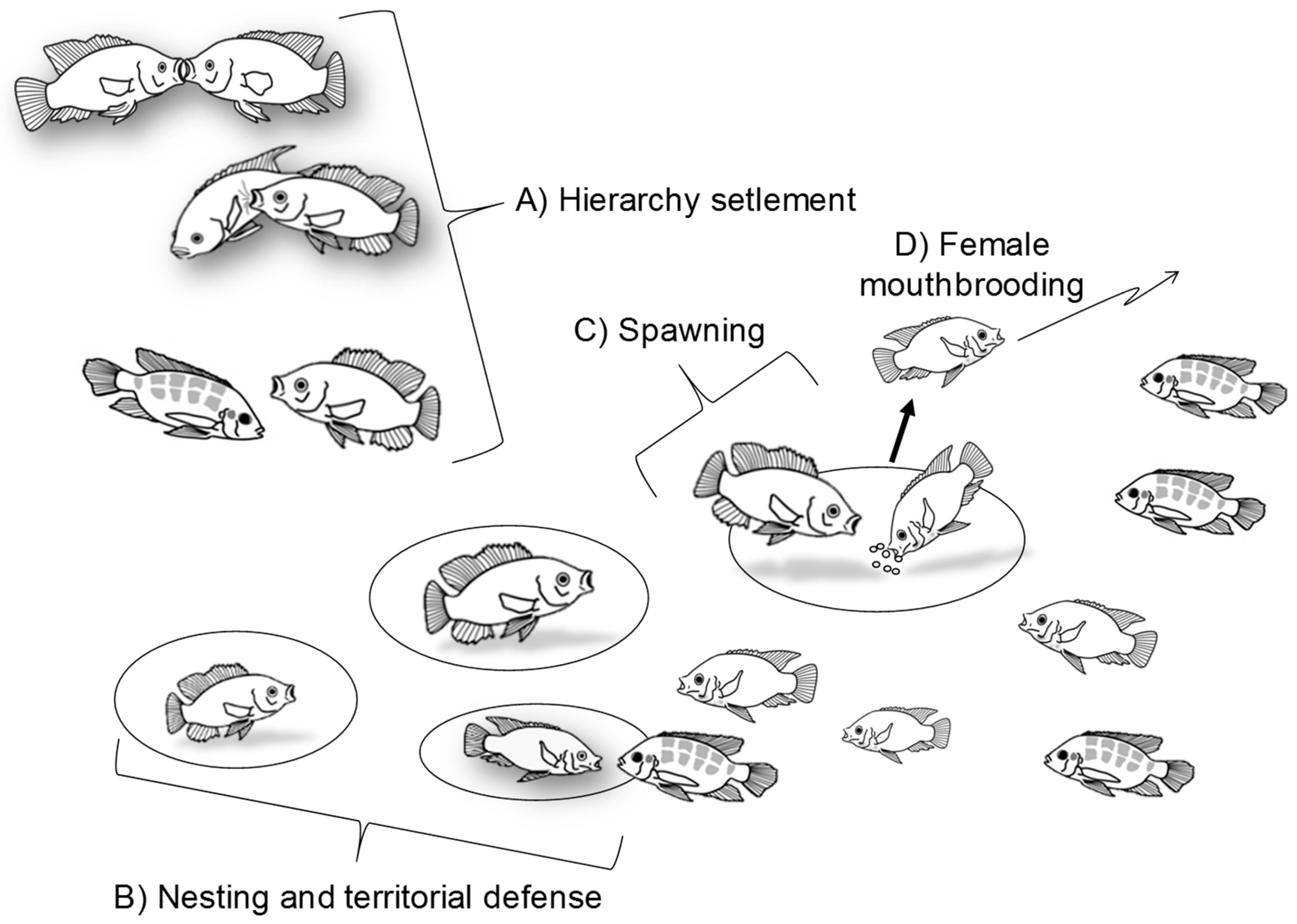
2. Nile Tilapia Social Behavior
3. Social Stress and Socially Controlled Growth
4. Impacts on Social Rank-Based Behavior
4.1. Body Size and Fighting Abilities
4.2. Stocking Density
4.3. Chemical Communication and Social Rank Signaling
4.4. Environment Lighting
5. Mitigation Technics
5.1. Environmental Enrichment
5.2. Body Tactile Stimulation
5.3. Environment Color
5.4. Preference Tests
6. Concluding Remarks
- Knowing communication channels and their role as social signaling to keep a good environment for social communication. Lack of information on social rank will increase aggressive interactions.
- The dynamics of social interaction, including information regarding the adaptive value of such behavior (e.g., fighting ability). In this sense, grading to gather same sized fish will group together individuals with the same fighting abilities. The consequence will be increased aggressions and, certainly, mortality will be higher than that already considered during fish management.
- Factors affecting aggressiveness (such as environment lighting and color) and physiological control, both neural and hormonal, of individual aggressiveness. Adequate control on such variables will help not to exaggerate the effects from aggressiveness.
- Strategies to counteract aggressive behaviors, such as body tactile stimulation, which will increase welfare and health in several ways. This is still a developing possibility for fish keeping and aquaculture.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, G.E.; Fernald, R.D.; Clayton, D.F. Genes and Social Behavior. Science 2008, 322, 896–900. [Google Scholar] [CrossRef]
- Teresa, F.B.; Gonçalves-de-Freitas, E. Reproductive Behavior and Parental Roles of the Cichlid Fish Laetacara araguaiae. Neotrop. Ichthyol. 2011, 9, 355–362. [Google Scholar] [CrossRef]
- Tanaka, H.; Frommen, J.G.; Koblmüller, S.; Sefc, K.M.; McGee, M.; Kohda, M.; Awata, S.; Hori, M.; Taborsky, M. Evolutionary Transitions to Cooperative Societies in Fishes Revisited. Ethology 2018, 124, 777–789. [Google Scholar] [CrossRef]
- Kasumyan, A.O.; Pavlov, D.S. Evolution of Schooling Behavior in Fish. J. Ichthyol. 2018, 58, 670–678. [Google Scholar] [CrossRef]
- Damsgård, B.; Huntingford, F. Fighting and Aggression. In Aquaculture and Behavior; Huntingford, F., Jobling, M., Kadri, S., Eds.; Wiley-Blackwell: Oxford, UK, 2012; p. 340. [Google Scholar]
- Paula, J.R.; Messias, J.P.; Grutter, A.S.; Bshary, R.; Soares, M.C. The Role of Serotonin in the Modulation of Cooperative Behavior. Behav. Ecol. 2015, 26, 1005–1012. [Google Scholar] [CrossRef]
- Triki, Z.; Bshary, R.; Grutter, A.S.; Ros, A.F.H. The Arginine-Vasotocin and Serotonergic Systems Affect Interspecific Social Behaviour of Client Fish in Marine Cleaning Mutualism. Physiol. Behav. 2017, 174, 136–143. [Google Scholar] [CrossRef]
- Desjardins, J.K.; Fernald, R.D. How Do Social Dominance and Social Information Influence Reproduction and the Brain? Integr. Comp. Biol. 2008, 48, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.F.; McGregor, P.K.; Latruffe, C. Know Thine Enemy: Fighting Fish Gather Information from Observing Conspecific Interactions. Proc. R. Soc. B Biol. Sci. 1998, 265, 1045–1049. [Google Scholar] [CrossRef]
- Keller-Costa, T.; Canário, A.V.M.; Hubbard, P.C. Chemical Communication in Cichlids: A Mini-Review. Gen. Comp. Endocrinol. 2015, 221, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-de-Freitas, E.; Teresa, F.B.; Gomes, F.S.; Giaquinto, P.C. Effect of Water Renewal on Dominance Hierarchy of Juvenile Nile Tilapia. Appl. Anim. Behav. Sci. 2008, 112, 187–195. [Google Scholar] [CrossRef]
- dos Santos Gauy, A.C.; Boscolo, C.N.P.; Gonçalves-de-Freitas, E. Less Water Renewal Reduces Effects on Social Aggression of the Cichlid Pterophyllum scalare. Appl. Anim. Behav. Sci. 2018, 198, 121–126. [Google Scholar] [CrossRef]
- Creel, S.; Dantzer, B.; Goymann, W.; Rubenstein, D.R. The Ecology of Stress: Effects of the Social Environment. Funct. Ecol. 2013, 27, 66–80. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Cortisol Concentrations and the Social Significance of Rank Instability among Wild Baboons. Psychoneuroendocrinology 1992, 17, 701–709. [Google Scholar] [CrossRef]
- Carvalho, R.R.; Palme, R.; da Silva Vasconcellos, A. An Integrated Analysis of Social Stress in Laying Hens: The Interaction between Physiology, Behaviour, and Hierarchy. Behav. Processes 2018, 149, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Barlow, G. The Cichlid Fishes: Nature’s Grand Experiment in Evolution, 1st ed.; Basic Books: New York, NY, USA, 2002. [Google Scholar]
- Balshine, S.; Sloman, K. Parental Care in Fishes. In Encyclopedia of Fish Physiology: From Genome to Environment; Farrel, A.P., Ed.; Academic Press: San Diego, CA, USA, 2011; Volume 1, pp. 670–677. [Google Scholar]
- Brandão, M.L.; Colognesi, G.; Bolognesi, M.C.; Costa-Ferreira, R.S.; Carvalho, T.B.; Gonçalves-de-Freitas, E. Water Temperature Affects Aggressive Interactions in a Neotropical Cichlid Fish. Neotrop. Ichthyol. 2018, 16, 1–8. [Google Scholar] [CrossRef]
- Huntingford, F.A.; Turner, A.K. (Eds.) Animal Conflict; Springer: Dordrecht, The Netherlands, 1987. [Google Scholar] [CrossRef]
- FAO. World Aquaculture 2015: A Brief Overview; FAO: Rome, Italy, 2017; Volume 1140. [Google Scholar]
- Lowe-McConnell, R.H. Observations on the Biology of Tilapia nilotica Linné in East African Waters. Rev. Zool. Bot. Afr. 1958, 57, 129–170. [Google Scholar]
- Gonçalves-de-Freitas, E.; Nishida, S.M. Sneaking Behavior of the Nile Tilapia. Bol. Técnico CEPTA 1998, 11, 71–79. [Google Scholar]
- Mendonça, F.Z.; Gonçalves-de-Freitas, E. Nest Deprivation and Mating Success in Nile Tilapia (Teleostei: Cichlidae). Rev. Bras. Zool. 2008, 25, 413–418. [Google Scholar] [CrossRef]
- Carvalho, T.; Gonçalves-de-Freitas, E. Sex Group Composition, Social Interaction, and Metabolism in the Fish Nile Tilapia. Braz. J. Biol. 2008, 68, 807–812. [Google Scholar] [CrossRef]
- Gonçalves-de-freitas, E.; Ferreira, A.C.; Paulista, U.E.; José, S. Female Social Dominance Does Not Establish Mating Priority in Nile Tilapia. Etologia 2004, 6, 33–37. [Google Scholar]
- Pinho-Neto, C.F.; Miyai, C.A.; Sanches, F.H.C.; Giaquinto, P.C.; Delicio, H.C.; Barcellos, L.J.G.; Volpato, G.L.; Barreto, R.E. Does Sex Influence Intraspecific Aggression and Dominance in Nile Tilapia Juveniles? Behav. Process. 2014, 105, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, C.M.D.; Volpato, G.L. Agonistic Profile and Metabolism in Alevins of the Nile Tilapia. Physiol. Behav. 1995, 57, 75–80. [Google Scholar] [CrossRef]
- de Verdal, H.; O’Connell, C.M.; Mekkawy, W.; Vandeputte, M.; Chatain, B.; Bégout, M.-L.; Benzie, J.A.H. Agonistic Behaviour and Feed Efficiency in Juvenile Nile Tilapia Oreochromis niloticus. Aquaculture 2019, 505, 271–279. [Google Scholar] [CrossRef]
- Falter, U. Description of the Color Patterns in Oreochromis niloticus (L) (Teleostei, Cichlidae). Ann. Soc. R. Zool. Belgique 1987, 117, 201–219. [Google Scholar]
- Volpato, G.L.; Luchiari, A.C.; Duarte, C.R.A.; Barreto, R.E.; Ramanzini, G.C. Eye Color as an Indicator of Social Rank in the Fish Nile Tilapia. Braz. J. Med. Biol. Res. 2003, 36, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, P.C.; Volpato, G.L. Chemical Communication, Aggression, and Conspecific Recognition in the Fish Nile Tilapia. Physiol. Behav. 1997, 62, 1333–1338. [Google Scholar] [CrossRef]
- Longrie, N.; Poncin, P.; Denoël, M.; Gennotte, V.; Delcourt, J.; Parmentier, E. Behaviours Associated with Acoustic Communication in Nile Tilapia (Oreochromis niloticus). PLoS ONE 2013, 8, e61467. [Google Scholar] [CrossRef] [PubMed]
- Longrie, N.; Van Wassenbergh, S.; Vandewalle, P.; Mauguit, Q.; Parmentier, E. Potential Mechanism of Sound Production in Oreochromis niloticus (Cichlidae). J. Exp. Biol. 2009, 212, 3395–3402. [Google Scholar] [CrossRef]
- Volpato, G.L.; Frioli, P.M.A.; Carrieri, M.P. Heterogeneous Growth in Fishes: Some New Data of Nile Tilapia Oreochromis niloticus and a General View about the Causal Mechanisms. Bol. Fisiol. Anim. 1989, 13, 7–22. [Google Scholar]
- Volpato, G.L.; Fernandes, M.O. Social Control of Growth in Fish. Braz. J. Med. Biol. Res. 1994, 27, 797–810. [Google Scholar]
- Carrieri, M.P.; Volpato, G.L. Does Snatching Frequency Really Indicate Food Ingestion in the Nile Tilapia? Physiol. Behav. 1991, 50, 489–492. [Google Scholar] [CrossRef]
- Fernandes, M.O.; Volpato, G.L. Heterogeneous Growth in the Nile Tilapia: Social Stress and Carbohydrate Metabolism. Physiol. Behav. 1993, 54, 319–323. [Google Scholar] [CrossRef]
- Enquist, M.; Leimar, O. Evolution of Fighting Behaviour: Decision Rules and Assessment of Relative Strength. J. Theor. Biol. 1983, 102, 387–410. [Google Scholar] [CrossRef]
- Nelissen, M.H.J. Does Body Size Affect the Ranking of a Cichild Fish in a Dominance Hierarchy? J. Ethol. 1992, 10, 153–156. [Google Scholar] [CrossRef]
- Beeching, S.C. Visual Assessment of Relative Body Size in a Cichlid Fish, the Oscar, Astronotus ocellatus. Ethology 1992, 90, 177–186. [Google Scholar] [CrossRef]
- Enquist, M.; Ljungberg, T.; Zandor, A. Visual Assessment of Fighting Ability in the Cichlid Fish Nannacara anomala. Anim. Behav. 1987, 35, 1262–1264. [Google Scholar] [CrossRef]
- Slavík, O.; Pešta, M.; Horký, P. Effect of Grading on Energy Consumption in European Catfish Silurus Glanis. Aquaculture 2011, 313, 73–78. [Google Scholar] [CrossRef]
- Boscolo, C.N.P.; Morais, R.N.; Gonçalves-de-Freitas, E. Same-Sized Fish Groups Increase Aggressive Interaction of Sex-Reversed Males Nile Tilapia GIFT Strain. Appl. Anim. Behav. Sci. 2011, 135, 154–159. [Google Scholar] [CrossRef]
- Barreto, T.N.; Boscolo, C.N.P.; Gonçalves-de-Freitas, E. Homogeneously Sized Groups Increase Aggressive Interaction and Affect Social Stress in Thai Strain Nile Tilapia (Oreochromis niloticus). Mar. Freshw. Behav. Physiol. 2015, 48, 309–318. [Google Scholar] [CrossRef]
- Garcia, F.; Romera, D.M.; Gozi, K.S.; Onaka, E.M.; Fonseca, F.S.; Schalch, S.H.C.; Candeira, P.G.; Guerra, L.O.M.; Carmo, F.J.; Carneiro, D.J.; et al. Stocking Density of Nile Tilapia in Cages Placed in a Hydroelectric Reservoir. Aquaculture 2013, 410–411, 51–56. [Google Scholar] [CrossRef]
- Ellis, T.; North, B.; Scott, A.P.; Bromage, N.R.; Porter, M.; Gadd, D. The Relationships between Stocking Density and Welfare in Farmed Rainbow Trout. J. Fish Biol. 2002, 61, 493–531. [Google Scholar] [CrossRef]
- Adams, C.E.; Turnbull, J.F.; Bell, A.; Bron, J.E.; Huntingford, F.A. Multiple Determinants of Welfare in Farmed Fish: Stocking Density, Disturbance, and Aggression in Atlantic Salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2007, 64, 336–344. [Google Scholar] [CrossRef]
- Ellison, A.R.; Uren Webster, T.M.; Rey, O.; Garcia de Leaniz, C.; Consuegra, S.; Orozco-terWengel, P.; Cable, J. Transcriptomic Response to Parasite Infection in Nile Tilapia (Oreochromis niloticus) Depends on Rearing Density. BMC Genom. 2018, 19, 723. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Nicolaiewsky, S.; De Souza, S.M.G.; Lulhier, F. Plasmatic Levels of Cortisol in the Response to Acute Stress in Nile Tilapia, Oreochromis niloticus (L.), Previously Exposed to Chronic Stress. Aquac. Res. 1999, 30, 437–444. [Google Scholar] [CrossRef]
- Maruska, K.P.; Fernald, R.D. Contextual Chemosensory Urine Signaling in an African Cichlid Fish. J. Exp. Biol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.G. Urine as a Social Signal in the Mozambique Tilapia (Oreochromis mossambicus). Chem. Senses 2005, 30 (Suppl. 1), i309–i310. [Google Scholar] [CrossRef] [PubMed]
- Barata, E.N.; Hubbard, P.C.; Almeida, O.G.; Miranda, A.; Canário, A.V. Male Urine Signals Social Rank in the Mozambique Tilapia (Oreochromis mossambicus). BMC Biol. 2007, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Keller-Costa, T.; Saraiva, J.L.; Hubbard, P.C.; Barata, E.N.; Canário, A.V.M. A Multi-Component Pheromone in the Urine of Dominant Male Tilapia (Oreochromis mossambicus) Reduces Aggression in Rivals. J. Chem. Ecol. 2016, 42, 173–182. [Google Scholar] [CrossRef]
- Lopes, A.C.C.; Villacorta-Correa, M.A.; Carvalho, T.B. Lower Light Intensity Reduces Larval Aggression in Matrinxã, Brycon amazonicus. Behav. Process. 2018, 151, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-de-Freitas, E.; Carvalho, T.B.; Oliveira, R.F. Photoperiod Modulation of Aggressive Behavior Is Independent of Androgens in a Tropical Cichlid Fish. Gen. Comp. Endocrinol. 2014, 207, 41–49. [Google Scholar] [CrossRef]
- Walton, J.C.; Weil, Z.M.; Nelson, R.J. Influence of Photoperiod on Hormones, Behavior, and Immune Function. Front. Neuroendocrinol. 2011, 32, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.D. Effects of Melatonin, Serotonin, and Naloxone on Aggression in Isolated Cichlid Fish (Aequidens pulcher). J. Pineal Res. 1986, 3, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Jasnow, A.M.; Huhman, K.L.; Bartness, T.J.; Demas, G.E. Short Days and Exogenous Melatonin Increase Aggression of Male Syrian Hamsters (Mesocricetus auratus). Horm. Behav. 2002, 42, 13–20. [Google Scholar] [CrossRef]
- Larson, E.T.; Winberg, S.; Mayer, I.; Lepage, O.; Summers, C.H.; Øverli, Ø. Social Stress Affects Circulating Melatonin Levels in Rainbow Trout. Gen. Comp. Endocrinol. 2004, 136, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Munley, K.M.; Rendon, N.M.; Demas, G.E. Neural Androgen Synthesis and Aggression: Insights From a Seasonally Breeding Rodent. Front. Endocrinol. (Lausanne) 2018, 9. [Google Scholar] [CrossRef]
- Castro, A.L.S.; Gonçalves-de-freitas, E.; Volpato, G.L.; Oliveira, C. Visual Communication Stimulates Reproduction in Nile Tilapia, Oreochromis niloticus (L.). Braz. J. Med. Biol. Res. 2009, 42, 368–374. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento Falsarella, L.; Brandão, M.L.; Gonçalves-de-Freitas, E. Fish Adjust Aggressive Behavior to Audience Size with Limited Information on Bystanders’ Fighting Ability. Behav. Process. 2017, 142, 116–118. [Google Scholar] [CrossRef]
- Carvalho, T.B.; Ha, J.C.; Gonçalves-de-Freitas, E. Light Intensity Can Trigger Different Agonistic Responses in Juveniles of Three Cichlid Species. Mar. Freshw. Behav. Physiol. 2012, 45, 91–100. [Google Scholar] [CrossRef]
- Carvalho, T.B.; Mendonça, F.Z.; Costa-Ferreira, R.S.; Gonçalves-de-Freitas, E. The Effect of Increased Light Intensity on the Aggressive Behavior of the Nile Tilapia, Oreochromis niloticus (Teleostei: Cichlidae). Zoologia 2013, 30, 125–129. [Google Scholar] [CrossRef]
- Fiszbein, A.; Cánepa, M.; Vázquez, G.R.; Maggese, C.; Pandolfi, M. Photoperiodic Modulation of Reproductive Physiology and Behaviour in the Cichlid Fish Cichlasoma dimerus. Physiol. Behav. 2010, 99, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Chavez, C.C.; Al-Khamees, S.; Campos-Mendoza, A.; Penman, D.J.; Migaud, H. Clock-Controlled Endogenous Melatonin Rhythms in Nile Tilapia (Oreochromis niloticus niloticus) and African Catfish (Clarias gariepinus). Chronobiol. Int. 2008, 25, 31–49. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M.; Kawanna, M. Effects of Photoperiod on Growth and Spawning Efficiency of Nile Tilapia (Oreochromis niloticus L.) Broodstock in a Recycling System. Aquac. Res. 2007, 38, 1242–1247. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental Enrichment: Increasing the Biological Relevance of Captive Environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Mason, G.; Clubb, R.; Latham, N.; Vickery, S. Why and How Should We Use Environmental Enrichment to Tackle Stereotypic Behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef]
- van de Weerd, H.A.; Day, J.E.L. A Review of Environmental Enrichment for Pigs Housed in Intensive Housing Systems. Appl. Anim. Behav. Sci. 2009, 116, 1–20. [Google Scholar] [CrossRef]
- Salvanes, A.G.V.; Braithwaite, V.A. Exposure to Variable Spatial Information in the Early Rearing Environment Generates Asymmetries in Social Interactions in Cod (Gadus morhua). Behav. Ecol. Sociobiol. 2005, 59, 250–257. [Google Scholar] [CrossRef]
- Salvanes, A.G.V.; Braithwaite, V.A. The Need to Understand the Behaviour of Fish Reared for Mariculture or Restocking. ICES J. Mar. Sci. 2006, 63, 346–354. [Google Scholar] [CrossRef]
- Salvanes, A.G.V.; Moberg, O.; Ebbesson, L.O.E.; Nilsen, T.O.; Jensen, K.H.; Braithwaite, V. a. Environmental Enrichment Promotes Neural Plasticity and Cognitive Ability in Fish. Proc. Biol. Sci. 2013, 280, 20131331. [Google Scholar] [CrossRef]
- Basquill, S.P.; Grant, J.W.A. An Increase in Habitat Complexity Reduces Aggression and Monopolization of Food by Zebra Fish (Danio Rerio); Prentice Hall Inc.: Upper Saddle River, NJ, USA, 1997; Volume 98. [Google Scholar]
- Barley, A.J.; Coleman, R.M. Habitat Structure Directly Affects Aggression in Convict Cichlids Archocentrus nigrofasciatus. Curr. Zool. 2010, 56, 52–56. [Google Scholar]
- Kadry, V.O.; Barreto, R.E. Environmental Enrichment Reduces Aggression of Pearl Cichlid, Geophagus brasiliensis, during Resident-Intruder Interactions. Neotrop. Ichthyol. 2010, 8, 329–332. [Google Scholar] [CrossRef]
- Torrezani, C.S.; Pinho-Neto, C.F.; Miyai, C.A.; Sanches, F.H.C.; Barreto, R.E. Structural Enrichment Reduces Aggression in Tilapia rendalli. Mar. Freshw. Behav. Physiol. 2013, 46, 183–190. [Google Scholar] [CrossRef]
- Barreto, R.E.; Carvalho, G.G.A.; Volpato, G.L. The Aggressive Behavior of Nile Tilapia Introduced into Novel Environments with Variation in Enrichment. Zoology 2011, 114, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Arnott, G.; Elwood, R.W. Information Gathering and Decision Making about Resource Value in Animal Contests. Anim. Behav. 2008, 76, 529–542. [Google Scholar] [CrossRef]
- Dunbar, R.I.M. The Social Role of Touch in Humans and Primates: Behavioural Function and Neurobiological Mechanisms. Neurosci. Biobehav. Rev. 2010, 34, 260–268. [Google Scholar] [CrossRef]
- Soares, M.C.; Oliveira, R.F.; Ros, A.F.H.; Grutter, A.S.; Bshary, R. Tactile Stimulation Lowers Stress in Fish. Nat. Commun. 2011, 2, 534. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.C.; dos Santos Gauy, A.C.; Gonçalves-de-Freitas, E. Tactile Stimulation Reduces Aggressiveness but Does Not Lower Stress in a Territorial Fish. Sci. Rep. 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Tallet, C.; Sy, K.; Prunier, A.; Nowak, R.; Boissy, A.; Boivin, X. Behavioural and Physiological Reactions of Piglets to Gentle Tactile Interactions Vary According to Their Previous Experience with Humans. Livest. Sci. 2014, 167, 331–341. [Google Scholar] [CrossRef]
- Schmied, C.; Waiblinger, S.; Scharl, T.; Leisch, F.; Boivin, X. Stroking of Different Body Regions by a Human: Effects on Behaviour and Heart Rate of Dairy Cows. Appl. Anim. Behav. Sci. 2008, 109, 25–38. [Google Scholar] [CrossRef]
- Clotfelter, E.D.; O’Hare, E.P.; McNitt, M.M.; Carpenter, R.E.; Summers, C.H. Serotonin Decreases Aggression via 5-HT1A Receptors in the Fighting Fish Betta splendens. Pharmacol. Biochem. Behav. 2007, 87, 222–231. [Google Scholar] [CrossRef]
- Zubizarreta, L.; Perrone, R.; Stoddard, P.K.; Costa, G.; Silva, A.C. Differential Serotonergic Modulation of Two Types of Aggression in Weakly Electric Fish. Front. Behav. Neurosci. 2012, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, S.; Laria, R.; Gray, S.M.; Hawryshyn, C.W. Functional Diversity in the Color Vision of Cichlid Fishes. BMC Biol. 2010, 8, 133. [Google Scholar] [CrossRef]
- Hofmann, C.M.; O’Quin, K.E.; Justin Marshall, N.; Cronin, T.W.; Seehausen, O.; Carleton, K.L. The Eyes Have It: Regulatory and Structural Changes Both Underlie Cichlid Visual Pigment Diversity. PLoS Biol. 2009, 7. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.M.; Volpato, G.L. Preference Index Supported by Motivation Tests in Nile Tilapia. PLoS ONE 2017, 12, e0175821. [Google Scholar] [CrossRef]
- Torres, I.F.A.; da Silva Ferreira, A.; de Souza e Silva, W.; Mesquita, F.O.; Luz, R.K. Effect of Environmental Color on Learning of Nile Tilapia. Appl. Anim. Behav. Sci. 2018, 209, 104–108. [Google Scholar] [CrossRef]
- Maia, C.M.; Volpato, G.L. Environmental Light Color Affects the Stress Response of Nile Tilapia. Zoology 2013, 116, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Volpato, G.L.; Bovi, T.S.; de Freitas, R.H.A.; da Silva, D.F.; Delicio, H.C.; Giaquinto, P.C.; Barreto, R.E. Red Light Stimulates Feeding Motivation in Fish but Does Not Improve Growth. PLoS ONE 2013, 8, e59134. [Google Scholar] [CrossRef] [PubMed]
- Volpato, G.L.; Duarte, C.R.A.; Luchiari, A.C. Environmental Color Affects Nile Tilapia Reproduction. Braz. J. Med. Biol. Res. 2004, 37, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Volpato, G.L.; Barreto, R.E. Environmental Blue Light Prevents Stress in the Fish Nile Tilapia. Braz. J. Med. Biol. Res. 2001, 34, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Volpato, G.; Gonçalves-de-Freitas, E.; Fernandes-de-Castilho, M. Insights into the Concept of Fish Welfare. Dis. Aquat. Organ. 2007, 75, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, M.S. The Science of Animal Suffering. Ethology 2008, 114, 937–945. [Google Scholar] [CrossRef]
- Volpato, G.L. Challenges in Assessing Fish Welfare. ILAR J. 2009, 50, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Luchiari, A.C.; do Amaral Duarte, C.R.; de Morais Freire, F.A.; Nissinen, K. Hierarchical Status and Colour Preference in Nile Tilapia (Oreochromis niloticus). J. Ethol. 2007, 25, 169–175. [Google Scholar] [CrossRef]
- Maia, C.M.; Volpato, G.L. A History-Based Method to Estimate Animal Preference. Sci. Rep. 2016, 6, 28328. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, F.Z.; Volpato, G.L.; Costa-Ferreira, R.S.; Gonçalves-de-Freitas, E. Substratum Choice for Nesting in Male Nile Tilapia Oreochromis niloticus. J. Fish Biol. 2010, 77, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.H.A.; Volpato, G.L. Motivation and Time of Day Affect Decision-Making for Substratum Granulometry in the Nile Tilapia Oreochromis niloticus. J. Appl. Ichthyol. 2013, 29, 239–241. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves-de-Freitas, E.; Bolognesi, M.C.; Gauy, A.C.d.S.; Brandão, M.L.; Giaquinto, P.C.; Fernandes-Castilho, M. Social Behavior and Welfare in Nile Tilapia. Fishes 2019, 4, 23. https://doi.org/10.3390/fishes4020023
Gonçalves-de-Freitas E, Bolognesi MC, Gauy ACdS, Brandão ML, Giaquinto PC, Fernandes-Castilho M. Social Behavior and Welfare in Nile Tilapia. Fishes. 2019; 4(2):23. https://doi.org/10.3390/fishes4020023
Chicago/Turabian StyleGonçalves-de-Freitas, Eliane, Marcela Cesar Bolognesi, Ana Carolina dos Santos Gauy, Manuela Lombardi Brandão, Percilia Cardoso Giaquinto, and Marisa Fernandes-Castilho. 2019. "Social Behavior and Welfare in Nile Tilapia" Fishes 4, no. 2: 23. https://doi.org/10.3390/fishes4020023
APA StyleGonçalves-de-Freitas, E., Bolognesi, M. C., Gauy, A. C. d. S., Brandão, M. L., Giaquinto, P. C., & Fernandes-Castilho, M. (2019). Social Behavior and Welfare in Nile Tilapia. Fishes, 4(2), 23. https://doi.org/10.3390/fishes4020023




