Comparative Analysis of the Blood Plasma Metabolome of Negligible, Gradual and Rapidly Ageing Fishes
Abstract
1. Introduction
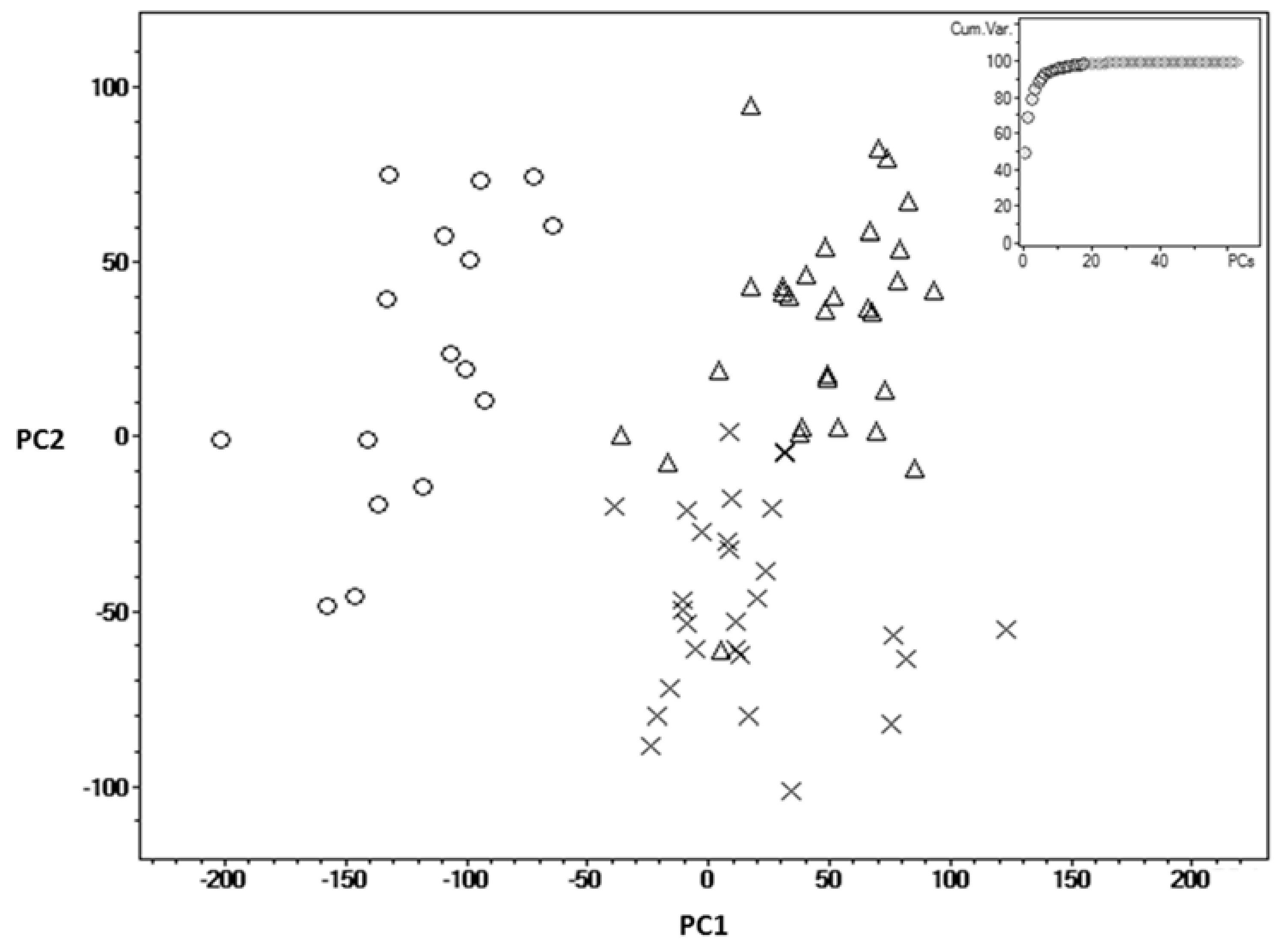
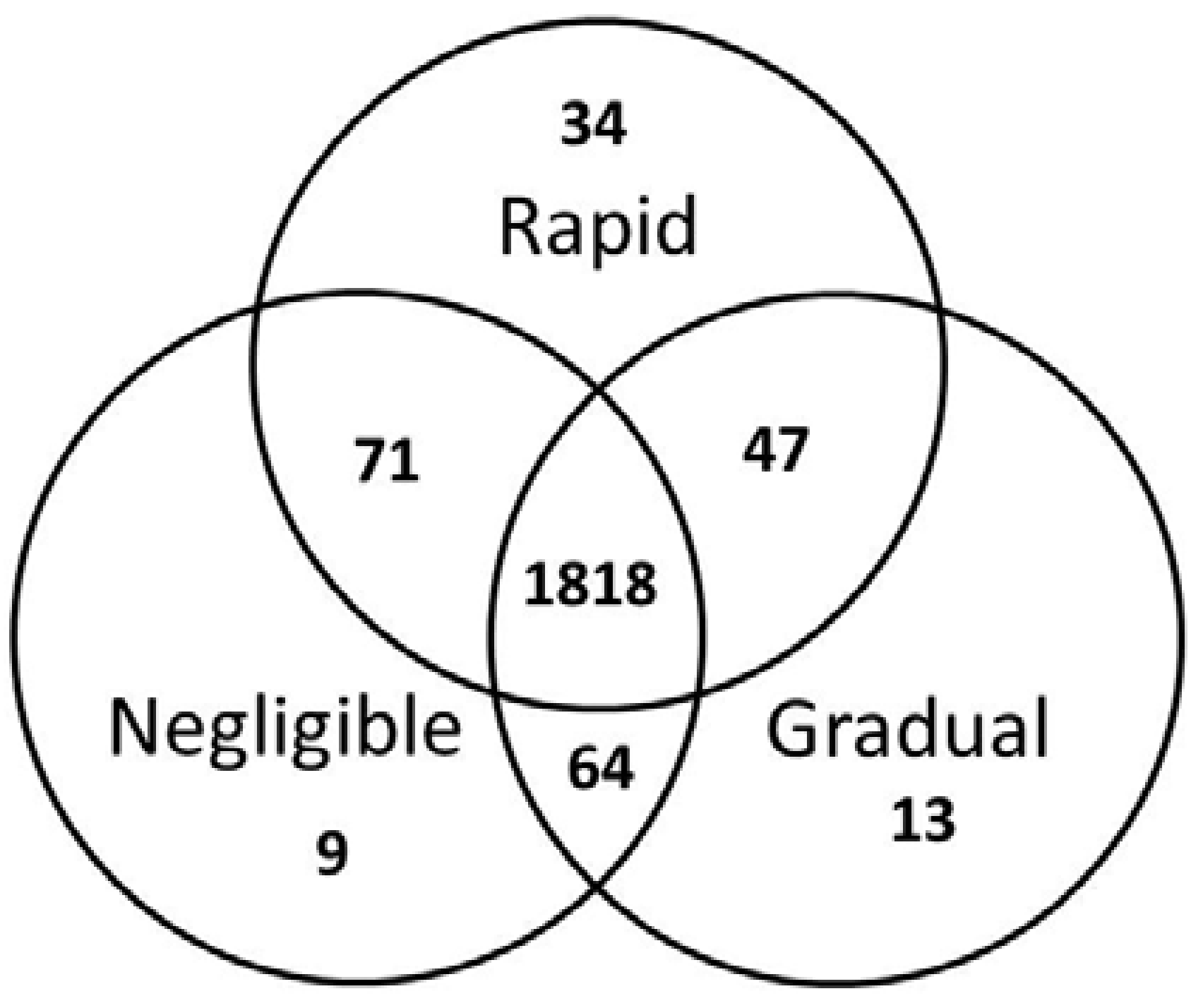
2. Results
3. Discussion
4. Materials and Methods
4.1. Fish, Experimental Conditions and Sampling
4.2. Blood Plasma Metabolite Extraction and Mass Spectrometry Analysis
4.3. Data Analysis
4.4. Mass Spectra Peak Annotation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Finch, C.E. Longevity, Senescence, and the Genome; University of Chicago Press: Chicago, IL, USA, 1990; ISBN 9780226248899. [Google Scholar]
- Finch, C.E. Variations in Senescence and Longevity Include the Possibility of Negligible Senescence. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1998, 53A, B235–B239. [Google Scholar] [CrossRef]
- Craig, T.; Smelick, C.; Tacutu, R.; Wuttke, D.; Wood, S.H.; Stanley, H.; Janssens, G.; Savitskaya, E.; Moskalev, A.; Arking, R.; et al. The Digital Ageing Atlas: Integrating the diversity of age-related changes into a unified resource. Nucleic Acids Res. 2015, 43, D873–D878. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Scheuerlein, A. Comparison of aging-related mortality among birds and mammals. Exp. Gerontol. 2001, 36, 845–857. [Google Scholar] [CrossRef]
- Finch, C.E.; Austad, S.N. History and prospects: Symposium on organisms with slow aging. Exp. Gerontol. 2001, 36, 593–597. [Google Scholar] [CrossRef]
- Finch, C.E. Update on slow aging and negligible senescence—A mini-review. Gerontology 2009, 55, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Cailliet, G.M.; Andrews, A.H.; Burton, E.J.; Watters, D.L.; Kline, D.E.; Ferry-Graham, L.A. Age determination and validation studies of marine fishes: Do deep-dwellers live longer? Exp. Gerontol. 2001, 36, 739–764. [Google Scholar] [CrossRef]
- Traniello, I.M.; Sîrbulescu, R.F.; Ilieş, I.; Zupanc, G.K.H. Age-related changes in stem cell dynamics, neurogenesis, apoptosis, and gliosis in the adult brain: A novel teleost fish model of negligible senescence. Dev. Neurobiol. 2014, 74, 514–530. [Google Scholar] [CrossRef]
- Fang, X.; Seim, I.; Huang, Z.; Gerashchenko, M.V.; Xiong, Z.; Turanov, A.A.; Zhu, Y.; Lobanov, A.V.; Fan, D.; Yim, S.H.; et al. Adaptations to a Subterranean Environment and Longevity Revealed by the Analysis of Mole Rat Genomes. Cell Rep. 2014, 8, 1354–1364. [Google Scholar] [CrossRef]
- Patnaik, B.K.; Mahapatro, N.; Jena, B.S. Ageing in fishes. Gerontology 1994, 40, 113–132. [Google Scholar] [CrossRef]
- Craig, J.F.; Kipling, C. Reproduction effort versus the environment; case histories of Windermere perch, Perca fluviatilis L., and pike, Esox lucius L. J. Fish Biol. 1983, 22, 713–727. [Google Scholar] [CrossRef]
- Forsman, A.; Tibblin, P.; Berggren, H.; Nordahl, O.; Koch-Schmidt, P.; Larsson, P. Pike Esox lucius as an emerging model organism for studies in ecology and evolutionary biology: A review. J. Fish Biol. 2015, 87, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Mishur, R.J.; Rea, S.L. Applications of mass spectrometry to metabolomics and metabonomics: Detection of biomarkers of aging and of age-related diseases. Mass Spectrom. Rev. 2012, 31, 70–95. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The human serum metabolome. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, Z.; Giegling, I.; Xie, L.; Hartmann, A.M.; Prehn, C.; Adamski, J.; Kahn, R.; Li, Y.; Illig, T.; et al. Schizophrenia shows a unique metabolomics signature in plasma. Transl. Psychiatry 2012. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, G.; Hao, H.; Huang, Q.; Yan, B.; Zha, W.; Gu, S.; Ren, H.; Zhang, Y.; Fan, X.; et al. Gas chromatography/time-of-flight mass spectrometry based metabonomic approach to differentiating hypertension- and age-related metabolic variation in spontaneously hypertensive rats. Rapid Commun. Mass Spectrom. 2008, 22, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wu, B.; Lin, Z.; Jin, H.; Huang, J.; Yang, Y.; Zhang, X.; Shen, Z.; Zhang, W. Metabonomic characterization of aging and investigation on the anti-aging effects of total flavones of Epimedium. Mol. Biosyst. 2009. [Google Scholar] [CrossRef] [PubMed]
- Nevedomskaya, E.; Meissner, A.; Goraler, S.; De Waard, M.; Ridwan, Y.; Zondag, G.; Van Der Pluijm, I.; Deelder, A.M.; Mayboroda, O.A. Metabolic profiling of accelerated aging ERCC1d/-mice. J. Proteome Res. 2010. [Google Scholar] [CrossRef]
- Lawton, K.A.; Berger, A.; Mitchell, M.; Milgram, K.E.; Evans, A.M.; Guo, L.; Hanson, R.W.; Kalhan, S.C.; Ryals, J.A.; Milburn, M.V. Analysis of the adult human plasma metabolome. Pharmacogenomics 2008. [Google Scholar] [CrossRef]
- Yu, Z.; Zhai, G.; Singmann, P.; He, Y.; Xu, T.; Prehn, C.; Römisch-Margl, W.; Lattka, E.; Gieger, C.; Soranzo, N.; et al. Human serum metabolic profiles are age dependent. Aging Cell 2012. [Google Scholar] [CrossRef]
- The Metabolomics Standards Initiative (MSI) Home Page. Available online: http://www.metabolomics-msi.org/ (accessed on 4 December 2018).
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007. [Google Scholar] [CrossRef]
- Dunn, W.B.; Erban, A.; Weber, R.J.M.; Creek, D.J.; Brown, M.; Breitling, R.; Hankemeier, T.; Goodacre, R.; Neumann, S.; Kopka, J.; et al. Mass appeal: Metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 2013, 9, 44–66. [Google Scholar] [CrossRef]
- Trifonova, O.; Lokhov, P.; Archakov, A. Postgenomics diagnostics: Metabolomics approaches to human blood profiling. OMICS 2013, 17, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D. Advances in metabolic profiling. Bioanalysis 2012, 4, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Lokhov, P.G.; Dashtiev, M.I.; Moshkovskii, S.A.; Archakov, A.I. Metabolite profiling of blood plasma of patients with prostate cancer. Metabolomics 2010, 6, 156–163. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Petroff, O.A.C. Book Review: GABA and Glutamate in the Human Brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef]
- Hamilton, M.T.; Areiqat, E.; Hamilton, D.G.; Bey, L. Plasma triglyceride metabolism in humans and rats during aging and physical inactivity. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, S97–104. [Google Scholar] [CrossRef]
- Greer, E.L.; Brunet, A. Signaling networks in aging. J. Cell Sci. 2008, 121, 407–412. [Google Scholar] [CrossRef]
- Igoe, R.S. Dictionary of Food Ingredients, 1st ed.; Springer US: New York, NY, USA, 1996; Volume 7, ISBN 9781441997135. [Google Scholar]
- Spiteller, G. Furan fatty acids: Occurrence, synthesis, and reactions. Are furan fatty acids responsible for the cardioprotective effects of a fish diet? Lipids 2005, 40, 755–771. [Google Scholar] [CrossRef]
- Brites, P.; Waterham, H.R.; Wanders, R.J.A. Functions and biosynthesis of plasmalogens in health and disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2004, 1636, 219–231. [Google Scholar] [CrossRef]
- Rockenfeller, P.; Koska, M.; Pietrocola, F.; Minois, N.; Knittelfelder, O.; Sica, V.; Franz, J.; Carmona-Gutierrez, D.; Kroemer, G.; Madeo, F. Phosphatidylethanolamine positively regulates autophagy and longevity. Cell Death Differ. 2015, 22, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Tesch, F.W. Age and Growth. In Methods for Assessment of Fish Production in Fresh Waters; Ricker, W.E., Ed.; Blackwell Sci. Pub. IBP: Hoboken, NJ, USA, 1968; pp. 93–123. [Google Scholar]
- AnAge: The Animal Ageing and Longevity Database. Available online: http://genomics.senescence.info/species/ (accessed on 4 December 2018).
- Carey, J.R.; Judge, D.S. Life Spans of Mammals, Birds, Amphibians, Reptiles, and Fish; Odense University Press: Odense, Denmark, 2000; ISBN 87-7838-539-3. [Google Scholar]
- Lokhov, P.G.; Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Archakov, A.I.; Shestakova, E.A.; Shestakova, M.V.; Dedov, I.I. Diagnosing impaired glucose tolerance using direct infusion mass spectrometry of blood plasma. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Human Metabolome Database. Available online: http://www.hmdb.ca (accessed on 4 December 2018).
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41. [Google Scholar] [CrossRef]
- METLIN. Available online: http://metlin.scripps.edu/ (accessed on 4 December 2018).
- Smith, A.; O’maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN A Metabolite Mass Spectral Database. Proc. Int. Congr. Ther. Drug Monit. Clin. Toxicol. 2005, 27, 747–751. [Google Scholar] [CrossRef]

| No. | Metabolite | HMDB ID | Mass Of Ion | Ion Form | Elemental Composition | p-Value * | Adjusted p-Value ** | |
|---|---|---|---|---|---|---|---|---|
| Measured (m/z) | Calculated (Da) | |||||||
| Increased in rapid ageing fishes—Oncorhynchus keta and Oncorhynchus gorbuscha | ||||||||
| 1 | Anserine | HMDB00194 | 263.1121 | 263.1115 | M+Na | C10H16N4O3 | 0.0004/0.0007 | |
| Balenine | HMDB05769 | 0.8/1.4 | ||||||
| Homocarnosine | HMDB00745 | 5 × 10−6/1.2 × 10−6 | ||||||
| 2 | a-Glutamylalanine | HMDB03764 | 437.1883 | 437.1878 | 2M+H | C8H14N2O5 | 2.5 × 10−9/5.1 × 10−10 | |
| g-Glutamylalanine | HMDB06248 | |||||||
| 3 | TG | N/A | 482.4210 | 482.4199 | M+2Na | C59H114O6 | 2.9 × 10−7/2 × 10−7 | |
| 482.4212 | M+H+Na | C61H112O6 | 5.8 × 10−4/4 × 10−4 | |||||
| 482.4224 | M+2H | C63H110O6 | ||||||
| 4 | DG | N/A | 571.5068 | 571.4932 | M+CH3OH+H | C33H62O5 | 1.2 × 10−13/2.8 × 10−13 | 2.4 × 10−10/5.6 × 10−10 |
| 5 | DG | N/A | 585.5222 | 585.5089 | M+CH3OH+H | C34H64O5 | 1.7 × 10−8/1.1 × 10−8 | 3.4 × 10−5/2.2 × 10−5 |
| 6 | DG | N/A | 599.5368 | 599.5245 | M+CH3OH+H | C35H66O5 | 9.6 × 10−14/3.1 × 10−14 | 2 × 10−10/6.2 × 10−11 |
| 7 | TG | N/A | 835.6687 | 835.6786 | M+Na | C52H92O6 | 2.3 × 10−8/2.2 × 10−13 | 4.6 × 10−5/4.4 × 10−10 |
| Increased in normal ageing fishes—Sander lucioperca and Perca fluviatilis | ||||||||
| 8 | 3-Methyl-5-pentyl-2-furanundecanoic acid | HMDB31005 | 695.5190 | 695.5221 | 2M+Na | C21H36O3 | 6.4 × 10−11/2.5 × 10−14 | 1.3 × 10−7/5 × 10−11 |
| 9 | PC | N/A | 716.5602 | 716.5589 | M+H | C40H78NO7P | 7.7 × 10−5/0.032 | 0.16/66 |
| 10 | 3,4-Dimethyl-5-pentyl-2-furanundecanoic acid | HMDB31126 | 723.5440 | 723.5534 | 2M+Na | C22H38O3 | 1.3 × 10−17/1.2 × 10−12 | 2.6 × 10−14/2.4 × 10−9 |
| 11 | PS | N/A | 790.5723 | 790.5592 | M+H | C42H80NO10P | 5.5 × 10−7/0.002 | 0.0011/4 |
| Increased in negligible ageing fishes—Esox Lucius and Acipenser ruthenus | ||||||||
| 12 | Hexadecanedioic acid | HMDB00672 | 287.2362 | 287.2217 | M+H | C16H30O4 | 2.4 × 10−5/0.01 | 0.048/20 |
| 13 | DG | N/A | 745.5786 | 745.5741 | M+Na | C47H78O5 | 0.002/0.02 | 4.0/40 |
| 14 | PC/PE | N/A | 758.5703 | 758.5694 | M+H | C42H80NO8P | 5.1 × 10−5/7.0 × 10−4 | 0.12/1.4 |
| 15 | PC/PE | N/A | 772.5870 | 772.5851 | M+H | C43H82NO8P | 0.01/0.007 | 20/14 |
| Ageing Rate | Rapid Ageing | Normal Ageing | Negligible Ageing | |||
|---|---|---|---|---|---|---|
| N = 20 | N = 31 | N = 31 | ||||
| Species | Oncorhynchus gorbuscha | Oncorhynchus keta | Perca fluviatilis | Sander lucioperca | Esox Lucius | Acipenser ruthenus |
| Number | 10 | 10 | 19 | 12 | 21 | 10 |
| Sex (male/female) | 5/5 | 5/5 | 7/8 | 3/6 | 8/13 | 8/2 |
| N/A* = 4 | N/A* = 2 | |||||
| Age (years) # | 2.4 ± 0.5 | 3.4 ± 0.5 | 6.7 ± 2.4 | 4.3 ± 1.9 | 6.1 ± 1.9 | 4.0 ± 0.4 |
| Mean Lifespan (years) & | 2 | 5 | 10 | 7 | 12 | 22 |
| Max Longevity (years) & | 3 | 7 | 22 | 16 | 30 | 46 |
| Pubertal timing (years) & | 1 | 3 | 2 | 3 | 2 | 3–4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifonova, O.P.; Maslov, D.L.; Mikhailov, A.N.; Zolotarev, K.V.; Nakhod, K.V.; Nakhod, V.I.; Belyaeva, N.F.; Mikhailova, M.V.; Lokhov, P.G.; Archakov, A.I. Comparative Analysis of the Blood Plasma Metabolome of Negligible, Gradual and Rapidly Ageing Fishes. Fishes 2018, 3, 46. https://doi.org/10.3390/fishes3040046
Trifonova OP, Maslov DL, Mikhailov AN, Zolotarev KV, Nakhod KV, Nakhod VI, Belyaeva NF, Mikhailova MV, Lokhov PG, Archakov AI. Comparative Analysis of the Blood Plasma Metabolome of Negligible, Gradual and Rapidly Ageing Fishes. Fishes. 2018; 3(4):46. https://doi.org/10.3390/fishes3040046
Chicago/Turabian StyleTrifonova, Oxana P., Dmitry L. Maslov, Anton N. Mikhailov, Konstantin V. Zolotarev, Kirill V. Nakhod, Valeriya I. Nakhod, Nataliya F. Belyaeva, Marina V. Mikhailova, Petr G. Lokhov, and Alexander I. Archakov. 2018. "Comparative Analysis of the Blood Plasma Metabolome of Negligible, Gradual and Rapidly Ageing Fishes" Fishes 3, no. 4: 46. https://doi.org/10.3390/fishes3040046
APA StyleTrifonova, O. P., Maslov, D. L., Mikhailov, A. N., Zolotarev, K. V., Nakhod, K. V., Nakhod, V. I., Belyaeva, N. F., Mikhailova, M. V., Lokhov, P. G., & Archakov, A. I. (2018). Comparative Analysis of the Blood Plasma Metabolome of Negligible, Gradual and Rapidly Ageing Fishes. Fishes, 3(4), 46. https://doi.org/10.3390/fishes3040046







