Effects of Harmful Algal Blooms on Fish: Insights from Prymnesium parvum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish and Husbandry
2.2. Respirometry
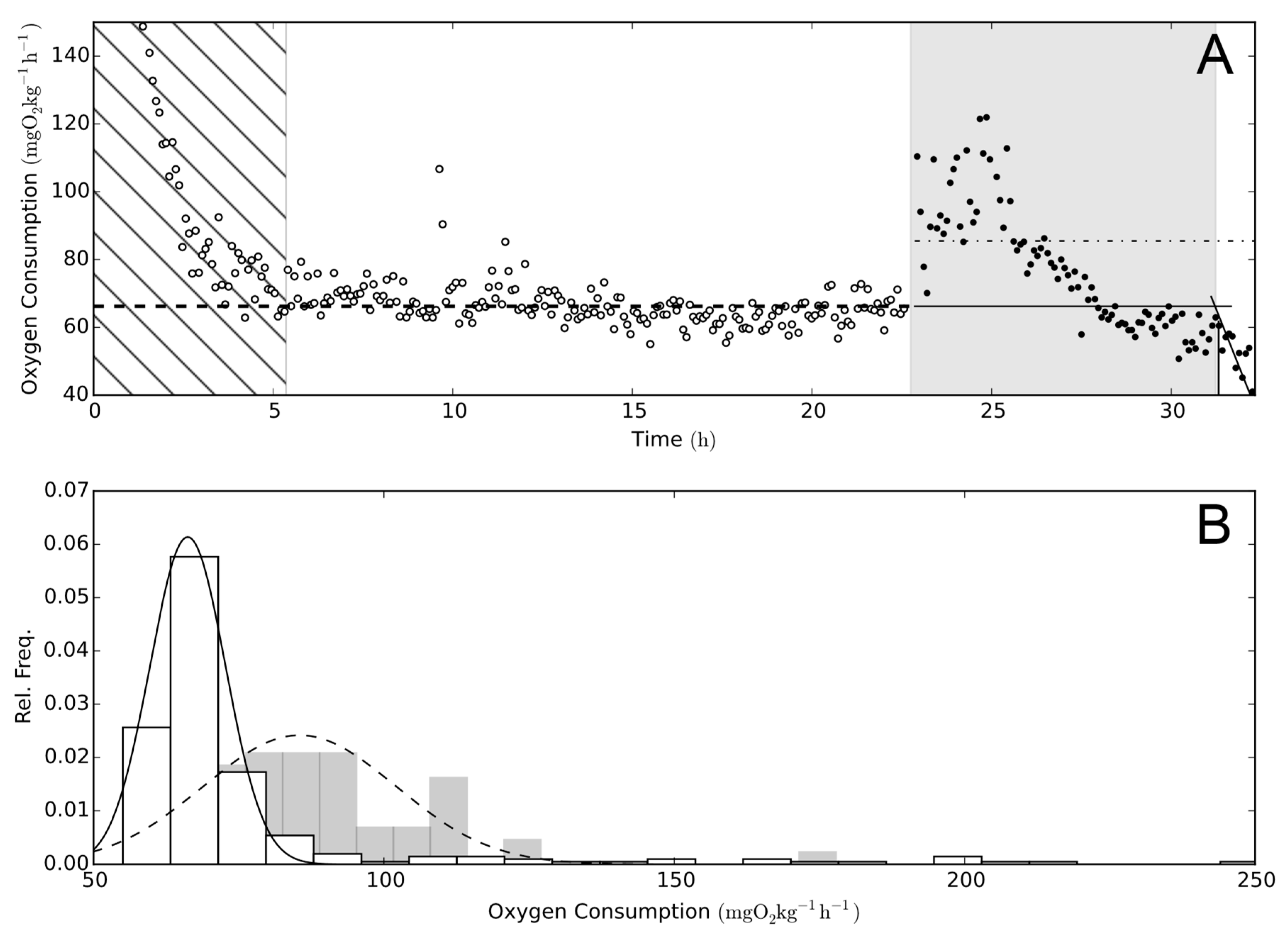
2.3. Background Oxygen Consumption
2.4. Ventilation Frequency
2.5. Loss of Equilibrium and Time to the Point of No Return
2.6. Algae Culture and Exposure
2.7. Hemolysis
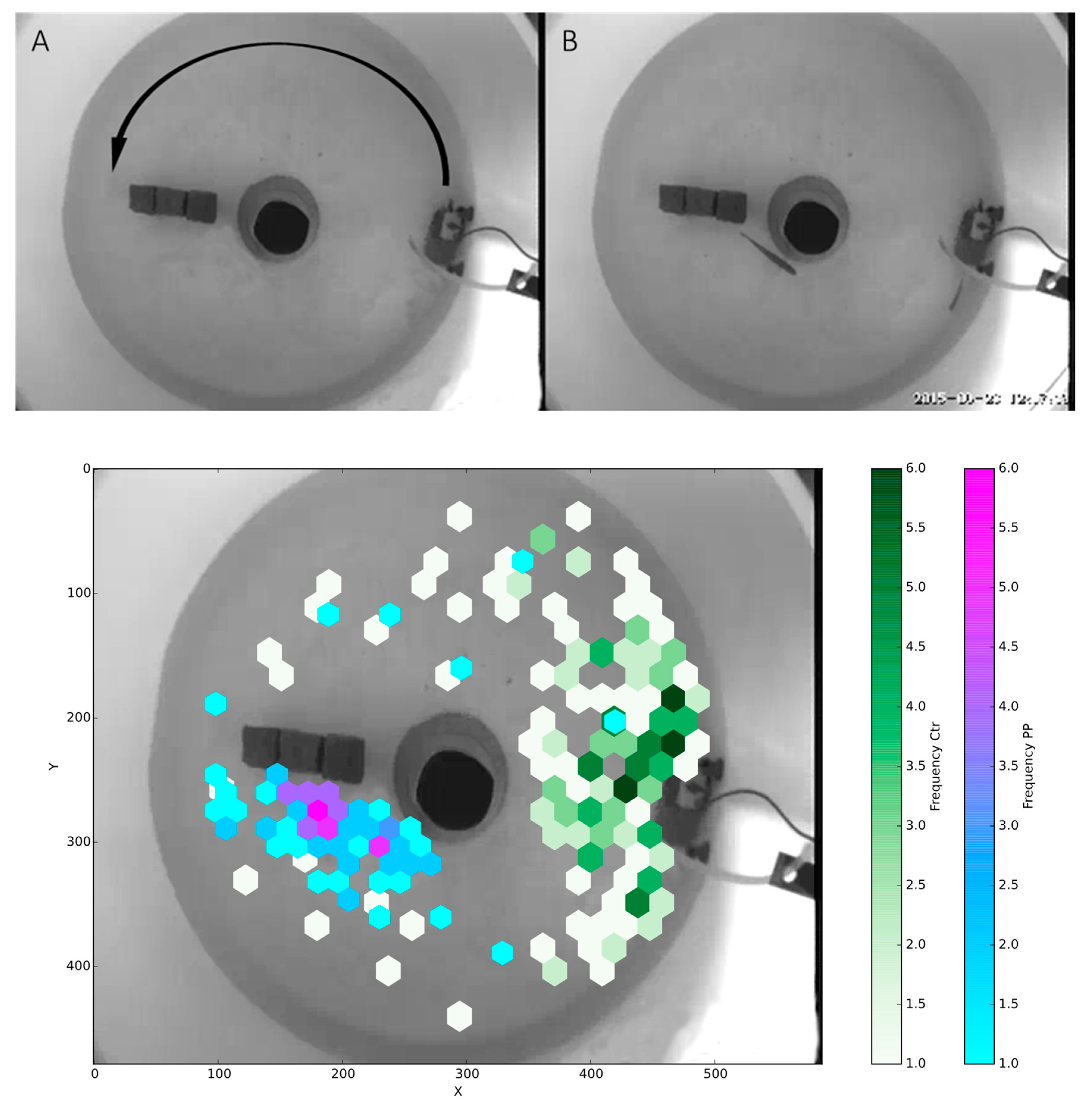
2.8. Effects on Swimming
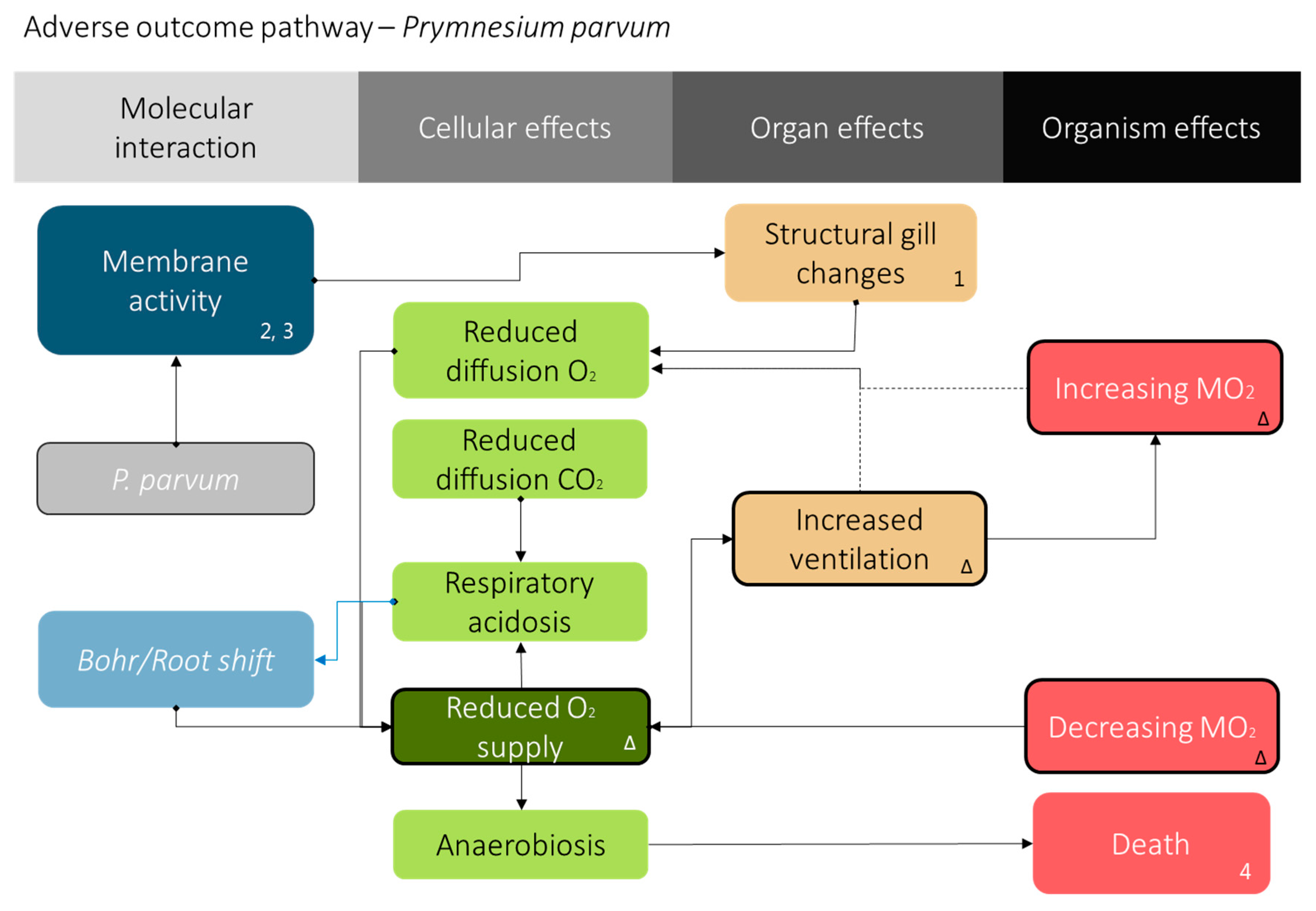
2.9. Mode of Action
2.10. Statistics
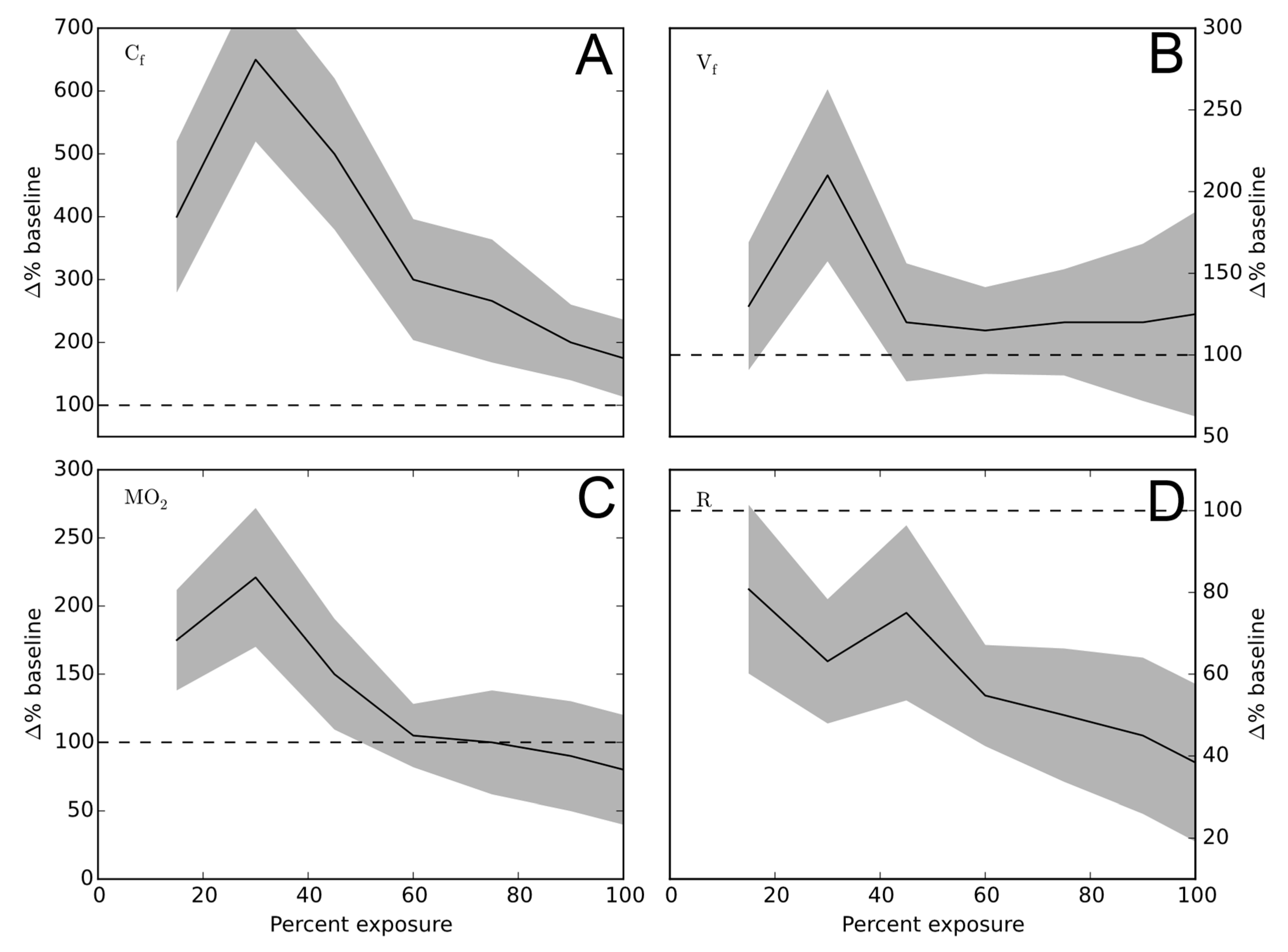
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Otterstorm, C.V.; Steemann-Nielsen, E. Two Cases of Extensive Mortality in Fishes Caused by the Flagellate Prymnesium Parvum Carter; Report Danish Biology Statistics: Copenhagen, Denmark, 1939; Volume 44, (non vidi). [Google Scholar]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Nazeer, M.; Wong, M.S.; Nichol, J.E. A new approach for the estimation of phytoplankton cell counts associated with algal blooms. Sci. Total Environ. 2017, 590, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Roelke, D.L.; Grover, J.P.; Brooks, B.W.; Glass, J.; Buzan, D.; Southard, G.M.; Fries, L.; Gable, G.M.; Schwierzke-Wade, L.; Byrd, M.; et al. A decade of fish-killing Prymnesium parvum blooms in Texas: Roles of inflow and salinity. J. Plankton Res. 2011, 33, 243–253. [Google Scholar] [CrossRef]
- Roelke, D.L.; Barkoh, A.; Brooks, B.W.; Grover, J.P.; David Hambright, K.; LaClaireII, J.W.; Moeller, P.D.R.; Patino, R. A chronicle of a killer alga in the west: Ecology, assessment, and management of Prymnesium parvum blooms. Hydrobiologia 2016, 764, 29–50. [Google Scholar] [CrossRef]
- Ulitzur, S. The amphiphatic nature of Prymnesium parvum hemolysin. Biochim. Biophys. Acta Biomembr. 1973, 298, 673–679. [Google Scholar] [CrossRef]
- Ulitzur, S.; Shilo, M. Mode of action of Prymnesium parvum ichthyotoxin. J. Protozool. 1966, 13, 332–336. [Google Scholar] [CrossRef]
- Yariv, J.; Hestrin, S. Toxicity of the extracellular phase of Prymnesium parvum cultures. Microbiology 1961, 24, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Blossom, H.E.; Rasmussen, S.A.; Andersen, N.G.; Larsen, T.O.; Nielsen, K.F.; Hansen, P.J. Prymnesium parvum revisited: Relationship between allelopathy, ichthyotoxicity, and chemical profiles in 5 strains. Aquat. Toxicol. 2014, 157, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Hishida, H.; Ishimatsu, I.; Oda, T. Effect of environmental hyperoxia on respiration of yellowtail exposed to Chattonella marina. Fish. Sci. 1999, 65, 84–90. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Maruta, H.; Oda, T.; Ozaki, M. A Comparison of physiological responses in yellowtail to fatal environmental hypoxia and exposure to Chattonella marina. Fish. Sci. 1997, 63, 557–562. [Google Scholar] [CrossRef]
- Lee, K.; Ishimatsu, A.; Sakaguchi, H.; Oda, T. Cardiac output during exposure to Chattonella marina and environmental hypoxia in yellowtail (Seriola quinqueradiata). Mar. Biol. 2003, 142, 391–397. [Google Scholar] [CrossRef]
- Binford, J.S., Jr.; Martin, D.F.; Padilla, G.M. Hemolysis induced by Prymnesium parvum toxin calorimetric studies. Biochim. Biophys. Acta 1973, 291, 156–164. [Google Scholar] [CrossRef]
- Doig, M.T.; Martin, D.F. Anticoagulant properties of a red tide toxin. Toxicon 1973, 11, 351–355. [Google Scholar] [CrossRef]
- Martin, D.F.; Padilla, G.M. Hemolysis induced by Prymnesium parvum toxin kinetics and binding. Biochim. Biophys. Acta 1971, 241, 213–225. [Google Scholar] [CrossRef]
- Martin, D.F.; Padilla, G.M.; Brown, P.A. Hemolysis induced by Prymnesium parvum toxin effect of primaquine treatment. Biochim. Biophys. Acta 1971, 249, 69–80. [Google Scholar] [CrossRef]
- Martin, D.F.; Padilla, G.M.; Heyl, M.G.; Brown, P.A. Effect of Gymnodinium breve toxin on hemolysis induced by Prymnesium parvum toxin. Toxicon 1972, 10, 285–290. [Google Scholar] [CrossRef]
- Bradbury, S.P.; Henry, T.R.; Niemi, G.J.; Carlson, R.W.; Snarski, V.M. Use of respiratory-cardiovascular responses of rainbow trout (Salmo gairdneri) in identifying acute toxicity syndromes in fish: Part 3. Polar narcotics. Environ. Toxicol. Chem. 1989, 8, 247–261. [Google Scholar] [CrossRef]
- Bradbury, S.P.; Carlson, R.W.; Niemi, G.J.; Henry, T.R. Use of respiratory-cardiovascular responses of rainbow trout (Oncorhynchus mykiss) in identifying acute toxicity syndromes in fish: Part 4. Central nervous system seizure agents. Environ. Toxicol. Chem. 1991, 10, 115–131. [Google Scholar] [CrossRef]
- McKim, J.M.; Bradbury, S.P.; Niemi, G.J. Fish acute toxicity syndromes and their use in the QSAR approach to hazard assessment. Environ. Health Perspect. 1987, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Graeb, B.D.S.; Suski, C.D.; Ostrand, K.G. Effects of suture material on incision healing, growth and survival of juvenile largemouth bass implanted with miniature radio transmitters: Case study of a novice and experienced fish surgeon. J. Fish Biol. 2003, 62, 1366–1380. [Google Scholar] [CrossRef]
- Beamish, F.W.H. Respiration of fishes with special emphasis on standard oxygen consumption: II. Influence of weight and temperature on respiration of several species. Can. J. Zool. 1964, 42, 177–188. [Google Scholar] [CrossRef]
- Fry, F.E.J. Effects of the environment on animal activity. In University of Toronto Studies; The University of Toronto Press: Toronto, ON, Canada, 1947. [Google Scholar]
- Steffensen, J.F. Some errors in respirometry of aquatic breathers: How to avoid and correct for them. Fish Physiol. Biochem. 1989, 6, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, M.B.S.; Bushnell, P.G.; Steffensen, J.F. Design and setup of intermittent-flow respirometry system for aquatic organisms. J. Fish Biol. 2016, 88, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, M.B.S.; Bushnell, P.G.; Christensen, E.A.F.; Steffensen, J.F. Sources of variation in oxygen consumption of aquatic animals demonstrated by simulated constant oxygen consumption and respirometers of different sizes. J. Fish Biol. 2016, 88, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Beamish, F.W.H. Influence of starvation on standard and routine oxygen consumption. Trans. Am. Fish. Soc. 1964, 93, 103–107. [Google Scholar] [CrossRef]
- Chabot, D.; Steffensen, J.F.; Farrell, A.P. The determination of standard metabolic rate in fishes. J. Fish Biol. 2016, 88, 81–121. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, J.F.; Bushnell, P.G.; Schurmann, H. Oxygen consumption in four species of teleosts from Greenland: No evidence of metabolic cold adaptation. Polar Biol. 1994, 14, 49–54. [Google Scholar] [CrossRef]
- Piiper, J.; Scheid, P. Maximum gas transfer efficacy of models for fish gills, avian lungs and mammalian lungs. Respir. Physiol. 1972, 14, 115–124. [Google Scholar] [CrossRef]
- Piiper, J.; Scheid, P. 4 Model analysis of gas transfer in fish gills. In Fish Physiology; Hoar, W.S., Randall, D.J., Eds.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Piiper, J.; Dejours, P.; Haab, P.; Rahn, H. Concepts and basic quantities in gas exchange physiology. Respir. Physiol. 1971, 13, 292–304. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Grieshaber, M.K. Critical Po2(s) in oxyconforming and oxyregulating animals: Gas exchange, metabolic rate and the mode of energy production. In The Vertebrate Gas Transport Cascade Adaptations to Environment and Mode of Life; Bicudo, J.E.P.W., Ed.; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Tang, P.-S. On the rate of oxygen consumption by tissues and lower organisms as a function of oxygen tension. Q. Rev. Biol. 1933, 8, 260–274. [Google Scholar] [CrossRef]
- Yeager, D.P.; Ultsch, G.R. Physiological regulation and conformation: A BASIC program for the determination of critical points. Physiol. Zool. 1989, 62, 888–907. [Google Scholar] [CrossRef]
- Claireaux, G.; Chabot, D. Responses by fishes to environmental hypoxia: Integration through Fry’s concept of aerobic metabolic scope. J. Fish Biol. 2016, 88, 232–251. [Google Scholar] [CrossRef] [PubMed]
- Shilo, M. The Toxic Principles of Prymnesium Parvum. In The Water Environment; Carmichael, W.W., Ed.; Springer: New York, NY, USA, 1981; pp. 37–47. [Google Scholar]
- Moran, A.; Ilani, A. The effect of prymnesin on the electric conductivity of thin lipid membranes. J. Membr. Biol. 1974, 16, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Satake, M. Yasumoto, T. Structures and partial stereochemical assignments for prymnesin-1 and prymnesin-2: Potent hemolytic and ichthyotoxic glycosides isolated from the red tide alga Prymnesium parvum. J. Am. Chem. Soc. 1999, 118, 479–480. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Meier, S.; Andersen, N.G.; Blossom, H.E.; Duus, J.Ø.; Nielsen, K.F.; Hansen, P.J.; Larsen, T.O. Chemodiversity of ladder-frame prymnesin polyethers in Prymnesium parvum. J. Nat. Prod. 2016, 79, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, F.; Parnas, I.; Reich, K. The action of the toxin of Prymnesium parvum carter on the guinea-pig ileum. Br. J. Pharmacol. Chemother. 1964, 22, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Henrikson, J.C.; Gharfeh, M.S.; Easton, A.C.; Easton, J.D.; Glenn, K.L.; Shadfan, M.; Mooberry, S.L.; Hambright, K.D.; Cichewicz, R.H. Reassessing the ichthyotoxin profile of cultured Prymnesium parvum (golden algae) and comparing it to samples collected from recent freshwater bloom and fish kill events in North America. Toxicon 2010, 55, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Shilo, M.; Aschner, M. Factors governing the toxicity of cultures containing the phytoflagellate Prymnesium parvum Carter. Microbiology 1953, 8, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Blossom, H.E.; Andersen, N.G.; Rasmussen, S.A.; Hansen, P.J. Stability of the intra- and extracellular toxins of Prymnesium parvum using a microalgal bioassay. Harmful Algae 2014, 32, 11–21. [Google Scholar] [CrossRef]
- Clemons, G.P.; Pinion, J.P.; Bass, E.; Pham, D.V.; Sharif, M.; Wutoh, J.G. A hemolytic principle associated with the red-tide dinoflagellate Gonyaulax monilata. Toxicon 1980, 18, 323–326. [Google Scholar] [CrossRef]
- Dorantes-Aranda, J.J.; Parra, L.M.G.; Alonso-Rodríguez, R.; Morquecho, L. Hemolytic activity and fatty acids composition in the ichthyotoxic dinoflagellate Cochlodinium polykrikoides isolated from Bahía de La Paz, Gulf of California. Mar. Pollut. Bull. 2009, 58, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.R. The Relation of size to rate of oxygen consumption and sustained swimming speed of Sockeye salmon (Oncorhynchus nerka). J. Fish. Res. Can. 1965, 22, 1491–1501. [Google Scholar] [CrossRef]
- Steffensen, J.F.; Johansen, K.; Bushnell, P.G. An automated swimming respirometer. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1984, 79, 437–440. [Google Scholar] [CrossRef]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; de Polavieja, G.G. idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743–748. [Google Scholar] [CrossRef]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, T.H.; Lyons, B.P.; Thain, J.E.; Law, R.J. Evaluating legacy contaminants and emerging chemicals in marine environments using adverse outcome pathways and biological effects-directed analysis. Mar. Pollut. Bull. 2013, 74, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Kramer, V.J.; Etterson, M.A.; Hecker, M.; Murphy, C.A.; Roesijadi, G.; Spade, D.J.; Spromberg, J.A.; Wang, M.; Ankley, G.T. Adverse outcome pathways and ecological risk assessment: Bridging to population-level effects. Environ. Toxicol. Chem. 2011, 30, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Vinken, M. The adverse outcome pathway concept: A pragmatic tool in toxicology. Toxicology 2013, 312, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, J.C.; Steffensen, J.F.; Aarestrup, K.; Frisk, M.; Etzerodt, A.; Jyde, M. Excess posthypoxic oxygen consumption in rainbow trout (Oncorhynchus mykiss): Recovery in normoxia and hypoxia. Can. J. Zool. 2011, 90, 1–11. [Google Scholar] [CrossRef]
- Bushnell, P.G.; Steffensen, J.F.; Johansen, K. Oxygen consumption and swimming performance in hypoxia-acclimated rainbow trout Salmo gairdneri. J. Exp. Biol. 1984, 113, 225–235. [Google Scholar] [CrossRef]
- Sloman, K.A.; Motherwell, G.; O’connor, K.; Taylor, A.C. The effect of social stress on the standard metabolic rate (SMR) of brown trout, Salmo trutta. Fish Physiol. Biochem. 2000, 23, 49–53. [Google Scholar] [CrossRef]
- Davis, J.C. Circulatory and Ventilatory Responses of Rainbow Trout (Salmo gairdneri) to Artificial Manipulation of Gill Surface Area. J. Fish. Res. Can. 1971, 28, 1609–1614. [Google Scholar] [CrossRef]
- Hughes, G.M. Respiratory Responses to Hypoxia in Fish. Am. Zool. 1973, 13, 475–489. [Google Scholar] [CrossRef]
- Lomholt, J.P.; Johansen, K. Hypoxia Acclimation in Carp: How It Affects O2 Uptake, Ventilation, and O2 Extraction from Water. Physiol. Zool. 1979, 52, 38–49. [Google Scholar] [CrossRef]
- Valenti, T.W., Jr.; James, S.V.; Lahousse, M.J.; Schug, K.A.; Roelke, D.L.; Grover, J.P.; Brooks, B.W. A mechanistic explanation for pH-dependent ambient aquatic toxicity of Prymnesium parvum carter. Toxicon 2010, 55, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Dafni, Z.; Shilo, M. The Cytotoxic Principle of the Phytoflagellate Prymnesium Parvum. J. Cell Biol. 1966, 28, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Inoue, K. The mechanism of the action of prymnesium toxin on membranes. Biochim. Biophys. Acta 1974, 352, 344–348. [Google Scholar] [CrossRef]
- Christensen, E.A.F.; Svendsen, M.B.S.; Steffensen, J.F. Plasma osmolality and oxygen consumption of perch Perca fluviatilis in response to different salinities and temperatures. J. Fish Biol. 2016, 90, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Vogelbein, W.K.; Lovko, V.J.; Shields, J.D.; Reece, K.S.; Mason, P.L.; Haas, L.W.; Walker, C.C. Pfiesteria shumwayae kills fish by micropredation not exotoxin secretion. Nature 2002, 418, 967–970. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svendsen, M.B.S.; Andersen, N.R.; Hansen, P.J.; Steffensen, J.F. Effects of Harmful Algal Blooms on Fish: Insights from Prymnesium parvum. Fishes 2018, 3, 11. https://doi.org/10.3390/fishes3010011
Svendsen MBS, Andersen NR, Hansen PJ, Steffensen JF. Effects of Harmful Algal Blooms on Fish: Insights from Prymnesium parvum. Fishes. 2018; 3(1):11. https://doi.org/10.3390/fishes3010011
Chicago/Turabian StyleSvendsen, Morten Bo Søndergaard, Nikolaj Reducha Andersen, Per Juel Hansen, and John Fleng Steffensen. 2018. "Effects of Harmful Algal Blooms on Fish: Insights from Prymnesium parvum" Fishes 3, no. 1: 11. https://doi.org/10.3390/fishes3010011
APA StyleSvendsen, M. B. S., Andersen, N. R., Hansen, P. J., & Steffensen, J. F. (2018). Effects of Harmful Algal Blooms on Fish: Insights from Prymnesium parvum. Fishes, 3(1), 11. https://doi.org/10.3390/fishes3010011



