Growth, Survival and Spore Formation of the Pathogenic Aquatic Oomycete Aphanomyces astaci and Fungus Fusarium avenaceum Are Inhibited by Zanthoxylum rhoifolium Bark Extracts In Vitro
Abstract
:1. Introduction
2. Results
2.1. Chemical Constituents of Chloroform–Methanol (9:1) Zr-b Extract
2.2. In Vitro Anti Oomycete and Antifungal Activity of Zr-b Extracts
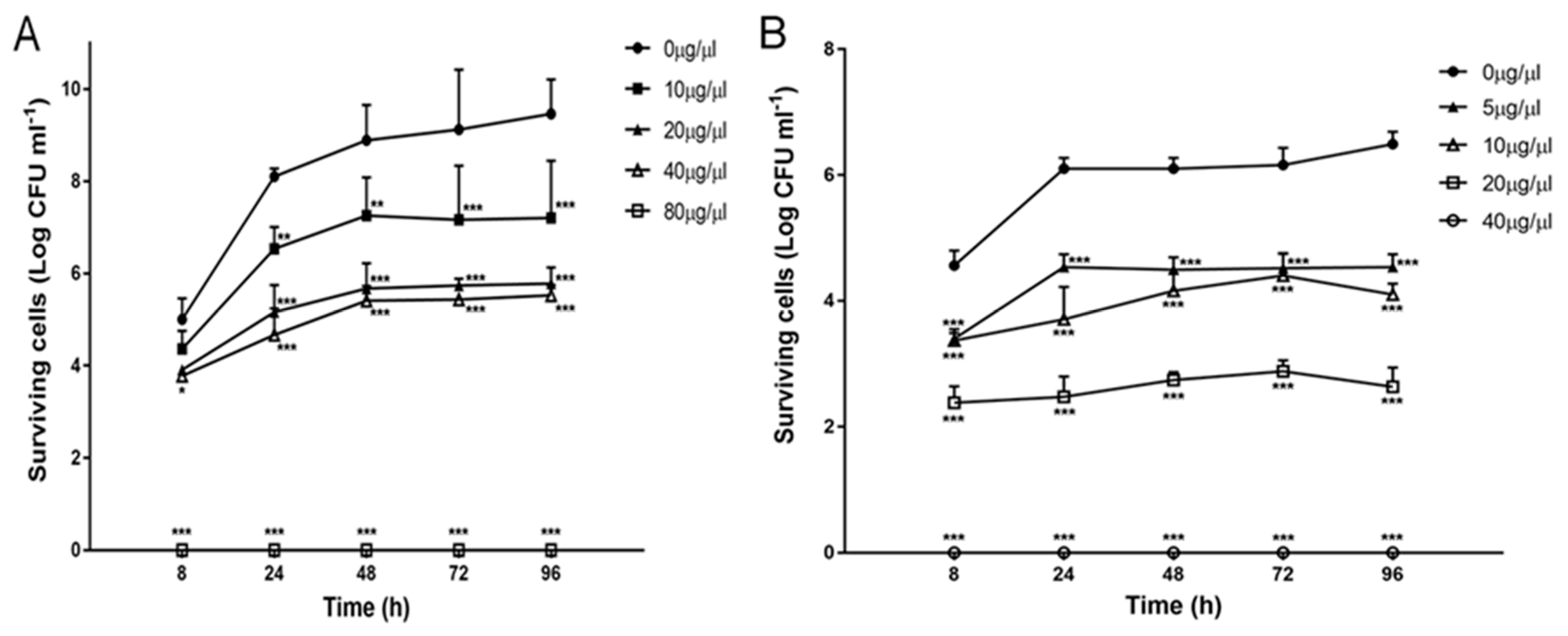
2.3. Effect of Zr-b Extracts on Oomycete and Fungal Growth and Survival
2.4. Effect of the Chloroform–Methanol (9:1) Zr-b Extract on Oomycete and Fungal Fitness
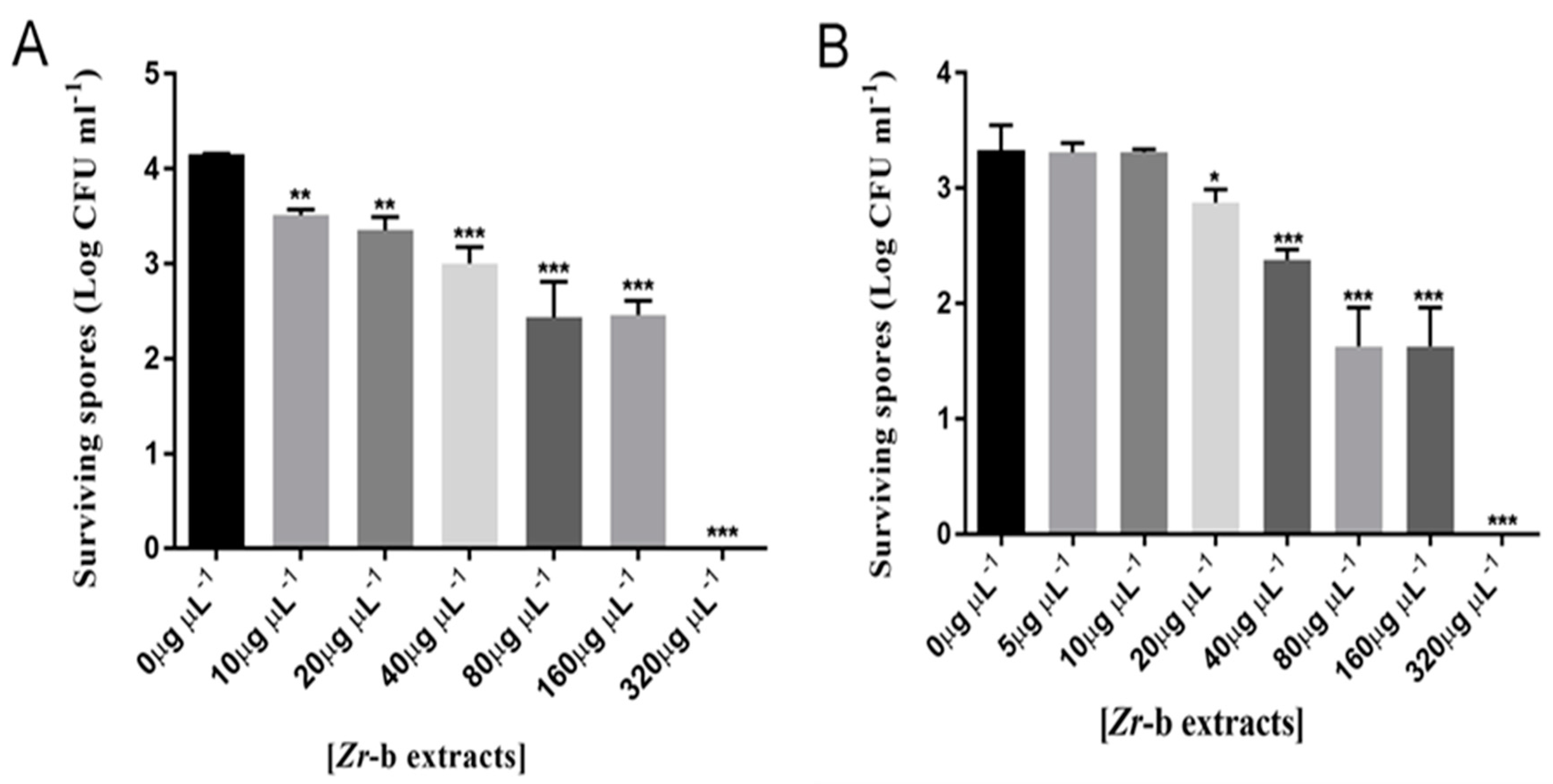
2.5. Effect of the Chloroform–Methanol (9:1) Zr-b Extract on Oomycete and Fungal Sporulation and Spore Germination
3. Discussion
4. Materials and Methods
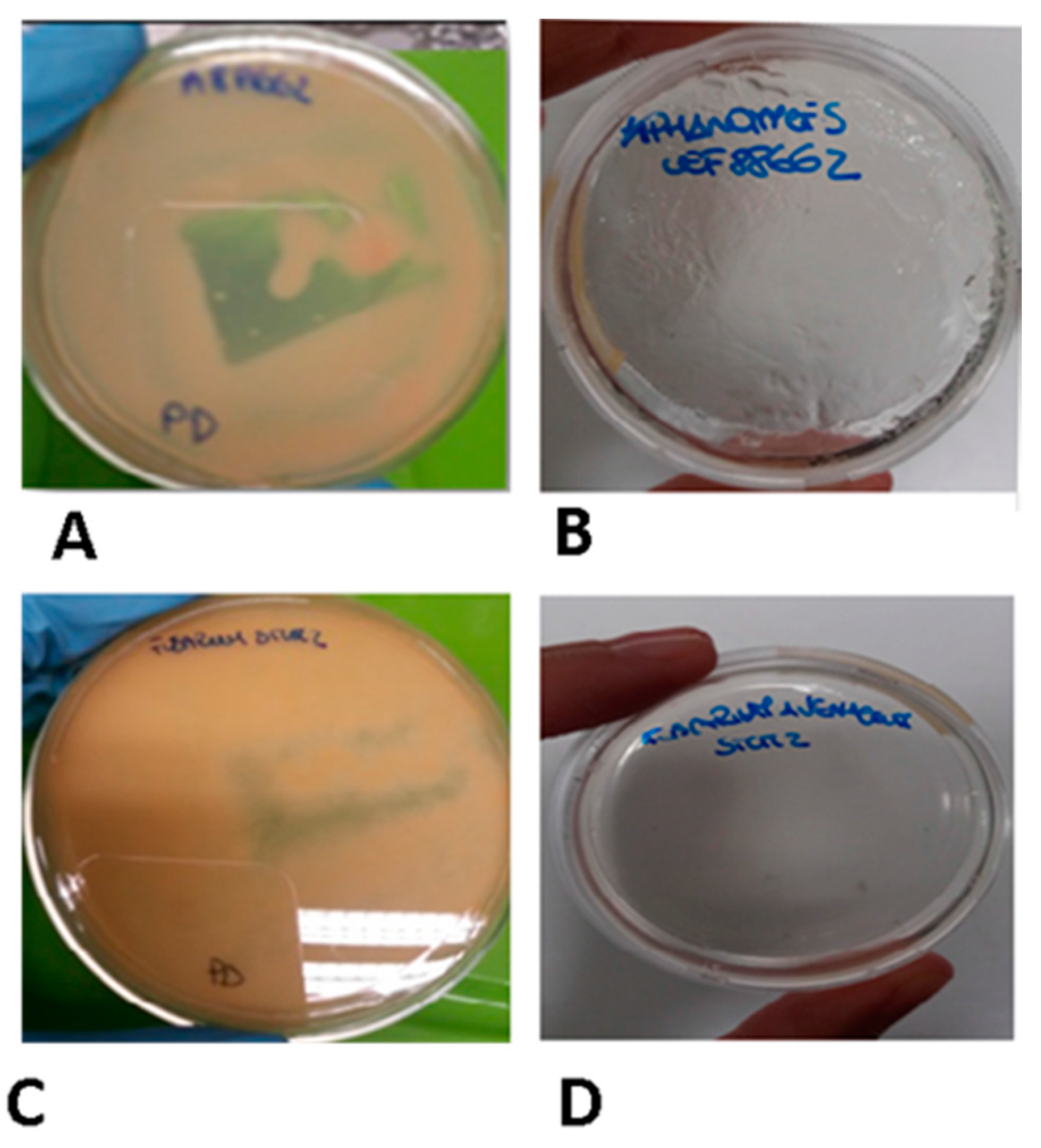
4.1. Microorganisms and Growth Conditions
4.2. Plant Material
4.3. Extraction and Characterization of Zanthoxylum rhoifolium bark
4.4. In Vitro Antioomycetidal and Antifungal Activity Assay of Zr-b Extracts with the Agar-Diffusion Method
4.5. Quantitative Evaluation of the Antioomycetidal and Antifungal Activities of Zr-b Extracts
4.6. Oomycete and Fungal Fitness Evaluation of the Chloroform–Methanol (9:1) Zr-b Extract
4.7. Assays of Oomycete and Fungal Sporulation with the Chloroform–Methanol (9:1) Zr-b Extract
4.8. Assays of Spore Germination with the Chloroform–Methanol (9:1) Zr-b Extract
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Zr-b | Zanthoxylum rhoifolium bark |
| PD | Potato-Dextrose |
| CC | Column Chromatography |
| TLC | Thin Layer Chromatography |
| NMR | Nuclear magnetic resonance |
| RP-HPLC | Reversed-phase high-performance liquid chromatography |
| TCZ | Tioconazole (PubChem CID: 5482) |
| MIC | minimum inhibitory concentration |
| MOC | minimum oomycetidal concentration |
| MFC | minimum fungicidal concentration. |
References
- Latijnhouwers, M.; de Wit, P.J.G.M.; Govers, F. Oomycetes and fungi: Similar weaponry to attack plants. Trends Microbiol. 2003, 11, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Jestoi, M.; Parikka, P. Fusarium avenaceum-The North European situation. Int. J. Food Microbiol. 2007, 119, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Nalim, F.A.; Elmer, W.H.; McGovern, R.J.; Geiser, D.M. Multilocus Phylogenetic Diversity of Fusarium avenaceum Pathogenic on Lisianthus. Phytopathology 2009, 99, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Alderman, D.J. Geographical spread of bacterial and fungal diseases of crustaceans. Rev. Sci. Tech. 1996, 15, 603–632. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Vey, A. Experimental study of Fusarium solani: Infections in Astacus leptodactylus and Pacifastacus leniusculus (Crustacea, Decapoda). Dis. Aquat. Org. 1988, 5, 215–223. [Google Scholar] [CrossRef]
- Makkonen, J.; Jussila, J.; Koistinen, L.; Paaver, T.; Hurt, M.; Kokko, H. Fusarium avenaceum causes burn spot disease syndrome in noble crayfish (Astacus astacus). J. Invertebr. Pathol. 2013, 113, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Vey, A. Infection fongines chez l’écrevisse Astacus leptodactylus Esch. Freshw. Crayfish 1978, 4, 403–410. [Google Scholar]
- Edgerton, B.F. Hazard analysis exotic pathogens of potential threat to european freshwater crayfish. Bull. Fr. Pêche Piscic. 2002, 367, 813–820. [Google Scholar] [CrossRef]
- Quaglio, F.; Morolli, C.; Galuppi, R.; Tampieri, M.P.; Marcer, F.; Rotundo, G. Pathological investigation on crayfish (Procambarus clarkii, Girard 1852) from canals in Padana Plain. Freshw. Crayfish 2006, 15, 365–375. [Google Scholar]
- Dörr, A.J.M.; Rodolfi, M.; Elia, A.C.; Scalici, M.; Garzoli, L.; Picco, A.M. Mycoflora on the cuticle of the invasive crayfish Procambarus clarkii. Fund. Appl. Limnol. 2012, 180, 77–84. [Google Scholar] [CrossRef]
- Edgerton, B.F.; Henttonen, P.; Jussila, J.; Mannonen, A.; Paasonen, P.; Taugbøl, T.; Edsman, L.; Souty-Grosset, C. Understanding the causes of disease in European freshwater crayfish. Conserv. Biol. 2004, 18, 1466–1474. [Google Scholar] [CrossRef]
- Edsman, L.; Nyström, P.; Sandström, A.; Stenberg, M.; Kokko, H.; Tiitinen, V.; Makkonen, J.; Jussila, J. Eroded swimmeret syndrome in female crayfish Pacifastacus leniusculus associated with Aphanomyces astaci and Fusarium spp. infections. Dis. Aquat. Org. 2014, 112, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Diéguez-Uribeondo, J.; García, M.A.; Cerenius, L.; Kozubíková, E.; Ballesteros, I.; Windels, C.; Weiland, J.; Kator, H.; Söderhäll, K.; Martín, M.P. Phylogenetic relationships among plant and animal parasites, and saprotrophs in Aphanomyces (Oomycetes). Fungal Genet. Biol. 2009, 46, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Souty-Grosset, C.; Holdich, D.M.; Noël, P.Y.; Reynolds, J.; Haffner, P. Atlas of crayfish in Europe, Muséum National d’Histoire Naturelle, Paris. Patrim. Nat. 2006, 64, 187. [Google Scholar]
- Makkonen, J.; Kokko, H.; Vainikka, A.; Kortet, R.; Jussila, J. Dose-dependent mortality of the noble crayfish (Astacus astacus) to different strains of the crayfish plague (Aphanomyces astaci). J. Invertebr. Pathol. 2014, 115, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Rahe, R.; Soylu, E. Identification of the pathogenic fungus causing destruction to Turkish crayfish stocks (Astacus leptodactylus). J. Invertebr. Pathol. 1989, 54, 10–15. [Google Scholar] [CrossRef]
- Unestam, T. Resistance to the crayfish plague in some American, Japanese and European crayfishes. Rep. Inst. Freshw. Res. 1969, 4, 202–209. [Google Scholar]
- Unestam, T. Defense reactions and susceptibility of Australian and New Guinea freshwater crayfish to European-crayfish-plague fungus. Aust. J. Exp. Biol. Med. Sci. 1976, 53, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Unestam, T.; Weiss, D.W. The host-parasite relationship between fresh-water crayfish and the crayfish disease fungus Aphanomyces astaci: Responses to infection by a susceptible and a resistant species. J. Gen. Microbiol. 1970, 60, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Aydin, H.; Kokko, H.; Makkonen, J.; Kortet, R.; Kukkonen, H.; Jussila, J. The signal crayfish is vulnerable to both the As and the PsI-isolates of the crayfish plague. Knowl. Manag. Aquat. Ecosyst. 2014, 413, 03. [Google Scholar] [CrossRef]
- Alderman, D.J.; Holdich, D.; Reeve, I. Signal crayfish as crayfish plague vectors in Britain. Aquaculture 1990, 86, 3–6. [Google Scholar] [CrossRef]
- Alderman, D.J.; Polglase, J.L.; Frayling, M.; Hogger, J. Crayfish plague in Britain. J. Fish Dis. 1984, 7, 401–405. [Google Scholar] [CrossRef]
- Bohman, P.; Nordwall, F.; Edsman, L. The effect of the large-scale introduction of signal crayfish on the spread of crayfish plague in Sweden. Bull. Fr. Pêche Piscic. 2006, 380–381, 1291–1302. [Google Scholar] [CrossRef]
- Holdich, D.M.; Reynolds, J.D.; Souty-Grosset, C.; Sibley, P.J. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 2009, 394–395, 11. [Google Scholar] [CrossRef]
- Ackefors, H. The culture and capture crayfish fisheries in Europe. World Aquac. Mag. 1998, 29, 18–67. [Google Scholar]
- Jussila, J.; Mannonen, A. Crayfisheries in Finland, a short overview. Bull. Fr. Pêche Piscic. 2004, 372–373, 263–273. [Google Scholar] [CrossRef]
- Jussila, J.; Makkonen, J.; Kokko, H. Peracetic acid (PAA) treatment is an effective disinfectant against crayfish plague (Aphanomyces astaci) spores in aquaculture. Aquaculture 2011, 320, 37–42. [Google Scholar] [CrossRef]
- Jussila, J.; Makkonen, J.; Vainikka, A.; Kortet, R.; Kokko, H. Latent crayfish plague (Aphanomyces astaci) infection in a robust wild noble crayfish (Astacus astacus) population. Aquaculture 2011, 321, 17–20. [Google Scholar] [CrossRef]
- Gherardi, F.; Aquiloni, L.; Diéguez-Uribeondo, J.; Tricarico, E. Managing invasive crayfish: Is there a hope? Aquat. Sci. 2011, 73, 185–200. [Google Scholar] [CrossRef]
- Rantamäki, J.; Cerenius, L.; Söderhäll, K. Prevention of transmission of the crayfish plague fungus (Aphanomyces astaci) to the freshwater crayfish Astacus astacus by treatment with MgCl2. Aquaculture 1992, 104, 11–18. [Google Scholar] [CrossRef]
- Lilley, J.H.; Inglis, V. Comparative effects of various antibiotics, fungicides and disinfectants on Aphanomyces invaderis and other saprolegniaceous fungi. Aquac. Res. 1997, 28, 461–469. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.A.K.; Hou, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Francis, G.; Becker, K. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal 2007, 1, 1371–1391. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.S.; Lopes, L.S.; Marques, R.B.; Figueiredo, K.A.; Costa, D.A.; Chaves, M.H.; Almeida, F.R. Antinociceptive effect of Zanthoxylum rhoifolium Lam. (Rutaceae) in models of acute pain in rodents. J. Ethnopharmacol. 2010, 129, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Jullian, V.; Bourdy, G.; Georges, S.; Maurel, S.; Sauvain, M. Validation of use of a traditional antimalarial remedy from French Guiana, Zanthoxilum rhoifolium Lam. J. Ethnopharmacol. 2006, 106, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, G.; Carrieri, R.; Tarantino, P.; Alfieri, M.; Leone, A.; De Tommasi, N.; Lahoz, E. Fungistatic activity of Zanthoxylum rhoifolium Lam. bark extracts against fungal plant pathogens and investigation on mechanism of action in Botrytis cinerea. Nat. Prod. Res. 2015, 29, 2251–2255. [Google Scholar] [CrossRef] [PubMed]
- Messmer, W.M.; Tin-Wa, M.; Fong, H.H.S.; Bevelle, C.; Farnswort, N.R.; Abraham, D.J.; Trojánek, J. Fagaronine, a new tumor inhibitor isolated from Fagara zanthoxyloids Lam. (Rutaceae). J. Pharm. Sci. 1972, 61, 1858–1859. [Google Scholar] [CrossRef] [PubMed]
- De A Gonzaga, W.; Weber, A.D.; Giacomelli, S.R.; Dalcol, I.I.; Hoelzel, S.C.; Morel, A.F. Antibacterial alkaloids from Zanthoxylum rhoifolium. Planta Med. 2003, 69, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, W.D.; Liu, R.H.; Zhang, C.; Shen, Y.H.; Li, H.L.; Liang, M.J.; Xu, X.K. Benzophenanthridine alkaloids from Zanthoxylum nitidum (Roxb.) DC, and their analgesic and anti-inflammatory activities. Chem. Biodivers. 2006, 3, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Bafi-Yeboa, N.F.A.; Arnason, J.T.; Baker, J.; Smith, M.L. Antifungal constituents of Northern prickly ash, Zanthoxylum americanum Mill. Phytomedicine 2005, 12, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Huang, Y.; Zhou, L.; Shi, X.; Guo, Z.; Wang, M.; Jiang, W. Chemical Composition and Antifungal Activity of the Fruit Oil of Zanthoxylum bungeanum Maxim. (Rutaceae) from China. J. Essent. Oil Res. 2009, 21, 174–178. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Singh, P.; Sundriyal, R.C. Chemical constituents and biological activities of the genus Zanthoxylum: A review. Afr. J. Pure Appl. Chem. 2011, 5, 412–416. [Google Scholar]
- Abad, M.J.; Ansuategui, M.; Bermejo, P. Active antifungal substances from natural sources. ARKIVOC 2007, 2007, 116–145. [Google Scholar]
- Dieguez-Hurtado, R.; Garrido-Garrido, G.; Prieto-Gonzalez, S.; Iznaga, Y.; Gonzalez, L.; Molina-Torres, J.; Curini, M.; Epifano, F.; Marcotullio, M.C. Antifungal activity of some Cuban Zanthoxylum species. Fitoterapia 2003, 74, 384–386. [Google Scholar] [CrossRef]
- Islam, A.; Sayeed, A.; Bhuiyan, M.S.A.; Mosaddik, M.A.G. In vitro Antimicrobial effect of three Terpenes, Isolated from the Bark of Zanthoxylum budrunga. Pak. J. Biol. Sci. 2001, 4, 711–713. [Google Scholar]
- Manandhar, A.; Tiwari, R.D. Antifungal efficacy of Zanthoxylum oil against Bipolaris sorokiniana (Sacc.) Shoem. Ecoprint 2005, 12, 91–93. [Google Scholar] [CrossRef]
- He, W.; Van Puyvelde, L.; De Kimpe, N.; Verbruggen, L.; Anthonissen, K.; Van der Flaas, M.; Bosselaers, J.; Mathenge, S.G.; Mudida, F.P. Chemical Constituents and Biological Activities of Zanthoxylum usambarense. Phytother. Res. 2002, 16, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Zheng, D.; Zhou, Z.; Yu, W.; Zhang, L.; Feng, L.; Liang, X.; Guan, W.; Zhou, J.; et al. Genomic evolution of Saccharomyces cerevisiae under Chinese rice wine fermentation. Genome Biol. Evol. 2014, 6, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Thouvenel, C.; Gantier, J.C.; Duret, P.; Fourneau, C.; Hocquemiller, R.; Ferreira, M.E.; de Arias, A.R.; Fournet, A. Antifungal compounds from Zanthoxylum chiloperone var. angustifolium. Phytother. Res. 2003, 17, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Cao, Y.Y.; Xu, Z.; Zhao, J.X.; Gao, P.H.; Qin, X.F.; Jiang, Y.Y. Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob. Agents Chemother. 2006, 50, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Zhang, W. New Lead Structures in Antifungal Drug Discovery. Curr. Med. Chem. 2011, 18, 733–766. [Google Scholar] [CrossRef] [PubMed]
- Grycová, L.; Dostál, J.; Marek, R. Quaternary protoberberine alkaloids. Phytochemistry 2007, 68, 150–175. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, F.; Barile, E.; Curir, P.; Dolci, M.; Lanzotti, V. Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity. Phytochem. Lett. 2008, 1, 44–48. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Valsaraj, R.; Pushpangadan, P.; Smitt, U.W.; Adsersen, A.; Christensen, S.B.; Sittie, A.; Nyman, U.; Nielsen, C.; Olsen, C.E. New anti-HIV-1, antimalarial, and antifungal compounds from Terminalia bellerica. J. Nat. Prod. 1997, 60, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Wachter, G.A.; Hoffmann, J.J.; Furbacher, T.; Blake, M.E.; Timmermann, B.N. Antibacterial and antifungal flavanones from Eysenhardtia texana. Phytochemistry 1999, 52, 1469–1471. [Google Scholar] [CrossRef]
- Fearnley, J. Bee Propolis; Souvenir Press Ltd.: London, UK, 2001; ISBN 9780285635227. [Google Scholar]
- Zheng, W.F.; Tan, R.X.; Yang, L.; Liu, Z.L. Two flavones from Artemisia giraldii and their antimicrobial activity. Planta Med. 1996, 62, 16–162. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, A.J.; Meyer, J.J. The antimicrobial activity of 3,5,7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens. J. Ethnopharmacol. 1997, 57, 177–1781. [Google Scholar] [CrossRef]
- Pagnussatt, F.A.; Del Ponte, E.M.; Garda-Buffon, J.; Badiale-Furlong, E. Inhibition of Fusarium graminearum growth and mycotoxin production by phenolic extract from Spirulina sp. Pest Biochem. Physiol. 2014, 108, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Kontoyiannis, D.P. Rationale for combination antifungal therapy. Pharmacotherapy 2001, 21, 149–164. [Google Scholar] [CrossRef]
- Maxim, D.; Bucşa, L.; Moza, M.I.; Chachula, O. Preliminary antifungal investigation of ten biocides against moulds from two different church frescoes. Ann. RSCB XVII 2012, 17, 139–146. [Google Scholar]
- Mironescu, M.; Georgescu, C.; Oprean, L. Comparative sporicidal effects of volatile oils. J. Agroaliment. Process. Technol. 2009, 15, 361–365. [Google Scholar]
- Häll, L.; Unestam, K. The effect of fungicides on survival of the crayfish plague fungus Aphanomyces astaci (Oomycetes) growing on fish scales. Mycopathologia 1980, 72, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 36, 493–496. [Google Scholar] [CrossRef]
- Varaldo, P.E. Antimicrobial resistance and susceptibility testing: An evergreen topic. J. Antimicrob. Chemother. 2002, 50, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, J.; Jussila, J.; Kortet, R.; Vainikka, A.; Kokko, H. Differing virulence of Aphanomyces astaci isolates and elevated resistance of noble crayfish Astacus astacus against crayfish plague. Dis. Aquat. Organ. 2012, 102, 129–136. [Google Scholar] [CrossRef] [PubMed]
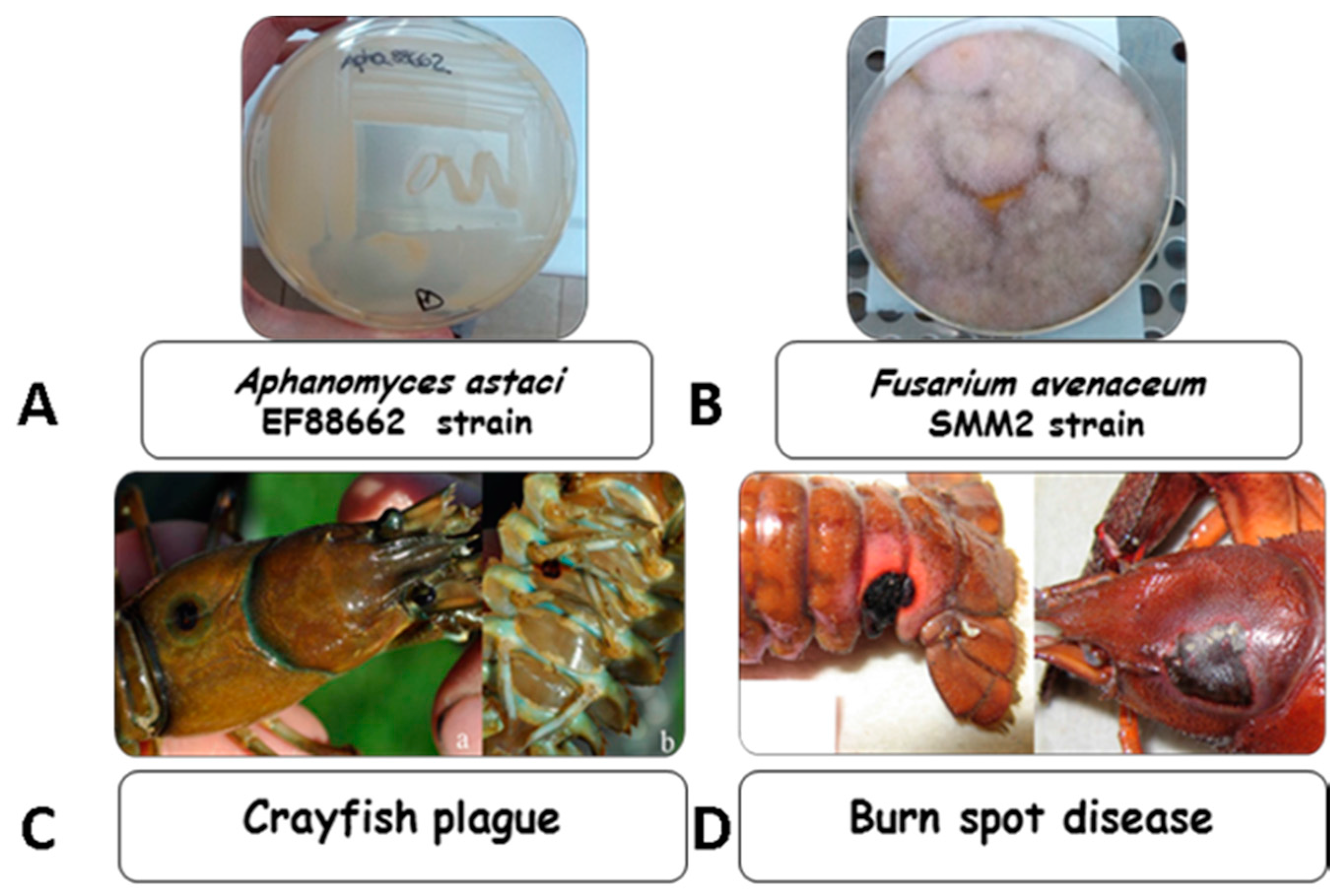
| Oomycete/Fungal Strain | n-Hexane (6 mg disc−1) | Chloroform (6 mg disc−1) | Chloroform–Methanol 9:1 (6 mg disc−1) | Methanol (6 mg disc−1) | Tioconazole (1.4 mg disc−1) |
|---|---|---|---|---|---|
| Aphanomyces astaci UEF88662 | 10.2 ± 0.9 | 11.4 ± 1.4 | 17.5 ± 3.5 | 10.2 ± 0.3 | 20.0 ± 4.1 |
| Fusarium avenaceum SMM2 | 13.1 ± 1.0 | 16.5 ± 2.1 | 47.5 ± 3.5 | 12.5 ± 3.5 | 40.1 ± 7.2 |
| Oomycete/Fungal Strain | n-Hexane | Chloroform | Chloroform–Methanol 9:1 | Methanol | Tioconazole | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MFC/MOC | MIC | MFC/MOC | MIC | MFC/MOC | MIC | MFC/MOC | MIC | MFC/MOC | |
| Aphanomyces astaci UEF88662 | 40 | 80 | 40 | 80 | 40 | 80 | 40 | 80 | 1.5 | 15 |
| Fusarium avenaceum SMM2 | 5 | 40 | 5 | 40 | 5 | 40 | 5 | 40 | 1 | 1.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagliarulo, C.; Sateriale, D.; Scioscia, E.; De Tommasi, N.; Colicchio, R.; Pagliuca, C.; Scaglione, E.; Jussila, J.; Makkonen, J.; Salvatore, P.; et al. Growth, Survival and Spore Formation of the Pathogenic Aquatic Oomycete Aphanomyces astaci and Fungus Fusarium avenaceum Are Inhibited by Zanthoxylum rhoifolium Bark Extracts In Vitro. Fishes 2018, 3, 12. https://doi.org/10.3390/fishes3010012
Pagliarulo C, Sateriale D, Scioscia E, De Tommasi N, Colicchio R, Pagliuca C, Scaglione E, Jussila J, Makkonen J, Salvatore P, et al. Growth, Survival and Spore Formation of the Pathogenic Aquatic Oomycete Aphanomyces astaci and Fungus Fusarium avenaceum Are Inhibited by Zanthoxylum rhoifolium Bark Extracts In Vitro. Fishes. 2018; 3(1):12. https://doi.org/10.3390/fishes3010012
Chicago/Turabian StylePagliarulo, Caterina, Daniela Sateriale, Elisa Scioscia, Nunziatina De Tommasi, Roberta Colicchio, Chiara Pagliuca, Elena Scaglione, Japo Jussila, Jenny Makkonen, Paola Salvatore, and et al. 2018. "Growth, Survival and Spore Formation of the Pathogenic Aquatic Oomycete Aphanomyces astaci and Fungus Fusarium avenaceum Are Inhibited by Zanthoxylum rhoifolium Bark Extracts In Vitro" Fishes 3, no. 1: 12. https://doi.org/10.3390/fishes3010012
APA StylePagliarulo, C., Sateriale, D., Scioscia, E., De Tommasi, N., Colicchio, R., Pagliuca, C., Scaglione, E., Jussila, J., Makkonen, J., Salvatore, P., & Paolucci, M. (2018). Growth, Survival and Spore Formation of the Pathogenic Aquatic Oomycete Aphanomyces astaci and Fungus Fusarium avenaceum Are Inhibited by Zanthoxylum rhoifolium Bark Extracts In Vitro. Fishes, 3(1), 12. https://doi.org/10.3390/fishes3010012







