1. Introduction
Organic aquaculture is a term usually understood as synonymous with ecological aquaculture and is a comprehensive method of farming fish and other marine species that adheres to organic principles [
1]. These principles can be summarized as sustainable resource management, environmental protection, principles of social responsibility and equity, and careful location selection to protect ecosystems, among others including the use of certified organic feed. Shrimp is the aquaculture product with the highest economic value around the world (USD 32 million), with a total production of almost 7 million tonnes in 2024, and although its organic production growth is very recent, with only 421 tonnes in 2018 [
2], some companies are producing organic shrimp —both
P. vannamei and
P. monodon—as an alternative strategy for diversification to serve a growing niche of consumers demanding organic products.
Compared to organic land-based farming, organic aquaculture is a very recent form of production. However, it is considered a rapidly growing sector due to the increasing demand for low-environmental-impact products [
1]. Different countries, regions, and organizations have developed different requirements for labeling shrimp as organic or eco-friendly, including concepts like density, welfare, soil practices, water quality, input limitation for reproduction, health management, and feeding (hormones, antibiotics, and feed ingredients) [
1]. The European Union regulates organic aquaculture through Regulation (EU) 2018/848, ensuring environmentally sustainable and high-welfare aquaculture practices [
3]. In 2020, the EU’s total organic aquaculture production reached 74,032 tonnes, accounting for 6.4% of the region’s total aquaculture output [
4]. Organic shrimp production is subject to strict guidelines, including organic feed requirements and sustainable stocking densities [
3]. The leading shrimp organic aquaculture producer within the EU is Italy. The sector has significant growth potential, supported by the EU’s Farm to Fork Strategy, which aims to expand organic aquaculture by 2030, ensuring economic viability and environmental sustainability [
4].
Organic ingredients are defined as raw materials produced in accordance with organic farming standards, excluding the use of synthetic chemicals, genetically modified organisms, and artificial additives. As outlined by Lembo and Mente and the European Commission, ingredients must be certified by authorized bodies, fully traceable, and sourced through environmentally sustainable practices that prioritize animal welfare and ecological integrity [
1,
3].
The market value of shrimp presents the highest commercial value in the global aquaculture market (USD 31.241 million in 2022) and continues to expand due to increasing global demand, making shrimp aquaculture one of the most profitable sectors in the seafood industry [
5]. Intensive shrimp farming has demonstrated high economic feasibility, with projected global market values reaching billions of dollars annually [
5]. This growth is driven by rising consumer preference for protein-rich seafood and advancements in sustainable farming techniques. The industry presents significant growth opportunities, particularly in Asia and Latin America, where good climatic conditions and innovative technological systems have improved production efficiency [
5]. With an increasing focus on sustainability and value-added products, shrimp aquaculture remains a highly lucrative market. Strategic investment in feed optimization, certified organic products, and efficient supply chains will be crucial in sustaining long-term profitability while meeting global demand for high-quality shrimp products [
5].
The feed used to raise animals is probably the main issue for developing organic aquaculture. European Union (EU) legislation protects the terms organic or ecological with a label that can only be used if the production is certified, and to obtain that label the feed used to raise organic shrimp must be certified as organic and conform to the minimum standards considered in the regulations, usually prohibiting GMOs, limiting pesticides and antibiotics, banning the use of synthetic amino acids, and many other requirements [
3]. Other certificates have similar criteria, typically allowing the use of sustainable raw materials due to the scarcity of organic raw materials on the market, mainly as a protein source, and particularly fish meal [
1].
Fish meal from sustainable sources can be used in organic shrimp diets, but price and scarcity make the use of other protein sources preferable. Several studies have been conducted with organic plant ingredients [
6,
7,
8]. A study by Browdy [
8] was the first to use an organic vegetable diet to feed
P. vannamei shrimp in ponds, and no significant differences were found between the experimental diet and a commercial diet containing fish meal in terms of growth or feed conversion ratio (FCR). Several studies on substitution by non-organic sources, such as those by Oujifard and colleagues [
9], have completely replaced fish meal with rice protein concentrate, but included 15% shrimp meal to ensure an aminoacidic balanced source.
Regarding animal sources not labeled as eco-friendly, several non-eco-friendly ingredients, both animal and vegetal, have been used in the past as an alternative to fish meal in shrimp diets [
10,
11,
12]. World rainbow trout aquaculture production in 2020 was 82 million tons [
13], therefore its by-products represent a valuable resource for circular economy practices in sustainable aquaculture feed.
Amaya [
12] reported good results without fish meal, using poultry by-products or vegetal mix. Some studies have been conducted with insects that, although not certified as organic, are likely to be labeled as organic in the near future [
10,
14,
15,
16]. Defatted insect meals are especially interesting due to their amino acid composition and high protein content, similar to that of fish meal. However, from an ecological perspective, whole, undefatted insect meals are more sustainable, especially compared to those defatted using a solvent [
17].
Another approach has been the use of used fermented corn protein concentrate, such as in a study by Galkanda-Arachchige [
18], but other studies showed the poorest results with several vegetal concentrates or foods fermented without fish meal [
19,
20,
21]. Good results were only obtained when including small quantities of fish meal [
22,
23,
24], but in other cases the inclusion of fish meal, chicken, and blood meal resulted in poorer growth [
25].
The development of organic shrimp aquaculture requires certified organic feed, and the requirements to obtain certification will become more demanding in the future. The scarcity of certified organic animal ingredients, particularly fish meal, poses a major challenge. Although plant-based eco-organic alternatives exist, their effectiveness in shrimp diets remains uncertain [
8,
21]. This study is essential to evaluate the limits of replacing fish meal with organic ingredients and ensuring sustainability, growth performance, and nutritional adequacy in organic shrimp farming.
According to this context, the main challenge to developing organic shrimp production is in obtaining organic diets based on organic ingredients, considering the lack of alternative organic animal ingredients available on the market, as Tefal [
26,
27,
28] has shown regarding fish.
To this end, four alternative ingredients with low ecological impact have been considered: Iberian pork by-products, due to their high availability, consistency, and quality of animal origin. Animal species generally contain good quality protein that contains all the essential amino acids. Organic trout by-products are currently less available on the market, but, a priori, present a very interesting profile, as we replace fish meal with organically produced fish by-products, thus contributing to the circular economy. Organic vegetable meals, the most available, represent the best non-by-product option, as they have lower trophic values, but also have amino acid profiles that could be less suitable. Some vegetables such as soya beans also contain good quality protein, but some vegetables do not and are deficient in one or more essential amino acids. Wheat is an example of a vegetable that is deficient in lysine and other essential amino acids. To use vegetables to provide all the essential amino acids there are two strategies: (a) the use of a vegetable that contains all of the essential amino acids, or (b) use a combination of vegetables that singly are deficient in one or more essential amino acids, but in combination supply all the amino acids. There is a third strategy that involves supplementation with synthetic amino acids, which is not permitted in the case of organic production, or insect protein, emerging meals with a still very high price but with a very promising future in aquaculture.
The aim of this research is to study the total replacement of fish meal with organic ingredients, both animal and vegetable, in shrimp feed for ecological and sustainable aquaculture, studying growth, nutritional parameters and digestibility.
2. Materials and Methods
2.1. Formulation and Manufacture of Experimental Diets
Five experimental diets were formulated and manufactured with raw materials from both plant and animal organic origin. The total fish meal in the control diet (CON) was replaced by Iberian pig viscera by-products (PIG), organic insect meal from
Hemetia illucens larvae (FLY), organic trout by-products (TRO), or organic wheat gluten (WHT), which all maintained isoprotein and isolipid dietary content. As different raw materials have different amounts of protein and fat, the values of oils and some ingredients were varied to maintain isoprotein and isolipid content. The ingredients and nutritional compositions of experimental diets are shown in
Table 1. Amino acid and fatty acid compositions are shown in
Table 2 and
Table 3, respectively.
All the organic ingredients came from organically certified producers with the EU organic label. Organic trout was self-processed to extract meat slices and the remaining parts were carefully cut, oven-dried, and ground into a suitable form for use in the feed. The remaining parts used for the Iberian pig meal were the liver, intestines, and heart, which were cut into small pieces. After oven-drying, they were ground into a suitable form for incorporation into the feed. Dry organic insect larvae were utilized after they were ground for inclusion in the diets. Dietary formulation and processing used organic raw components labeled and approved following Regulation (EU) 2018/848 [
29]. The feed was extruded with two nozzle sizes: 2 mm for shrimp weighing between 2 and 12 g and 3 mm for those weighing more than 12 g. For shrimp weighing less than 2 g, the 2 mm feed was ground and sieved to obtain 1 mm crumbs. Once completed, they were packaged and stored in a commercial refrigerator at 4 °C.
The diets used in the trial were manufactured in the feed factory facilities of the Universitat Politècnica de València (UPV) by a cooking extrusion process, using a semi-industrial double-screw extruder (CLEXTRAL BC 45, St. Etienne, France) from the Animal Technology and Science Institute. The following processing parameters were used: a screw speed of 100 rpm, a pressure range of 20–30 atm, and a temperature of 110 °C.
The dry ingredients were ground in a mill with a 1 mm mesh screen. For the mixture, we mixed the macro-ingredients on one side and the micro-ingredients (calcium phosphate, lecithin, and vitamins) on the other. The micro-ingredient mixture was mixed with a small portion of the macro-ingredient mixture, increasing the quantity of the macro-ingredient mixture in successive proportions. Finally, the oils were added to the horizontal screw mixer.
2.2. Experimental Design
Shrimp growth trials were conducted in the facilities of the Wet Aquaculture Laboratory of the Universitat Politècnica de València. Shrimp used for this study came as larvae from the White Panther hatchery in Austria. Before the beginning of the experiment, larvae were kept in an adaptation period and fed with a commercial diet until they reached 1 g.
The shrimp were placed in 30 plastic rectangular tanks with a capacity of 90 L, with independent and constant aeration and temperature regulation. Tanks were included within an indoor RAS system, which allowed a water flow of 40% per hour. Water was purified using a mechanical filter, a foam fractionator, and a biofilter.
Fifteen shrimps, with an average weight of approximately 1 g, were placed in each 90 L tank, which equates to 167 shrimp/m
3. Shrimp from six tanks were fed with one of the 5 experimental diets (CON, PIG, FLY, TRO, and WHT). All shrimps were individually weighed every 14 days, and the daily rations were changed depending on liveweight and biomass. The growth was estimated using the specific growth rate parameter (SGR) in the following equation:
where the initial body weight is BWi, BW
f is the final body weight, and days are the number of days between both weights.
The daily feeding rate varied from 10% for average weights less than 4 g, 8% for weights between 4 and 7 g, and 5% after the average weight reached 7 g. Feeding was carried out manually and divided into three doses per day during the first period and into two doses per day in the second period. The experiment lasted a total of 83 days.
For feed conversion ratio (FCR), feed intake (FI), ingested protein retention (IPR), and ingested fat retention (IFR) estimates, the final biomass was corrected to consider the biomass of dead shrimp during the trial:
The biomass of dead shrimp was estimated from the average weight between sampling periods and the number of dead shrimps.
2.3. Chemical and Biochemical Analysis
Water quality parameters, including temperature, oxygen levels, and N compounds (ammonia, nitrites, and nitrates), were monitored daily. These parameters remained within the optimal range for the species’ development: temperature 28 °C ± 1, pH 7.92, O
2 6.36 mg/L, salinity 30 ± 1 psu, and NO
3− 26.3 mg/L. Temperature, salinity, pH, and O
2 were measured using a multiparametric device (HANNA, HI19829 Model, Woonsocket, RI, USA). Ammonia and nitrites were measured using the Baumgarten protocol [
30] and remained lower than the detection limit of the method throughout the growth experiment (N-NH
4+ < 0.001 and N-NO
2− < 0.001 mg/L).
All biochemical analyses were developed in the laboratories of the Animal Technology and Science Institute, where samples of ingredients, diets, shrimp, and their feces were analyzed.
The diet’s approximate composition (
Table 1), as well as the whole shrimp, were examined using the methods described in AOAC [
31] and analyzed for the following metrics: dry matter (105 °C to constant weight); ash (incinerated at 550 °C for five hours); crude protein (determined by the direct combustion method DUMAS using LECO CN628, Geleen, The Netherlands); and crude lipid (extracted with methyl-ether using ANKOMXT10 Extractor (Macedon, NY, USA)). Each analysis was carried out in triplicate.
A Waters HPLC system (Waters 474, Waters, Milford, MA, USA) composed of two pumps (Model 515, Waters), an autosampler (Model 717, Waters), a fluorescence detector (Model 474, Waters), and a temperature-control module was used to analyze the levels of amino acids (AA) in the diets and in the shrimp using a procedure previously described by Bosch [
32].
Before hydrolysis, aminobutyric acid was introduced as an internal standard. AQC was used to derivatize AA (6-aminoquinolyl-N-hydroxysuccinimidyl carbamate). After oxidation with performic acid, methionine and cysteine were identified individually as methionine sulphone and cystic acid. AA was converted to methionine and cystine after it was separated with a reverse-phase C-18 column by Waters Acc—Tag (150 mm 3.9 mm).
Table 3 shows the essential amino acid (EAA) content of experimental diets. All amino acid analyses were carried out in duplicate.
For the determination of fatty acids, a direct method of synthesis of fatty acid methyl esters (FAME) was performed. Fatty acids were analyzed in a Focus Gas Chromatograph (Thermo, Milan, Italy), equipped with a split/splitless injector and an ionization detector of flame. The separation was performed on an SPTM 2560 capillary column (Supelco, PA, USA). Shrimp meat fat analysis was carried out by extracting 10–30 mg of crude fat from each sample. First, 1 mL of tridecanoic acid (C13:0) was used. Next, 0.7 mL of 10 N KOH and 5.3 mL of methanol were added. The tubes were incubated at 55 °C in a thermoblock for an hour and a half, and were subjected to vigorous shaking for 5 s every 20 min. After cooling to room temperature in a water bath, 1.5 mL of HPLC-grade hexane was added to the reaction tubes, vortexed, and centrifuged at 1006×
g for 5 min. The hexane layer, containing the FAMEs, was then placed in vials for analysis by gas chromatography. Fatty acids were identified by comparing their retention times with those of a standard of fatty acid methyl esters (47885-U) from Supelco
® (Bellefonte, PA, USA) and quantified using C13:0 as an internal standard [
33]. Initial and final shrimp samples were analyzed to evaluate the nutritional value of each diet and to estimate the nutrient retention in the shrimp.
2.4. Enzymatic Activity Analysis
Regarding the evaluation of the activity of digestive enzymes, this study was carried out through the analysis of the enzymes trypsin and chymotrypsin at the level of the hepatopancreas. For sampling, the shrimp were slaughtered 3 h after sampling, and the cephalothorax part was cut from 12 shrimps per treatment (3 shrimps per bucket and 4 replicates/treatment). The weight of the cephalothorax was recorded, and it was stored at a temperature of −20 °C. Digestive enzyme analyses and trypsin and chymotrypsin activities were measured using 50 mM BApNA (N-α-benzoyl-L-arginine-p-nitroanilide hydrochloride, Merck KGaA, Darmstadt, Germany) and 50 mM GApNA (N-glutaryl-L-phenylalanine-p-nitroanilide, Merck KGaA, Darmstadt, Germany) as substrates, respectively. The absorbance at 405 nm was measured using an Ex multiscan spectrophotometer (Thermolab Systems, Helsinki, Finland), where a unit of activity was defined as 1 μg of p-nitroaniline released per minute.
2.5. Digestibility Study
After the feeding trial, the shrimp were individually placed in 80 L glass aquariums to continue with the digestibility trial and 3 replicates were made for each feed to obtain feces. Changes of water in each aquarium were carried out to maintain good water quality, twice per week, with a continuous filtration system during the test period. For the technique, two aquariums were used for each batch of shrimp. In one, the shrimp were fed and approximately 30 min later they were moved to a second aquarium, clean of food remains and molting, to ensure that only feces were collected. Feces were collected daily by manually siphoning the aquariums after feeding (at 8:30 a.m. and 2:00 p.m.). This process requires a lot of staff time but ensures proper collection of feces. The collected fecal material was stored at −20 °C, dried weekly in an oven at 55 °C with crucibles, and stored in hermetic plastic jars until reaching 4 to 5 g to carry out the analyses of nutritional components and markers.
Apparent digestibility of the diet was estimated indirectly using chromic oxide (Cr
2O
3) as an inert and indigestible marker, which was added to the mixtures shown in
Table 1 at 5 g/kg. Additionally, its concentration was measured in the diet and feces to analyze dry matter, crude protein, and energy in both. An atomic absorption spectrometer was used to determine the amount of chromium oxide in diets and feces after acid digestion (Perkin Elmer 3300, Perkin Elmer, Boston, MA, USA). The apparent digestibility coefficients (ADCs) of the diets were calculated according to Cho [
34].
2.6. Economic Indexes
For the economic study, two economic efficiency indicators were estimated. Economic conversion (EC) was calculated using the following formula:
And Profit Index (PI), which takes into account increase in profit, was calculated using the formula
2.7. Statistical Analyses
Growth data, nutrient utilization, biometric parameters, body composition, amino acid retention, protein and fat retention, and digestibility data were treated using analysis of variance (ANOVA). The Student–Newman–Keuls test was used to evaluate specific differences between diets. Levene’s test was used to check for homogeneity of variance, and the Chi-square and Kolmogorov–Smirnov tests were used to check for normality. If the variables did not pass the normality test, data were transformed using ASIN or SQRT functions before performing the ANOVA test. Initial bodyweight was used as a covariate when analyzing Final Weight, Weight Gain, or SGR. Data were considered statistically significant when p < 0.05, and data are shown as the mean with standard error of the mean (±SEM). Statistical analyses of the data were performed with Statgraphics Statistical Graphics System, version Centurion 18—X64.
3. Results
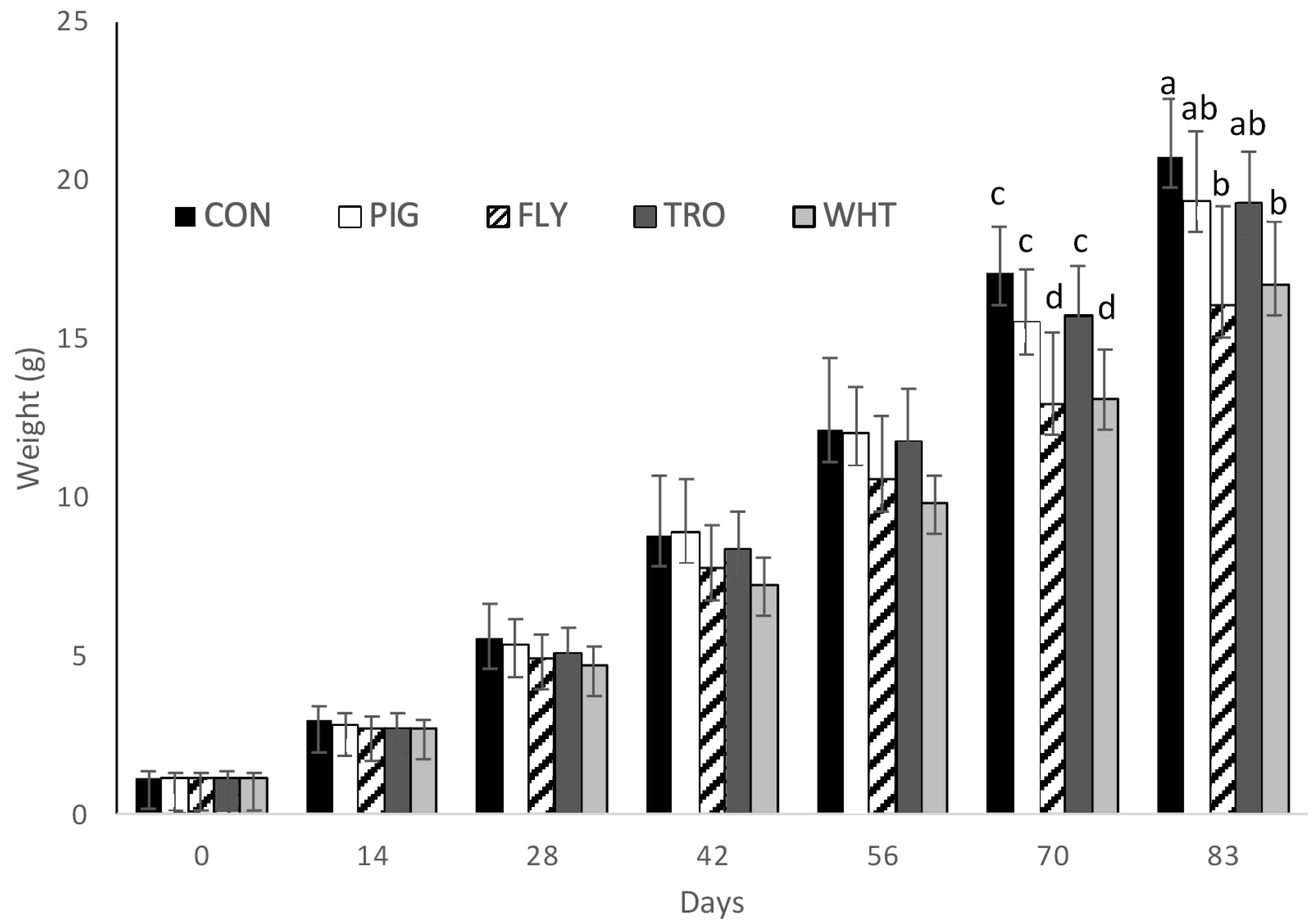
Growth of
Penaeus vannamei fed with the different organic experimental diets (CON; PIG; TRO; FLY; WHT) can be observed in
Figure 1. Differences among treatments began on day 70, where shrimp fed with the FLY and WHT diets grew less than those on the rest of the treatments. On the final day 83, significant differences in weight appeared between shrimp fed with the CON diet and shrimp fed with the FLY and WHT diets, but not with PIG and TRO.
The results of shrimp growth and nutritional parameters are shown in
Table 4. No significant differences were seen among treatments in terms of survival, specific growth rate (SGR), feed intake (FI), and feed conversion ratio (FCR). However, there were statistical differences in the final weight; shrimp fed with the control diet (CON) had the highest final weight (20.7 g), with no differences between those fed with Iberian pig by-product (PIG) and organic trout by-product (TRO) diets (both 19.3 g), while the lowest final weights (16.7 g and 16.0 g) were in shrimp fed with the organic vegetal mixture (WHT) and organic insect meal (FLY) diets, respectively. Likewise, weekly gains in shrimp fed CON (1.65 g/week) were higher than in shrimp fed WHT (1.32 g/week) and FLY (1.26 g/week), but similar to shrimp fed PIG (1.54 g/week) and TRO (1.53 g/week).
Body composition and protein and fat retention efficiencies are presented in
Table 5. All shrimp showed increased dry matter and protein from the beginning, but the percentage of fat was similar. Shrimp fed with the FLY diet had significantly higher dry matter and ash (25.75 and 2.90%, respectively), followed by shrimp fed with the TRO diet (25.39 and 2.69%, respectively). No differences in body fat appeared, but there were significant differences in body protein, with the highest values in shrimp fed FLY and TRO (both 20.5%) and lowest in shrimp fed WHT (19.7%).
Regarding retention efficiencies, no significant differences were found between all the organic diets and the ranges were 29–33% in IPR and 6–7% in IFR.
Table 6 shows amino acid composition of whole shrimp expressed as g per 100 g wet weight. For the essential amino acids, there were no significant differences in histidine, lysine, phenylalanine, or threonine in non-essential amino acids, except for alanine and proline, which were higher (1.27% and 1.28%, respectively) in shrimp fed with the FLY diet. Shrimp fed with TRO presented the highest level of methionine (0.52%) and those fed the FLY diet had the highest content of arginine, isoleucine, leucine, and valine (2.04, 0.85, 1.46, and 1.00%, respectively).
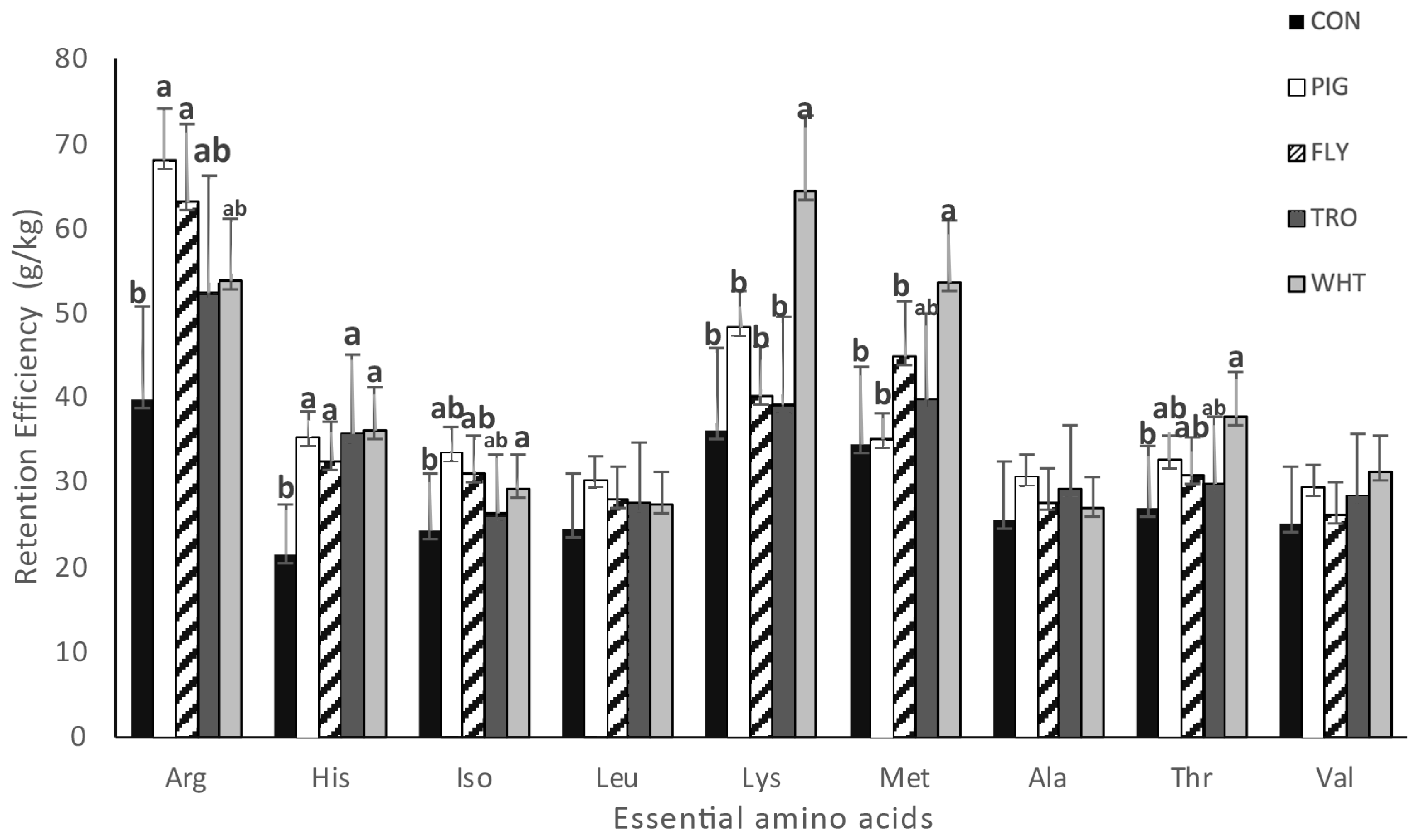
In amino acid retention efficiency, significant differences were found between shrimp fed with different diets for most essential amino acids (
Figure 2). Shrimp fed with the WHT diet had the highest retention efficiency of lysine (64.4%), methionine (53.6%), and threonine (37.8%), and shrimp fed CON presented the lowest values in some amino acids.
In the case of Lysine, shrimp fed with the WHT diet presented significant differences across all treatments. Regarding methionine values, shrimp fed the WHT diet presented significant differences to the shrimp fed with all treatments, except the shrimp fed with the TRO diet. Finally, retention efficiency of arginine showed significant differences; shrimp fed with the CON diet had lower values (39.8%) than shrimp fed with the PIG (68.0%) and FLY (63.0%) diets.
As shown in
Table 7, significant differences in enzymatic activity were observed. Shrimp fed with the FLY diet showed higher trypsin activity (0.17 U/g) compared to those fed with WHT, TRO and PIG diets. Similarly, shrimp fed with the FLY diet also showed higher chymotrypsin activity (0.62 U/g) than those fed with the PIG diet.
Apparent digestibility coefficients (
Table 7) revealed some differences, the FLY diet presented significantly lower values for dry matter, crude protein and gross energy (33.5, 68.9 and 64.9%, respectively) compared to the other diets. The ADC of dry matter registered higher values in the PIG and CON diets (58.6 and 56.7%, respectively), and intermediate values in TRO and WHT diets (49.4 and 46.3%, respectively). No differences can be observed on protein and energy ADC for the rest of diets.
The cost of organic experimental diets (
Table 8) was different in the function of the price of ingredients. The cost was highest for FLY due to it consisting of very expensive organic insect meal, followed by WHT and CON because of the high prices of organic wheat gluten and soybean, and fish meal, respectively, making the PIG and TRO diets cheaper due to the low prices of by-products. Economic conversion and profit index were similar in all diets except in FLY, which presented the highest value of EC and lowest of PI. When comparisons were made without FLY, new differences appeared and PIG and TRO presented better EC and PI values than CON and WHT.
4. Discussion
The exploration of alternative raw materials to protein derived from fish meal is complicated when they need to be certified as organic. Organic certification regulations promote the use of organic vegetable meals, animal by-products, which are preferable when they come from organic production, and insect production, which is seen as a good alternative to animal protein.
Shrimp survival can vary depending on many factors: initial and final weight, management, diet, and the use of probiotics, among others [
35]. In the present test, it ranged between 58.6% and 71%, typical values for this species considering the entire growth range [
35]. The estimated biomass data from mortality was considered in the calculations so as not to distort the FI and FCR results.
The growth, measured as SGR, of the organic diets was between 88 and 95% of the SGR obtained with the CON diet (
Table 4), with similar SGR values (3.19–3.43%/day) to the values obtained by Liao [
19] and Hlordzi [
23] with a control diet, but these authors grew shrimp to lower weights (5.7–7.5 g). When weekly gain is considered, the growth obtained with the best diets (CON, PIG, and TRO) ranged from 1.53 to 1.65 g/w, which was in the same interval as values cited by other authors [
12,
20,
21,
22] and with a similar final weight. Although some tendencies were observed on days 42 and 56, growth differences between diets only appeared on day 70 (
Figure 1).
Previously, only one study has researched the growth of shrimp on organic plant-based diets [
8]. Comparison of results is limited by several factors. The feed composition used by Browdy et al. [
8] includes 581 g/kg of soybean meal, 100 g/kg of pea meal, and 20 g/kg of squid and liquid fish meal. In the present study, the VEG diet has 214 g kg
−1 of wheat gluten and 225 g kg
−1 of soybean meal, which is responsible for the low lysine content (
Table 2). In contrast, soybean and pea meals are richer in lysine, and it could be assumed that the diet used by Browdy et al. [
8] was not deficient in lysine. Browdy et al. [
8] developed their trial in earth ponds and reported a lower weekly gain (1.43 g/w) than the current trial but a better FCR (1.33), perhaps due to natural productivity and diet. Likewise, the organic plant diet included a small amount of squid meal and liquid fish solubles, and the good results may have originated from natural nutrients.
Currently, the growth of shrimp fed diets containing Iberian pig viscera (PIG) and organic trout by-products (TRO) was good, with no differences in relation to the control diet. These results agree with Amaya [
12], who obtained similar shrimp growth with diets in which the protein source was a mix of soybean and poultry by-product or even only soybean. However, in the present study the vegetal diet (WHT) initiated lower growth due to the insufficient lysine content of the diet. Likewise, Galkanda-Arachchige [
18] reported good results with diets without fish meal and fermented corn protein, but the duration of the trial was only 8 weeks and the shrimps’ final weights were lower (3.8–4.7 g), so it is possible that differences did not appear. On the other hand, some authors obtained poor results with a total substitution of fish meal by soybean [
21], by
Rhodobacter fermented protein [
19], or by fermented soybean [
20]. Although the substitution was not total (5–10% fish meal), Manikandan and Felix [
22] reported better growth with single-cell protein than the control diet, which is a promising result. Likewise, organic insect meal showed a bad result (FLY), in agreement with Cummins Jr [
36] who cited lower growth (final weight 8 g) with a complete substitution of fish meal with
Hermetia meal, and even with partial substitution (9–12 g), in relation to control diets (16 g).
Concerning FCR, the results were not as expected. The lowest value (1.83) was obtained with the diet TRO; however, no significant differences appeared between experimental diets, and FCR values were in general similar to those obtained by other authors, even with lower final weights [
18,
19,
20,
21,
22,
23], but clearly worse than those cited by Amaya [
12] (around 1.1–1.2).
In general, similar values of FCR have been found when fish meal was replaced by alternative ingredients, but some differences can be cited. For example, Liao [
19] obtained lower FCR values of 1.71 and 1.58 with 12 and 16% fish meal level, respectively, than diets including
Rhodobacter sphaeroides protein, which showed an FCR of 2.24. Hlordzi [
23] tried a 37.5% fish meal substitution (15% fish meal) by hydrolyzed fish protein meal (4%), obtaining a promising FCR of 1.5 in relation to 1.8–2.2 in other diets. Other alternative vegetable sources [
18], such as fermented corn protein concentrate [
21], soy protein concentrate [
20], and fermented soybean meal, did not show differences in FCR between diets, and values were similar or higher (2.1–2.9) than for the shrimps fed with WHT diets of the present study (2.1). In the case of insect meal, FCR values were worse with high inclusion (4.0–4.5), in comparison with the control diet (FCR around 2.0), and much higher than in the current trial with organic insect meal (2.4).
When alternative protein sources, primarily plant-based, are recommended, the amino acid profile can be limiting. Raw materials such as soy flour provide more complete profiles than other plant-based sources. Wheat gluten is limiting in this case, due to its lysine content. In non-organic feeds, supplementation with synthetic amino acids is generally required. However, in organic aquaculture, this is difficult because organically certified synthetic amino acids are not usually available [
26]. Nevertheless, Nunes [
37] did not find global differences in the performance of shrimp fed diets containing several levels of fish meal (0, 6, 12, and 18%) supplemented with methionine (0.58, 0.69 and 0.82%), but considering only diets without fish meal, a methionine level of 0.58% gave a lower growth in comparison with 0.69%. In the current trial, diets of FLY and WHT, containing 0.48% and 0.40% met, gave a lower growth than the rest of the diets containing 0.59–0.68% met.
The diets were designed to have 35% crude protein and 10% lipids, using only organic raw materials and without supplementation with amino acids. But the amino acid needs were not considered; consequently, this WHT diet has some amino acid deficiencies. According to recommendations for meeting nutritional needs, lysine should be above 1.6% (
Table 2) [
38,
39]. This is almost achieved in the case of the PIG diet (1.44), but was deficient in the case of the WHT diet (1.07). Assuming a lysine content in wheat gluten of 0.5% and with a lysine content of 2.6% in organic soybean meal (47% protein), it can be hypothesized that by varying the WHT diet by increasing the soybean meal up to 450 g/kg, decreasing the wheat gluten to 89 g/kg, and decreasing the organic wheat to 332 g/kg, we would obtain a WHT diet formulation with enough lysine to not be limiting, reaching the required 1.6% and maintaining the 35% of crude protein required. But with the current composition of the WHT diet, the main reason for the lower growth is the lysine content.
On the other hand, experimental organic diets presented different levels of essential fatty acids (
Table 3), mainly EPA and DHA, which could have some effect on growth. The National Research Council [
39] recommends minimum levels of LC-PUFA of 0.25–0.5%, which is provided by LNA in diets. But previous studies have demonstrated the inability of shrimp to elongate and desaturate PUFA into HUFA [
40], and in some studies it seems that this does not affect growth performance [
41]. Other studies have shown that EPA and DHA fatty acid levels above 1.7% and 2.7% appear advisable to optimize growth [
42]. CON, PIG, and FLY presented similar values, and TRO slightly lower, but WHT had the lowest values, which could also partially explain its worse growth results.
Protein composition is usually a stable parameter, in the present study it varies slightly between 19.7% dm and 20.5% dm of body composition (
Table 5), as expected and in agreement with other studies with alternative proteins, where the protein composition was very similar and between 20.0% and 21.5% in shrimp weighing 10 g [
24], similar in small shrimp of 6–9 g with 17.5–19.1% [
23] but higher than in big shrimp of 23.3 g, with 16.1–16.9% protein [
22]. The significant differences in body protein found between shrimps fed with FLY treatment and those fed with WHT do not have enough importance to offer a clear result; for example, in the experiments of Cummins Jr., fish meal was replaced by insect meal in different proportions until total substitution, and a variation of between 18 and 20% protein was obtained without any significant differences or trends being noted. In the present study, all values are very close to 20.
IPR, a ratio that offers information on protein quality without distinguishing between amino acid profile or digestibility, ranged between 29% (CON) and 33.5% (TRO), but no significant differences among groups were found, which partially agrees with the only results obtained by Hlordzi [
18] with a hydrolyzed fish protein, which report a good amino acid profile and good digestibility, obtaining an IPR that improved with dietary inclusion from 23 to 35%.
If experimental organic diets were not supplemented with amino acids then essential AAs were not balanced (
Table 2) and, for example, arginine, lysine, methionine, threonine, and valine presented at lower levels in the WHT diet. Nevertheless, the final composition of shrimp did not reflect this (
Table 6) because no differences were found in the same Aas, such as lysine and threonine, and in others, such as methionine, the differences were small. Despite the lower lysine content in the diet already mentioned, amino acid retention (
Figure 2) is a good way to approach this matter. Shrimp fed the WHT diet showed the highest values of lysine, methionine, and threonine retention as a response to lower amounts of these amino acids in its composition, which means more effective retention in shrimp fed the WHT diet to cover requirements. This could mean that higher levels of methionine and threonine in the WHT diet could be desirable to maximize growth, in addition to the already known deficiency of lysine in the WHT diet. This effect has also been observed in several studies [
26,
27,
28] in organic diets for seabream and seabass, containing lower amounts of a particular amino acid, methionine, in most cases.
Conversely, when the amino acid profile of essentials is well balanced, as expected in shrimp fed with the CON diet, retention of essential amino acids could be lower and the retention of essential amino acids may decrease due to reduced catabolic recycling. In such cases, amino acids are more efficiently utilized for protein synthesis, minimizing the need for excess retention and resulting in improved growth or feed conversion efficiency in shrimp. The poor digestibility of the FLY diet seems to have some influence on the bad performance in the shrimp fed it, which would partially agree with Tefal et al. [
26], because seabass organic diets containing insect meal had a lower digestibility, but not with Tefal et al. [
28], because seabream organic diets with insect meal presented the highest digestibility. Organic animal ingredients (PIG and TRO) had good digestibility, such as the organic vegetal diet (WHT), the results for which are in agreement with findings of high digestibility with fermented soybean [
20] and single-cell protein [
22]. In this study, the influence of the different diets in digestibility could be partially explained by enzyme activity.
Furthermore, chymotrypsin activity was higher than trypsin activity in shrimp, which agrees with other studies [
43,
44,
45]. Although in the past trypsin was considered the main peptidase, chymotrypsin is nowadays recognized as the one with the highest activity [
46]. Their activities have been shown to be a sensitive key in the protein digestion process, strongly modulated by the quality and quantity of food proteins, because trypsin shows a preference for amide substrates with arginine and lysine, and chymotrypsin for carbonyl groups of tyrosine, phenylalanine, tryptophan and leucine [
44].
In this study, trypsin activity was significantly higher in shrimp fed with the FLY diet than the other shrimp fed with alternative protein ingredients, which could indicate greater difficulty in digesting the insect meal protein. This is probably caused by the probable chitin content of insect-based meals, which may impact digestive enzyme reactions. Increased chitin has been shown to have a negative influence on protein digestibility, at least in vitro [
47].
Furthermore, in
P. monodon, an increase in chitin has also been shown to decrease the digestibility of protein and lipids, although to a lesser extent [
48]. A higher trypsin activity was found in low-protein diets, but no differences appeared in relation animal-to-plant protein ratio [
49], which partially corroborates results for similar trypsin activity in diets of CON, PIG, TRO, and WHT.
Omont [
45] cited higher trypsin activity in shrimp fed with marine algae in comparison with other plant ingredients (wheat), and although no data for amino acid composition was presented, the authors commented that it could be due to the amount of lysine and arginine. However, in the present study several experimental diets contained different levels of arginine and lysine and similar trypsin activity was obtained, except in the shrimp fed the WHT diet, which had intermediate levels of these amino acids.
In the case of chymotrypsin activity, differences only appeared between the shrimp fed with the FLY and PIG diets, which showed lower values, indicating that shrimp fed with the PIG diet required less digestive effort than the shrimp fed with the FLY diet. Other authors [
45] have not found differences in chymotrypsin activity using several plant sources with similar levels of fish meal. For a more complete understanding, the interaction of different raw materials with digestibility and enzymes should also be studied with studies on intestinal health and immunology.
Alternative protein ingredients hold interest for aquaculture in improving sustainability, both environmental and economic. The environmental advantages of reducing the use of fish meal and reutilizing by-products are obvious, but economic benefits depend on the price of raw materials. In the case of organic feeding some ingredients can be more expensive, which makes an economic evaluation necessary. The economic productivity parameters shown in
Table 9, in terms of kilograms per hectare, include some extrapolations that should be interpreted with caution. These experiments were conducted in RAS systems with small tanks, and caution should be taken in extrapolating to production sizes. Furthermore, ingredient prices can vary on the market; by-products do not have an established price, unlike other, more common raw materials. Insect meal also has a high current price, which could decrease in the future with the development of the industry.
Any different mechanism can explain the good results using organic ingredients because these products do not contain different nutrients to conventional ingredients. Iberian and trout by-products provided similar results to fish meal diets, but the low content of some EAAs, such as arginine and methionine, or the lower lysine content in the WHT diet, could explain the poor results for the wheat organic diet and partially for the INS diet. Furthermore, protein digestibility may also influence the INS diet.