Abstract
Although marine caves are among the most species-diverse habitats in the Mediterranean Sea, most available studies have focused on their sessile fauna. This study provides the first quantitative assessment of motile fauna in 27 marine caves across four geographical subareas of the Aegean and Ionian Seas, using a rapid assessment visual census protocol, applied through 3 min time transects in each ecological cave zone. Multivariate analysis revealed that the motile community structure of the cave entrance was differentiated from that of the semidark and dark zones. Deeper caves were distinct from shallower ones while caves of the east Aegean differed from those around Crete Island. A total of 163 taxa were recorded, 27 of which are reported herein for the first time in marine caves of the eastern Mediterranean Sea, while three species (two native and one introduced) are recorded in Greek waters for the first time, enriching our knowledge on the permanent and occasional cave residents. Seventeen species were introduced, comprising more than half of the total fish abundance in the southeasternmost cave. Our limited knowledge of the motile fauna of Mediterranean marine caves coupled with the continued spread of introduced species highlights the urgent need for monitoring and conservation actions, especially within marine protected areas.
Keywords:
motile species; introduced species; visual census; sea caves; ecological zone; Aegean Sea; Ionian Sea; Greece Key Contribution:
A visual census protocol can be applied to rapidly assess the motile community structure of eastern Mediterranean marine caves, a species-diverse habitat differentiated depending on the depth and geographic area.
1. Introduction
The rocky coasts of the Mediterranean Sea are characterized by the presence of numerous marine caves, which can be either semi-submerged or entirely submerged []. Marine caves rank among the richest habitats in terms of biodiversity in the Mediterranean Sea, with approximately 2400 taxa reported from 350 caves (mostly semi-submerged and/or shallow) documented to date [].
Most studies on marine cave fauna have focused on sessile communities, e.g., [,,,,,]. Although efforts to study motile fauna in Mediterranean marine caves date back to the 1960s [,], most available studies are either qualitative or limited to specific taxonomic groups [,,,,,,,,]. Recent reviews on marine cave fishes and crustaceans point to a rich diversity of motile species that find refuge in these habitats [,,]. In addition, the exploration of marine caves in previously under-studied areas has led to several new records of rarely observed species [,,], suggesting that marine cave biodiversity is greater than previously estimated.
However, most studies on motile cave fauna have been conducted in the western and central Mediterranean [,,,,,], leaving significant knowledge gaps in the eastern and southern sectors of the basin. These areas are experiencing rapid ecological shifts as they have been impacted by mass mortality events and biological invasions over the past decades [,]. Marine caves are threatened by multiple global and local pressures [], such as the effects of climate change, coastal infrastructure development, unregulated tourism, and marine pollution [,,,]. Recent studies have highlighted the growing numbers of introduced species in marine caves of the eastern and southern Mediterranean [,,], with the term “introduced species” being used to include non-indigenous, cryptogenic (species with unclear native or introduced origin), crypto-expanding (species with unclear natural or human-mediated expansion), and species with questionable status []. Specifically, within only eight years, the number of introduced species inhabiting Mediterranean marine caves has more than doubled []. Approximately half of these species (60 species or 47.6%) are motile, with the majority recorded in the eastern basin (50), particularly in the Levantine (45) and Aegean (20) ecoregions. Most of these introduced species originate from the Indo-Pacific and have entered the Mediterranean Sea through the Suez Canal []. In light of such abrupt ecological changes, there is an urgent need to obtain quantitative data on the population structure of motile fauna in eastern Mediterranean caves, to better understand the potential responses of native biodiversity to cumulative pressures []. Systematic sampling in such environments is particularly challenging due to sharp ecological gradients, limited visibility, and time and space constraints of cave habitats [,]. Marine caves are characterized by steep environmental gradients, such as the decrease in light levels and hydrodynamism, accompanied by increased oligotrophy towards the inner zones, caused by their unique topographic and morphological features [,]. These gradients structure the cave biota into three distinct ecological zones: the well-lit entrance zone (CE), dominated by macroalgae (mostly rhodophytes); the intermediate semidark zone (SD), dominated by sciaphilic animals; and the inner dark zone (D), where both diversity and biotic cover diminish []. While such environmental gradients are primarily known to influence sessile communities, increasing evidence indicates notable spatial variability among motile taxa, particularly fish assemblages. Most fish species tend to occur in the entrance and semidark zones of caves, whereas only a limited number of larger or hyperbenthic species, along with small epibenthic and cryptobenthic fishes, are typically found in the innermost dark sections ([] and references therein).
Beyond this small-scale heterogeneity, cave assemblages significantly differ among Mediterranean biogeographic regions, supporting the view that caves are highly fragmented habitats, functioning as isolated “islands” that host unique and often isolated populations [].
In this study, a novel visual census protocol was developed and applied to rapidly and quantitatively assess the motile faunal community in 27 marine caves of the eastern Mediterranean Sea. Specifically, we tested whether motile community structure (i) shows high similarity between equivalent ecological zones across different caves; (ii) differs significantly among geographic subareas and among caves within each subarea; and (iii) exhibits similar patterns for species with high (e.g., fish) and low mobility (e.g., crustaceans, mollusks). Given the high numbers of introduced fishes observed in the studied caves, we also investigated whether the ratios of introduced to native fishes (in terms of both species richness and abundance) correspond with the known southeast-to-northwest gradient observed in the Mediterranean Sea []. In addition, a broader assessment of motile biodiversity in the studied caves is presented, combining visual census data with qualitative sampling, being the first large-scale study of this kind in the most vulnerable sector of the Mediterranean basin.
2. Materials and Methods
2.1. Study Area
Twenty-seven marine caves in the eastern Mediterranean, distributed across ten islands and spanning four geographical subareas of Greece (east Aegean, central Aegean, Ionian Sea and Crete), were surveyed for their motile fauna during different sampling periods between May and December of the years 2020 and 2022 (Figure 1, Table 1) by a single observer during a single dive per cave. Eleven of these caves had fully submerged entrances, whereas sixteen had semi-submerged entrances. The maximum entrance depth ranged from 3.5 to 32 m for submerged caves and from 3.5 to 20 m for semi-submerged ones; cave lengths ranged from 11 to 163 m (Table 1). All studied caves had an entrance and a semidark zone, while eleven also featured a dark zone. Most of the studied caves (20) are located within national parks (National Marine Park of Zakynthos and National Park of Samaria) and/or protected areas of the European Union’s Natura 2000 network (Table 1), including nineteen designated as Habitats Directive Sites and two as Birds Directive Sites.
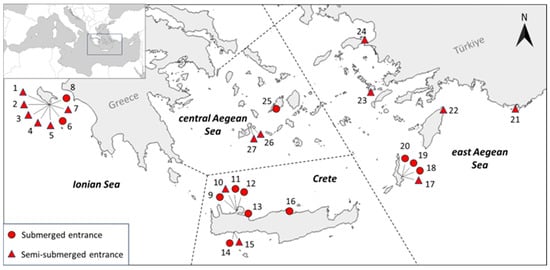
Figure 1.
Location of the studied marine caves with fully submerged (circles) and semi-submerged (triangles) entrances and given reference numbers (RN) (see Table 1). Biogeographical subareas (Ionian Sea, central Aegean, east Aegean, and Crete) based on [] and modified. The inset map (upper left) indicates the location of the surveyed area within the Mediterranean Sea.

Table 1.
Reference number (RN), location, depth, year of sampling, and protection status (Natura 2000 code; * for National Parks) of the 27 studied marine caves. Oct: October; Nov: November; Dec: December; Jun: June; Aug: August.
2.2. Sampling
A visual census protocol was developed and applied to rapidly and efficiently assess the motile faunal communities in marine caves. According to this protocol, a single scientist/scientific diver recorded both species richness and abundance of all observed motile taxa within a 3 min visual survey transect conducted in each of the three ecological cave zones (i.e., entrance, semidark, and dark) while SCUBA diving. Motile species were recorded on cave walls, crevices, overhangs, the cave bed, and ceiling (when present in submerged caves) within each zone. Basic topographic and morphological features of each cave (e.g., submersion level, depth) were also recorded after the visual census. This protocol was designed for non-decompression single-dive surveys in marine caves up to a depth of 30 m, considering the logistical constraints of diving time at such depths (16 to 20 min maximum). This approach allowed for a safe exit from inner cave zones under a precautionary approach and standardized research effort across caves with different topographic features (i.e., depth and varying extent of ecological zones). Overall, a maximum time of nine minutes (three minutes per ecological zone) is required for each survey, with less time needed in caves lacking a dark zone. For deeper caves or those with complex morphology, supplemental diving rules need to be considered.
In the present study, the visual census protocol was consistently applied once in each cave, by the same scientific diver (M.D.), who always entered first, during morning to mid-day hours, to minimize disturbance to the motile fauna and ensure consistency across observations. A second diver accompanied the lead, being responsible for maintaining time intervals between zones and ensuring diving safety. Following the visual census, the team of diving scientists recorded the presence and abundance of motile taxa observed outside the time transects. They also collected qualitative samples for morphological identification (approximately 76 specimens) and close-up photos when in situ identification was not feasible (e.g., for small-sized taxa), to produce a biodiversity inventory for the studied caves. Conspicuous taxa (mostly fishes) were identified during the dives, while small-sized taxa (e.g., mollusks) were mainly identified from collected samples.
2.3. Statistical Analyses
Data on the abundance of all motile taxa recorded within time transects were square-root transformed, and a triangular similarity matrix was generated using the Bray–Curtis similarity index []. A two-way non-parametric multivariate analysis of variance (PERMANOVA) [] was performed, with the factor ‘Cave’ (random, 27 levels—see Table 1 for site names) nested within the factor ‘Subarea’ (fixed, 4 levels—Ionian, Crete, east Aegean, central Aegean). To visualize multivariate patterns, non-metric multidimensional scaling (nMDS) and cluster analysis were applied. The SIMPROF test was used to examine the null hypothesis of no meaningful clustering among samples (significance level: 5%, confidence level: 95%) [,]. A Spearman rank order correlation was used to investigate the monotonic relationship of species abundance with the resulting multivariate community patterns. Species that exhibited correlation values greater than 0.6 (and p < 0.05) were overlaid onto the nMDS plot as vectors [,]. Similarity percentages (SIMPER) analysis was performed to estimate the contribution of individual taxa to dissimilarity patterns []. PERMANOVA, nMDS, and SIMPER analyses were also independently conducted for fish and invertebrate taxa, following the above-mentioned design. If there was no significant variation in community structure among caves within the same geographic subarea, a one-way PERMANOVA was applied, based on pooled abundance data from caves within each subarea to examine potential differences in community structure across the three cave ecological zones. Multivariate, non-parametric resemblance analysis was performed using the software PRIMER-6 [] with the PERMANOVA+ add-on package [].
3. Results
3.1. Species Richness
Within the time transects, 70 motile taxa belonging to seven phyla were identified: Chordata (37), Arthropoda (14), Echinodermata (11), Mollusca (4), Annelida (2), Platyhelminthes (1), and Cnidaria (1). These were identified to the species (55), genus (9), family (4), and class (2) levels by a single observer during a single dive per cave (Table 2).

Table 2.
List of all taxa recorded during the visual census in the studied caves by major taxonomic group (in bold). *: taxa recorded within time transects; ǂ: taxa recorded out of transect. Gammogobius/Corcyrogobius: refer to the species Gammogobius steinitzi Bath, 1971 and/or Corcyrogobius liechtensteini (Kolombatovic, 1891), two small-sized cryptobenthic fish with similar color patterns, often difficult to be distinguished visually by the observer within the time transects.
The taxa recorded within transects comprised 77.8% of the total motile fauna recorded in situ; an additional 20 taxa were observed out of time transects (Table 2). Osteichthyes represented more than half (52.8%) of the taxa which were recorded within transects. At a cave level, the number of motile taxa recorded in transects ranged from 6 to 18, representing 33.3% to 91.7% of the total in situ observed fauna (Figure S1), with a mean of 61.2% ± 2.8 (SE).
The highest number of taxa was recorded in the semidark zone (45), followed by the cave entrance (42), and the dark zone (30). Osteichthyes were the most frequently observed group in all three zones, representing 73.8% of the motile fauna in the cave entrance, 55.5% in the semidark, and 36.6% in the dark zone. The most common taxa were the cardinal fish Apogon imberbis (present in all caves), followed by the polychaete Hermodice carunculata (in 22 caves), the sea urchin Arbacia lixula and holothurians (each recorded in 21 caves).
Species richness per zone (recorded within transects) ranged from 2 to 12 taxa, comprising 16.7% to 100% of the total taxa observed in situ in each respective zone (mean 65.1% ± 3.3 SE, Figure S2). The lowest percentage values (16.7%) were observed in the semidark zones of Blue 2 and Polyaigos caves, while the highest values (100%) were observed in the entrance zones of six caves (Sulfur 1, Sulfur 2, Marathia, Achata 2, Seal’s 2 and Polyaigos), the semidark zone of one cave (Alikes), and the dark zone of two caves (Sulfur 2 and Cathedral) (Table 3). On average, the proportion of motile taxa recorded within transects to the total recorded taxa was highest in the entrance zone (75.1%), followed by the dark (64.4%) and the semidark zone (49.7%) (Table 3).

Table 3.
Species richness and abundance per ecological zone recorded in transect in the studied caves. The percentage of species richness and abundance recorded in transects in relation to the total (in and out of transects) is presented in brackets. CE: cave entrance; SD: semidark zone; D: dark zone; SE: standard error.
3.2. Abundance
The abundance of motile taxa recorded within transects per cave ranged from 14 to 2411 individuals, comprising between 35.5% and 99.5% of all individuals observed in situ (mean 76.6% ± 4.1 SE, Figure S3). Osteichthyes were the most abundant group comprising 64.2% of all individuals recorded, followed by Arthropoda (32.3%), Echinodermata (2.1%), and Annelida (1.2%). The remaining groups (Mollusca, Cnidaria, and Platyhelminthes) comprised 0.2% of the total abundance. Apogon imberbis was the most abundant fish in most caves (19), followed by atherinids (6) and Anthias anthias, which dominated in the two deepest caves (Shrimp and Neptune’s cave). The semidark zone often presented the highest transect abundance (in 15 caves). In total, the highest transect abundance in a single cave was observed in the dark zone (up to 1311 individuals of mostly Plesionika narval), followed by the semidark zone (1054 individuals of mostly Atherina spp.) and the cave entrance (324 individuals of mostly Anthias anthias). The proportion of transect-recorded abundance relative to the total abundance was highest in the dark zone (81.6%), followed by the entrance (77.9%) and the semidark zone (72.6%) (Table 3, Figure S4).
3.3. Resemblance Patterns
Two-way PERMANOVA analysis results indicated that motile community structure significantly differed among the geographic subareas (pseudo-F = 1.622; df = 3; p = 0.021); no significant differences were evident among individual caves within subareas (pseudo-F = 1.154; df = 23; p = 0.083). Pairwise comparisons showed significant differentiation between the east Aegean and Crete (t = 1.428, p = 0.032), with Apogon imberbis, Atherina spp., Chromis chromis, Plesionika narval, Hermodice carunculata, and Arbacia lixula contributing 52.2% to this differentiation (Tables S1–S3). The average similarity within each area ranged from 20.3% for the east Aegean to 27.3% for the central Aegean. Within all geographic areas, significant differences in community structure were detected between the cave entrance and the inner semidark and dark zones (see Tables S4–S7) (Ionian: pseudo-F = 2.682, df = 2, p = 0.001; Crete: pseudo-F = 2.690, df = 2, p = 0.002; east Aegean: pseudo-F = 1.699, df = 2, p = 0.041; central Aegean: pseudo-F = 2.192, df = 2, p = 0.038). Apogon imberbis and Plesionika narval showed the strongest positive associations with the semidark and dark cave zones, while Coris julis, Chromis chromis, Thalassoma pavo, and Arbacia lixula were more closely associated with the cave entrance (Figure 2).
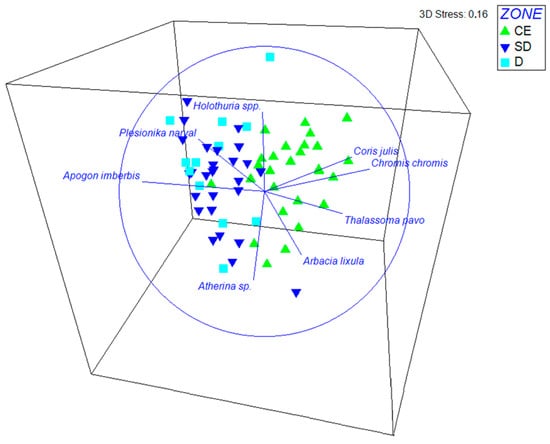
Figure 2.
Non-metric multidimensional scaling (nMDS) three-dimensional plot showing the dissimilarities among motile taxa recorded in transects at different ecological zones and the species contributing the most to this differentiation. CE: cave entrance; SD: semidark zone; D: dark zone.
Cluster analysis indicated that the species composition of the deepest caves with fully submerged entrance (Neptune’s and Shrimp cave) was significantly different from all other caves (Figure 3), with an average dissimilarity of 80.3% (SIMPER and SIMPROF analyses). This differentiation was primarily driven by the shrimp Plesionika narval (45.4%) and the fish Anthias anthias (10.5%).
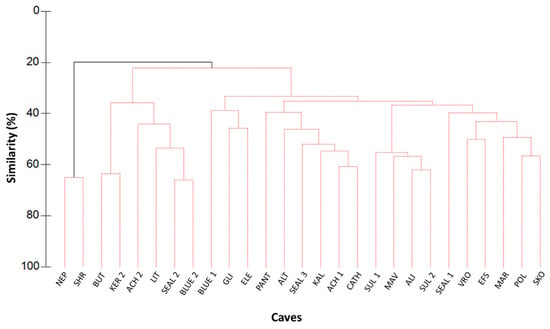
Figure 3.
Cluster dendrogram with the percent similarity among all studied caves (NEP: Neptune’s; SHR: Shrimp; BUT: Butterfly; KER 2: Keri 2; ACH 2: Achata 2; LIT: Lithistid; SEAL 2: Seal’s 2; BLUE 2: Blue 2; BLUE 1: Blue 1; GLI: Glika Nera; ELE: Elephant; PANT: Pantieronisi; ALT: Altar; SEAL 3; Seal’s 3; KAL: Kalymnos; ACH 1: Achata 1; CATH: Cathedral; SUL 1: Sulfur 1; MAV: Mavros Kavos; ALI: Alikes; SUL 2: Sulfur 2; SEAL 1: Seal’s 1; VRO: Vronti; EFS: Efstathios; MAR: Marathia; POL: Polyaigos; SKO: Skotino). Red lines indicate non-significant differentiation; Black lines indicate significant differentiation.
SIMPER analysis also showed that Neptune’s and Shrimp caves had an average similarity of 64.8%, mainly due to P. narval (72.1% contribution), while all other caves clustered with an average similarity of 31.9%, primarily due to A. imberbis (52.3% contribution). When fish and motile invertebrate taxa were analyzed separately, two-way PERMANOVA indicated no differentiation among the four subareas or between the caves within the same geographic subarea. However, for fish species alone, PERMANOVA detected significant differences in community structure between the entrance and inner zones across all subareas: Ionian: pseudo-F = 3.139, df = 2, p = 0.001; Crete: pseudo-F = 3.314, df = 2, p = 0.004; central Aegean: pseudo-F = 2.941, df = 2, p = 0.034; east Aegean: pseudo-F = 2.101, df = 2, p = 0.030 (see Tables S8–S13). Apogon imberbis was predominantly associated with inner zones, whereas Coris julis, Chromis chromis, and Thalassoma pavo were mostly related to the cave entrance (Figure 4).
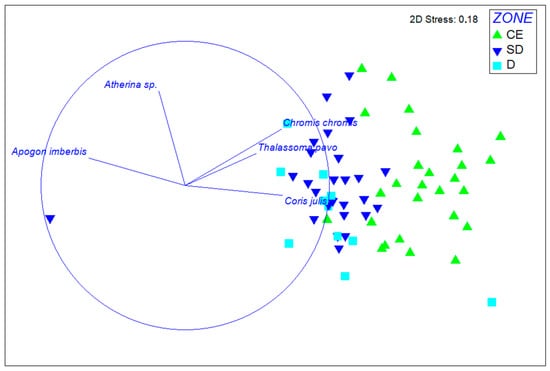
Figure 4.
Non-metric multidimensional scaling (nMDS) two-dimensional plot showing the dissimilarities among the fish taxa recorded in transects at different ecological zones and the fish species contributing the most to this differentiation. CE: cave entrance; SD: semidark zone; D: dark zone.
3.4. Biodiversity Inventory
A total of 163 motile taxa belonging to seven phyla were identified: Chordata (57), Mollusca (51), Arthropoda (30), Echinodermata (18), Platyhelminthes (4), Annelida (2), and Cnidaria (1). These were identified to the species (142), genus (9), family (9), superfamily (1), class (1), and order (1) levels, and included 12 protected and 17 introduced species (Table 4). These taxa were identified visually during the time transects and through out-of-transect in situ observations (1–3 dives per cave and 1–3 observers per dive), and by examining the supplementary collected material (photos and samples).
The total number of motile species recorded per cave ranged from 9 to 54 taxa. The Lithistid cave in southern Crete and Seal’s 3 cave on Samos Island (eastern Aegean) were the most species-rich sites, with 54 and 49 taxa recorded, respectively.

Table 4.
Complete list of all motile taxa recorded in the studied caves by major taxonomic group (in bold). *: Introduced species; ǂ: Protected species; °: species morphologically identified with collected specimens; f: species recorded for the first time in eastern Mediterranean marine caves (black colored) and in Mediterranean marine caves (orange colored). Colored circles indicate the presence of taxa in different ecological zones (white: entrance zone; gray: semidark zone; black: dark zone); numbers in brackets indicate the number of caves where each taxon was recorded; blue triangles in squares indicate the geographical subarea each taxon was recorded (left: Ionian; bottom: Crete; right: east Aegean; top: central Aegean).
Table 4.
Complete list of all motile taxa recorded in the studied caves by major taxonomic group (in bold). *: Introduced species; ǂ: Protected species; °: species morphologically identified with collected specimens; f: species recorded for the first time in eastern Mediterranean marine caves (black colored) and in Mediterranean marine caves (orange colored). Colored circles indicate the presence of taxa in different ecological zones (white: entrance zone; gray: semidark zone; black: dark zone); numbers in brackets indicate the number of caves where each taxon was recorded; blue triangles in squares indicate the geographical subarea each taxon was recorded (left: Ionian; bottom: Crete; right: east Aegean; top: central Aegean).
| Phylum Cnidaria | Gastropoda (cont.) |
|---|---|
| Scyphozoa | Polycera quadrilineata (O. F. Müller, 1776) f |
| Pelagia noctiluca (Forsskål, 1775) f | Pseudofusus rolani (Buzzurro & Ovalis, 2005) ° f |
| Phylum Platyhelminthes | Similiphora similior (Bouchet & Guillemot, 1978) |
| Planocera sp. ° | Spurilla neapolitana Bergh, 1889 f |
| Prostheceraeus moseleyi Lang, 1884 f | Steromphala rarilineata (Michaud, 1829) ° f |
| Monobiceros langi Faubel, 1984 ° f | Tarantinaea lignaria (Linnaeus, 1758) f |
| Thysanozoon brocchii (Risso, 1818) | Trapania lineata Haefelfinger, 1960 ° |
| Phylum Mollusca | Tritia incrassata (Strøm, 1768) f |
| Cephalopoda | Tylodina perversa (Gmelin, 1791) ° |
| Octopus vulgaris Cuvier, 1797 f | Umbraculum umbraculum ([Lightfoot], 1786) |
| Sepia officinalis Linnaeus, 1758 f | Bivalvia |
| Gastropoda | Pectinidae sp. |
| Aplus gaillardoti (Puton, 1856) ° f | Phylum Annelida |
| Aplus scacchianus (R. A. Philippi, 1844) ° | Polychaeta |
| Berthella aurantiaca (Risso, 1818) ° f | Bonellia viridis Rolando, 1822 |
| Berthella ocellata (Delle Chiaje, 1830) f | Hermodice carunculata (Pallas, 1766) |
| Berthellina edwardsii (Vayssière, 1897) ° f | Phylum Arthropoda |
| Bittium sp. | Malacostraca |
| Bittium latreillii (Payraudeau, 1826) ° | Brachycarpus biunguiculatus (Lucas, 1846) |
| Calliostoma laugieri (Payraudeau, 1826) ° | Calcinus tubularis (Linnaeus, 1767) |
| Caloria elegans (Alder & Hancock, 1845) f | Carupa tenuipes Dana, 1852 * ° |
| Cerithium scabridum R. A. Philippi, 1848 * ° | Dardanus calidus (Risso, 1827) |
| Cerithiidae sp. | Dromia personata (Linnaeus, 1758) ° |
| Charonia seguenzae (Aradas & Benoit, 1871) ǂ | Galathea strigosa (Linnaeus, 1761) |
| Clanculus corallinus (Gmelin, 1791) ° | Gonioinfradens giardi (Nobili, 1905) * f |
| Clanculus cruciatus (Linnaeus, 1758) ° | Herbstia condyliata (Fabricius, 1787) ° |
| Conomurex persicus (Swainson, 1821) * | Herbstia spp. |
| Cratena peregrina (Gmelin, 1791) f | Hemimysis spp. ° |
| Dendrodoris grandiflora (Rapp, 1827) f | Lysmata nilita Dohrn & Holthuis, 1950 |
| Diaphorodoris papillata Portmann & Sandmeier, 1960 f | Lysmata seticaudata (Risso, 1816) |
| Episcomitra cornicula (Linnaeus, 1758) ° | Maja sp. |
| Ergalatax junionae Houart, 2008 * ° | Mysida spp. |
| Euthria cornea (Linnaeus, 1758) ° | Pagurus anachoretus Risso, 1827 |
| Facelina annulicornis (Chamisso & Eysenhardt, 1821) ° f | Pagurus chevreuxi (Bouvier, 1896) ° |
| Facelina rubrovittata (A. Costa, 1866) f | Paguroidea spp. |
| Felimare gasconi (Ortea, 1996) ° f | Palaemon serratus (Pennant, 1777) |
| Felimida krohni (Vérany, 1846) f | Palinurus elephas (Fabricius, 1787) ǂ |
| Fissurellidae sp. | Paractaea monodi Guinot, 1969 ° f |
| Flabellina affinis (Gmelin, 1791) | Paragalene longicrura (Nardo, 1869) |
| Flabellinidae sp. | Percnon gibbesi (H. Milne Edwards, 1853) * |
| Fusinus sp. ° | Plesionika narval (Fabricius, 1787) |
| Hexaplex trunculus (Linnaeus, 1758) | Scyllarides latus (Latreille, 1803) ǂ |
| Homalopoma sanguineum (Linnaeus, 1758) ° | Scyllarus pygmaeus (Spence Bate, 1888) ǂ |
| Luria lurida (Linnaeus, 1758) ǂ | Siriella gracilipes Nouvel, 1942 ° |
| Muricopsis cristata (Brocchi, 1814) ° | Stenopus spinosus Risso, 1827 |
| Naria spurca (Linnaeus, 1758) ǂ | Urocaridella pulchella Yokes & Galil, 2006 * ° |
| Peltodoris atromaculata Bergh, 1880 | Xantho pilipes A. Milne-Edwards, 1867 ° |
| Phyllidia flava Aradas, 1847 ° f | Pycnogonida |
| Pisania striata (Gmelin, 1791) | Pantopoda sp. ° |
| Plocamopherus ocellatus Rüppell & Leuckart, 1828 * | |
| Phylum Echinodermata | Osteichthyes (cont.) |
| Crinoidea | Gammogobius steinitzi Bath, 1971 |
| Antedon mediterranea (Lamarck, 1816) ° | Gobiidae sp. |
| Asteroidea | Gobius bucchichi Steindachner, 1870 |
| Coscinasterias tenuispina (Lamarck, 1816) | Gobius vittatus Vinciguerra, 1883 |
| Hacelia attenuata Gray, 1840 | Grammonus ater (Risso, 1810) |
| Marthasterias glacialis (Linnaeus, 1758) | Lepadogaster candolii Risso, 1810 |
| Ophidiaster ophidianus (Lamarck, 1816) ǂ | Lepadogaster lepadogaster (Bonnaterre, 1788) |
| Ophiuroidea | Marcelogobius splechtnai (Ahnelt & Patzner, 1995) |
| Ophiactis sp. ° | Microlipophrys nigriceps (Vinciguerra, 1883) |
| Ophioderma longicaudum (Bruzelius, 1805) f | Mugilidae spp. |
| Ophiopsila aranea Forbes, 1843 ° f | Mullus surmuletus Linnaeus, 1758 |
| Ophiothrix fragilis (Abildgaard in O.F. Müller, 1789) | Muraena helena Linnaeus, 1758 |
| Ophiuroidea sp. | Oblada melanurus (Linnaeus, 1758) |
| Echinoidea | Parablennius gattorugine (Linnaeus, 1758) |
| Arbacia lixula (Linnaeus, 1758) ° | Parablennius rouxi (Cocco, 1833) |
| Centrostephanus longispinus (Philippi, 1845) ǂ | Parupeneus forsskali (Fourmanoir & Guézé, 1976) * |
| Diadema setosum (Leske, 1778) * | Pempheris rhomboidea Kossmann & Räuber, 1877 * |
| Paracentrotus lividus (Lamarck, 1816) ǂ | Phycis phycis (Linnaeus, 1766) |
| Sphaerechinus granularis (Lamarck, 1816) | Pterois miles (Bennett, 1828) * |
| Stylocidaris affinis (Philippi, 1845) | Sargocentron rubrum (Forsskål, 1775) * |
| Holothuroidea | Sciaena umbra Linnaeus, 1758 ǂ |
| Holothuria (Platyperona) sanctori Delle Chiaje, 1823 | Scorpaena maderensis Valenciennes, 1833 |
| Holothuria spp. | Scorpaena notata Rafinesque, 1810 |
| Phylum Chordata | Scorpaena porcus Linnaeus, 1758 |
| Osteichthyes | Scorpaena scrofa Linnaeus, 1758 |
| Anthias anthias (Linnaeus, 1758) | Scorpaenodes arenai Torchio, 1962 |
| Apogon imberbis (Linnaeus, 1758) | Serranus cabrilla (Linnaeus, 1758) |
| Atherina spp. | Serranus scriba (Linnaeus, 1758) |
| Blenniidae spp. | Siganus luridus (Rüppell, 1829) * |
| Boops boops (Linnaeus, 1758) | Siganus rivulatus Forsskål & Niebuhr, 1775 * |
| Chromis chromis (Linnaeus, 1758) | Sparisoma cretense (Linnaeus, 1758) |
| Conger conger (Linnaeus, 1758) | Spicara smaris (Linnaeus, 1758) |
| Corcyrogobius liechtensteini (Kolombatovic, 1891) | Symphodus mediterraneus (Linnaeus, 1758) |
| Coris julis (Linnaeus, 1758) | Symphodus sp. |
| Diplodus annularis (Linnaeus, 1758) | Synodus saurus (Linnaeus, 1758) f |
| Diplodus puntazzo (Walbaum, 1792) | Thalassoma pavo (Linnaeus, 1758) |
| Diplodus sargus (Linnaeus, 1758) | Thorogobius ephippiatus (Lowe, 1839) |
| Diplodus vulgaris (Geoffroy Saint-Hilaire, 1817) | Torquigener flavimaculosus Hardy & Randall, 1983 * |
| Enchelycore anatina (Lowe, 1838) * | Tripterygion delaisi Cadenat & Blache, 1970 |
| Epinephelus costae (Steindachner, 1878) | Tripterygion melanurus Guichenot, 1850 |
| Epinephelus marginatus (Lowe, 1834) ǂ | Tripterygion tripteronotum (Risso, 1810) |
3.5. Introduced Species
Seventeen introduced species were recorded, belonging to four phyla: Chordata (8), Arthropoda (4), Mollusca (4), and Echinodermata (1). The lionfish Pterois miles was the most frequently encountered introduced species, recorded in ten caves across the east Aegean, Crete, and the Ionian Sea, followed by Sargocentron rubrum in six caves of the east Aegean, and Pempheris rhomboidea in four caves of the east Aegean and Crete (Figure 5, Table 4). The highest number of introduced species was recorded in the easternmost caves of the east Aegean and Crete, with Blue 2 cave (Kastellorizo Island) hosting the most (ten species).
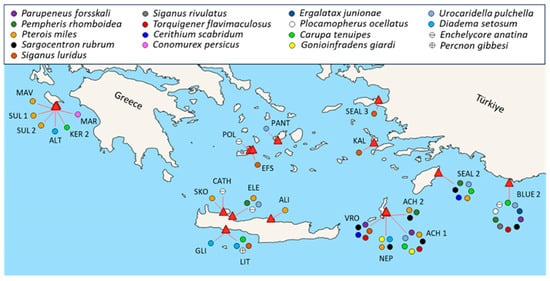
Figure 5.
Introduced species recorded in the studied caves in and out of transects labelled with different colors (MAV: Mavros Kavos; SUL 1: Sulfur 1; SUL 2: Sulfur 2; ALT: Altar; KER 2: Keri 2; MAR: Marathia; SKO: Skotino; CATH: Cathedral; ALI: Alikes; GLI: Glika Nera; LIT: Lithistid; VRO: Vronti; NEP: Neptune’s; ACH 1: Achata 1; ACH 2: Achata 2; BLUE 2: Blue 2; SEAL 2: Seal’s 2; KAL: Kalymnos; SEAL 3: Seal’s 3; PANT: Pantieronisi; POL: Polyaigos; EFS: Efstathios). Caves with no introduced species are not presented.
Fishes constituted the most abundant introduced group in most caves. Blue 2, the southeasternmost studied cave, presented the highest overall abundance of introduced species, with more than 200 individuals of P. rhomboidea in its semidark zone and over 200 juveniles in its entrance zone, comprising approximately 53% of the cave’s total fish abundance (Figure 6).
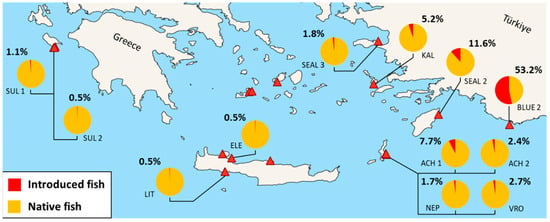
Figure 6.
Percent ratio of introduced to native fish species recorded in transect for the studied marine caves (SUL 1: Sulfur 1; SUL 2: Sulfur 2; ELE: Elephant; LIT: Lithistid; VRO: Vronti; NEP: Neptune’s; ACH 1: Achata 1; ACH 2: Achata 2; BLUE 2: Blue 2; SEAL 2: Seal’s 2; KAL: Kalymnos; SEAL 3: Seal’s 3). Caves with no introduced species are not presented.
4. Discussion
This study provides the first large-scale biodiversity assessment of motile fauna in 27 marine caves across the eastern Mediterranean, using a standardized quantitative methodology based on a rapid visual census protocol, complemented by qualitative material (photos and specimens). These caves were surveyed for the first time for their motile fauna, revealing rich biodiversity, with 163 taxa identified. Motile community structure exhibited considerable variation at a large spatial scale (i.e., among the four surveyed geographic subareas). In contrast, the lack of significant differentiation among caves within the same subareas suggests a more homogenized pattern in motile communities, likely due to the high mobility of most taxa, possible differences at local species pools and environmental filters. This finding contrasts with the widely accepted concept of cave “individuality” for sessile communities [,,,]. Species with distinct ecological niches and traits had a significant effect on the community structure across ecological zones. Reef-associated species dominated the cave entrance (e.g., Coris julis, Chromis chromis, Thalassoma pavo, and Arbacia lixula), while nocturnal (Apogon imberbis) and deeper-water species (Anthias anthias and Plesionika narval) prevailed in darker cave zones and deeper caves.
Consistent with previous faunistic zonation schemes for sessile communities in Mediterranean marine caves [,,], it was observed that species typical of darker cave zones tend to shift towards the cave entrance with increasing depth. Examples include Thorogobius ephippiatus, Stenopus spinosus, and Plesionika narval, which were encountered closer to the entrance in deeper caves.
Apogon imberbis was the most widespread and abundant fish in most caves, echoing findings from Italy and France [,], where it comprised around 75% of the total fish density in caves []. However, in deeper caves, A. imberbis was usually replaced by Anthias anthias, while atherinids dominated the entrance and semidark zones of some semi-submerged caves forming large schools close to the surface. Among decapods, Stenopus spinosus was the most common (in 17 caves), followed by Brachycarpus biunguiculatus (in 15 caves) and Dromia personata (in 11 caves), in contrast with earlier studies where Palaemon serratus and Herbstia condyliata were more prominent [,].
Based on our findings and prior faunal categorization schemes, e.g., [,,,,,,], motile taxa in the studied marine caves of the eastern Mediterranean can be assigned into the following ecological groups in relation to their degree of association with the cave environment: (1) accidental visitors (e.g., Pelagia noctiluca), often carried by currents and trapped inside the cave; (2) diurnal reef-associated species (e.g., Diplodus sargus, Coris julis, and Chromis chromis), which can occasionally be found in caves, usually close to the entrance; (3) trophic associates dependent on prey (typically but not exclusively) found within caves (e.g., Peltodoris atromaculata feeding on sponges); (4) cryptic habitat dwellers or “cave within cave” species, i.e., species associated with cryptic cave-like “mesolithial habitats”, such as crevices, empty holes of endolithic bivalves, overhangs, and fissures (e.g., Gammogobius steinitzi, Scyllarus pygmaeus, and the introduced Urocaridella pulchella); (5) nocturnal shelter-seekers, i.e., species finding shelter inside caves during daytime (e.g., Apogon imberbis, mysids, and the introduced Pempheris rhomboidea, and Sargocentron rubrum); (6) deeper-water species (e.g., Grammonus ater, Scorpaenodes arenai, and Stylocidaris affinis); (7) speleophilic species (typically but not exclusively) inhabiting and reproducing in marine caves (e.g., Stenopus spinosus and Palaemon serratus); and (8) cave-exclusive species (stygobionts), not observed in other environments than caves. Species belonging to the latter category were not observed in the studied marine caves, but they are known from other studies, e.g., [,]. It should be noted that some taxa could be assigned to more than one category (e.g., Muraena helena is both cryptic habitat dweller and nocturnal shelter-seeker). Categories 4–7 include species considered to exhibit secondary stygobiosis, since they originate from external marine environments and only secondarily inhabit marine caves [,].
In this study, the biodiversity inventory from the 27 studied marine caves revealed several rarely reported, protected, and introduced species, including new records for the study area and the marine cave habitat, thereby filling biogeographical and ecological knowledge gaps.
4.1. Rarely Reported Species
Overall, 17.8% of the identified taxa (29 species) were recorded for the first time in marine caves of the eastern Mediterranean Sea (Table 2), while 9.8% (16 species) were recorded for the first time in Mediterranean marine caves [,,,,,]. Some notable examples are presented below along with photographic evidence (Figure 7).

Figure 7.
The Messina rockfish Scorpaenodes arenai eating the shrimp Plesionika narval in a deep marine cave of Zakynthos Island ((A): front view; (B): side view); the flatworm Prostheceraeus moseleyi photographed at the entrance of a cave in Saria Island (C); two individuals of the decapod Brachycarpus biunguiculatus (D) and the collected nudibranch Felimare gasconi from a shallow marine cave of Crete (E). Photos by M. Digenis (A,B,E), T. Dailianis (C), and M. Ragkousis (D).
One of the most striking examples of rarely reported species was the finding of the Messina rockfish Scorpaenodes arenai in the same cave where it was first recorded in 2015 (Shrimp cave, Zakynthos Island), representing the shallowest Mediterranean records of this deep-water species and the only site where it can be observed through SCUBA diving []. Two individuals were sighted in an upside-down position in the semidark and dark zones of the cave (Figure 7A,B). The rockfish was recorded for the first time as feeding on the shrimp Plesionika narval, shedding light on its trophic habits inside marine caves.
The shrimp Brachycarpus biunguiculatus was observed almost exclusively in the semidark and dark zones of 15 caves, with up to 14 individuals in a single shallow cave in southern Crete (Figure 7D). Due to its highly cryptic habits and its nocturnal behavior, this species seeks shelter in dark environments such as caves, where it is mostly recorded in the Mediterranean Sea [,]. The frequent presence of B. biunguiculatus in 55% of the herein studied caves indicates that the species is a lot more common than previously thought.
Two of the recorded species, the flatworm Prostheceraeus moseleyi and the nudibranch Felimare gasconi, are herein reported for the first time in Greek waters as well as in the cave environment increasing the known fauna of Mediterranean marine caves. The flatworm P. moseleyi was observed in the entrance and semidark zones of two caves on Karpathos Island (Achata 2 and Vronti cave), and more recently at the entrance of a cave on the nearby Saria Island (35.854402° N, 27.193404° E), during another expedition (authors’ personal observation). This flatworm is easily distinguished from other congeneric Mediterranean species by its unique color patterns [] (Figure 7C). The nudibranch F. gasconi was collected from the dark zone of Lithistid cave in Crete (Table 2, Figure 7E). This dark blue dorid is characterized by a notum edged with a yellow line, white markings in front of the rhinophores and behind the gill tuft, and a prominent thick white longitudinal line running dorsally [].
Among the identified motile taxa, twelve are protected under the Bern and Barcelona Conventions and the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) (Table 4). Protected taxa were recorded in most studied caves (25) across all geographical areas, with 1–3 protected species found per cave, highlighting the high conservation value of Mediterranean marine caves [,].
4.2. Introduced Species in Marine Caves
Approximately 10% of all motile species identified in the studied caves were introduced, with the highest richness and abundance in southeastern areas. This pattern is consistent with their known distribution in other marine habitats [,,,]. Several of these species were recorded for the first time in marine caves or in specific areas. The introduced portunid crab Carupa tenuipes, which can be distinguished by its pale orange coloration and fingers dark brown distally and along their inner margins [] (Figure 8A), was recorded for the first time in the Ionian Sea, within the semidark zone of Keri 2 cave on Zakynthos Island. The decapod Gonioinfradens giardi and the gastropod Conomurex persicus are herein firstly reported from the marine cave habitat, raising the total number of introduced species in Mediterranean marine caves to 128 []. Two individuals of the portunid crab G. giardi, were recorded in the semidark zone of Neptune’s cave on Karpathos Island, as far as 70 m from its entrance (Figure 8B). This species can be distinguished by the shape of its frontal lobes with two spines medially on the upper surface of its palms set close apart, its distinctive body color pattern, the small ancillary teeth between four well-developed teeth at the anterolateral border of the carapace, and light-banded dark-tipped anterolateral spines [,,]. Another interesting finding was the presence of the shrimp Urocaridella pulchella in five caves, with up to 40 individuals in caves on Crete and Karpathos Islands (Figure 8E), mostly on soft substrate. This decapod was recently reported from Aegean and Ionian marine caves [,].

Figure 8.
The introduced crabs Carupa tenuipes (A) and Gonioinfradens giardi (B) from the dark zone in a cave of Rhodes (Seal 2) and Karpathos Islands (Neptune’s cave), respectively; juveniles of the sweeper fish Pempheris rhomboidea (C) from the cave entrance of Blue 2 cave in Kastellorizo Island; school of Siganus spp. passing in front of Glika Nera cave entrance in south Crete (D); the shrimp Urocaridella pulchella (E) from the semidark zone of a cave in south Crete. Photos by M. Ragkousis (A,E), M. Digenis (B,C), and V. Gerovasileiou (D).
Several of the introduced fish species had considerably high abundances in the studied caves. Large aggregations of the well-established sweeper fish Pempheris rhomboidea were observed in the Blue 2 cave (Kastellorizo Island), with up to 400 individuals, half of which were juveniles (Figure 8C). Large schools of Siganus spp. were observed near the entrance of a cave in southern Crete (Figure 8D). Pempheris rhomboidea as well as some other introduced fishes (Sargocentron rubrum), undertake diurnal migrations to feed in outer environments, possibly enriching the oligotrophic cave interior with organic matter, in a manner similar to that of native species such as Apogon imberbis [,,,]. In contrast, other introduced species, such as Pterois miles, may negatively affect the cave ecosystem through predation and competition with native predators, as observed in [,] and through personal observations.
4.3. Marine Caves as Nursery Grounds
During the surveys, some species were found to be more common than expected, displaying interesting and little-known behavioral traits related to the marine cave habitat. For instance, the sea urchin Arbacia lixula was recorded in 78% of the surveyed caves (21 caves), constituting the most frequently reported echinoderm (Table 4). Although commonly regarded as a diurnal herbivore feeding primarily on crustose algae, it is actually an omnivore with a strong tendency toward carnivory, occasionally reaching high densities in barren states such as dark caves, where it avoids competition with the more dominant sea urchin Paracentrotus lividus [,]. In addition, small and medium sized individuals of A. lixula, being more vulnerable to predation, compensate for their less-efficient morpho-functional defenses against predators by adopting cryptic behavior and seeking refuge in concealed microhabitats [,,,]. This behavior likely explains the presence of A. lixula in all ecological cave zones, with juveniles observed as far as 160 m away from the entrance in the Elephant cave (Crete).
Mediterranean marine caves seem to serve as nursery grounds for certain species, such as Antedon mediterranea, a crinoid only sporadically reported from caves [,]. In this study, although A. mediterranea was observed in low numbers (in only four caves), we recorded a remarkably high abundance with numerous juveniles in the semidark zone of a submerged cave with freshwater inflow in southern Crete (personal observation). This observation probably represents the first documented case of caves functioning as a breeding ground for this echinoderm species.
Additionally, the cave interior may serve as an egg-laying site for cuttlefish, as eggs of Sepia spp. were recorded in the semidark zone of three caves distributed from the east to the central Aegean and Crete. Although other cephalopods, such as octopuses, have previously been reported to lay eggs in caves [], this appears to be the first documented observation of cuttlefish eggs in Mediterranean marine caves.
4.4. Limitations of the Applied Methodology
The applied methodology enabled the identification of a high number of motile species through a standardized quantitative protocol requiring only one observer and non-decompression single-dive surveys. Nevertheless, since the proposed rapid assessment protocol is time limited—due to the logistic constraints of diving in space-limited and dark environments—it does not allow for a complete assessment of motile cave biota. The in-transect/total species richness percentage ratio differed among zones likely due to the varying light level and spatial extent of each zone. Specifically, the highest percentage at the cave entrance was probably due to the better visibility and its smaller size while the lower percentages in the dark zone are attributed to the poorest visibility and low species richness. The semidark zone presented the lowest proportion of in-transect to total species richness and abundance due to its largest size and higher heterogeneity in terms of resources and micro-habitats [,,,]. Thus, lower ratios are expected to be recorded in caves with extensive semidark zones, lower visibility (e.g., caves with sulfur springs and microbial mats) or increased surface complexity with several micro-habitats (e.g., submerged ceilings, fissures, and overhangs). The high small-scale heterogeneity of the cave habitat affects species diversity and distribution, with different species occupying specific sections and micro-habitats within caves []. To better capture this variability, the use of modified transect methods and quadrat sampling across distinct ‘sub-habitats’ (e.g., walls, ceilings, and floor) has been suggested, although these approaches typically require multiple dives or extended dive times [,,].
In this study, taxa that could not be reliably identified in situ at species level were recorded at higher taxonomic ranks (e.g., Atherina spp., Clanculus spp.). Cryptic and small sized species such as cryptic habitat dwellers (e.g., Microlipophrys nigriceps, Corcyrogobius liechtensteini, Marcelogobius splechtnai, Gammogobius steinitzi) are likely underrepresented, especially in less-studied caves, such as deeper caves or caves with complex morphology. The combined approach adopted in this work, which included photographic documentation and qualitative sampling, seems to mitigate the restrictions of the time-limited visual census, as evidenced by the high total number of species recorded. However, to accurately assess the distribution and abundance of cryptobenthic fishes in marine caves, more targeted methodologies should be considered, such as the use of anesthetic agents and quadrat sampling [].
Annual, seasonal, and diurnal variations are also expected to influence the species richness and abundance of motile taxa, especially for occasional visitors and/or highly mobile species (e.g., Atherina spp. and mysids). Although our visual census protocol was applied across different years and periods (Table 1), the majority of surveys (63%, corresponding to 17 caves) were carried out during the warm season (typically April to September in the Mediterranean Sea) and cosistently during morning to midday hours.
Although having a single observer entering first in the cave is expected to reduce disturbance, some highly mobile species (e.g., fishes) may still relocate toward outer or inner cave sections or hide in micro-habitats before being noticed. Finally, the involvement of multiple observers can introduce variability in species detection and data collection []. Thus, to minimize such bias, the same diver consistently applied the visual census protocol in all surveyed caves.
5. Conclusions
The assessment of motile cave fauna in a large number of marine caves in the eastern Mediterranean Sea enabled—for the first time—the quantitative characterization of their motile assemblages, employing a standardized rapid visual census protocol. Through the suggested methodology, more than 60% on average of the motile cave fauna observed in situ can be recorded, providing information on species richness and abundance. Such data are vital for assessing the ecological quality of marine caves [,] and for informing long-term monitoring initiatives, especially in marine protected areas.
The high number of new species records for marine caves in the eastern Mediterranean, and for cave habitats overall, demonstrates that our knowledge regarding cave biota is far from complete. In order to understand the distribution pathways of current and future cave inhabitants, it is important to examine the association between motile species assemblages in caves and nearby habitats such as rocky reefs. Given the unique biodiversity of marine caves, their low ecological resilience, and the increasing occurrence of several introduced species, this key habitat emerges as particularly vulnerable. Notably, six of the identified introduced species are listed among the worst Mediterranean invasives [], and recent studies have highlighted the urgent need to investigate their potential ecological impacts on cave biota []. The population explosion of certain introduced species and their increasing abundance in caves of southeastern subareas, as seen in [,,,] and the present study, indicate a rapid shift in the cave ecosystem. Therefore, the protection of certain caves should be prioritized. Among these caves is the Shrimp cave of Zakynthos Island, a currently uninvaded cave within an otherwise invaded area, which represents the shallowest known Mediterranean habitat of the deep-water Messina rockfish S. arenai. Additionally, the Lithistid cave in southern Crete and Seal’s 3 cave on Samos Island stand out as the two most species-rich caves studied. In this context, the long-term monitoring of the motile cave communities through seasonal surveys is recommended, particularly within newly established high-priority protected areas. However, the absence of historical baseline restricts our understanding of change in cave communities. This study provides a baseline for future comparisons of cave communities between invaded and uninvaded caves [], and for future monitoring areas that are still unaffected or only minimally impacted in view of the continuous expansion of thermophilic introduced species due to climate change.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10080383/s1. Figure S1: Number of motile taxa recorded within time transects compared with all taxa recorded in situ during a single dive survey in each studied cave. Figure S2: Number of motile taxa recorded within time transects in the cave entrance (CE), the semidark (SD), and the dark zone (D) compared with all taxa recorded in situ during a single dive survey in each studied cave. Figure S3: Abundance recorded within time transects compared with all taxa recorded in situ during a single dive survey in each studied cave. Figure S4: Abundance recorded within time transects in the cave entrance (CE), the semidark (SD), and the dark zone (D) compared with the total abundance recorded in situ during a single dive survey in each studied cave. Table S1: Dissimilarity percentage analysis (SIMPER) showing the contribution of motile taxa to the average dissimilarity (%) between the subareas east Aegean and Crete. Table S2: Pair-wise tests for the two-way PERMANOVA analysis conducted with the factor ‘Cave’ nested as random within the fixed factor ‘Subarea’ for the total of motile taxa recorded in transect. Table S3: Construction of pseudo-F ratio from mean squares from the two-way PERMANOVA analysis conducted with the factor ‘Cave’ nested as random within the fixed factor ‘Subarea’ for the total of motile taxa recorded in transect. Table S4: Construction of pseudo-F ratios from mean squares from the one-way PERMANOVA analysis conducted with the factor ‘Zone’ within each subarea for the total of motile taxa recorded in transect. Table S5: Dissimilarity percentage analysis (SIMPER) showing the contribution of motile taxa to the average dissimilarity (%) between the cave entrance (CE) and the semidark zone (SD). Table S6: Dissimilarity percentage analysis (SIMPER) showing the contribution of motile taxa to the average dissimilarity (%) between the cave entrance (CE) and the dark zone (D). Table S7: Dissimilarity percentage analysis (SIMPER) showing the contribution of motile taxa to the average dissimilarity (%) between the semidark zone (SD) and the dark zone (D). Table S8: Pair-wise tests for the two-way PERMANOVA analysis conducted with the factor ‘Cave’ nested as random within the fixed factor ‘Subarea’ for fishes recorded in transect. Table S9: Construction of pseudo-F ratio from mean squares from the two-way PERMANOVA analysis conducted with the factor ‘Cave’ nested as random within the fixed factor ‘Subarea’ for fishes recorded in transect. Table S10: Construction of pseudo-F ratios from mean squares from the one-way PERMANOVA analysis conducted with the factor ‘Zone’ within each subarea for fishes recorded in transect. Table S11: Dissimilarity percentage analysis (SIMPER) showing the contribution of fish species to the average dissimilarity (%) between the cave entrance (CE) and the semidark zone (SD). Table S12: Dissimilarity percentage analysis (SIMPER) showing the contribution of fish species to the average dissimilarity (%) between the cave entrance (CE) and the dark zone (D). Table S13: Dissimilarity percentage analysis (SIMPER) showing the contribution of fish species to the average dissimilarity (%) between the cave entrance (CE) and the dark zone (D).
Author Contributions
Conceptualization and methodology, M.D. and V.G.; sampling: M.D., M.R. and V.G.; identified samples, M.D. and V.G.; formal analysis, M.D., C.D. and V.G.; writing—original draft preparation, M.D.; writing—review and editing, V.G., M.R., C.D. and S.K.; funding acquisition, M.D., S.K. and V.G. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Hellenic Foundation for Research and Innovation (HFRI) under the 4th Call for HFRI PhD Fellowships (Fellowship Number: 10597). Part of the sampling activities was funded through the projects “First Call for HFRI Research Projects to support faculty members and researchers and the procurement of high-cost research equipment grant” (Project ALAS—‘ALiens in the Aegean—a Sea under siege’, project number: HFRI-FM17-1597) (3/2020-3/2023), and the “STUDIOTOPIA—Art meets Science in the Anthropocene (2019–2022)” residency program, hosted by Onassis Stegi and co-funded by the Creative Europe programme of the European Union. Additional support for the 2021 sampling campaign was provided to V.G. by the project “Centre for the study and sustainable exploitation of Marine Biological Resources (CMBR)” (MIS 5002670), implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) and co-financed by Greece and the EU (European Regional Development Fund).
Institutional Review Board Statement
The research has been accepted for implementation by the Research Ethics and Deontology Committee of the Ionian University (Approval code: 1036, Approval date: 2023-04-06).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are contained within the article and its Supplementary Materials.
Acknowledgments
The authors are grateful to Dimitris Podaras and Maria Naletaki for their assistance with the identification of echinoderm and decapod samples, respectively; Fabio Crocetta for his help with mollusk identification; and Pierre Chevaldonné for verifying the identification of mysid specimens. We also thank Thanos Dailianis for providing one of the photos presented (Figure 7C) and for his contribution to some of the sampling campaigns. We are grateful to the Management Units of the Southeastern Aegean Protected Areas, Samaria National Park, and the Protected Areas of Western Crete, and Zakynthos and Ainos National Parks and Protected Areas of the Ionian islands of the Natural Environment and Climate Change Agency (NECCA) for granting sampling permissions in marine caves within these protected areas. We also wish to thank Dennis Mohr and the staff of Nero Sport Diving Center (Limni Keriou, Zakynthos), Nikos Giannoulakis from the Chania Diving Center (Chania, Crete), and Dinos Protopapas from the Karpathos Diving Center (Pigadia, Karpathos) for their valuable expertise and support during fieldwork in Zakynthos, Crete and in Karpathos Islands, respectively. Finally, we warmly thank Carlos Navarro-Barranco, Juan Sempere-Valverde, Sahar Chebaane, Alfredo Marchiò, and Ioannis Rallis for their assistance during fieldwork.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giakoumi, S.; Sini, M.; Gerovasileiou, V.; Mazor, T.; Beher, J.; Possingham, H.P.; Abdulla, A.; Çinar, M.E.; Dendrinos, P.; Gucu, A.C.; et al. Ecoregion-based conservation planning in the Mediterranean: Dealing with large-scale heterogeneity. PLoS ONE 2013, 8, e76449. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Bianchi, C.N. Mediterranean marine caves: A synthesis of current knowledge. In Oceanography and Marine Biology; Taylor & Francis: Oxford, UK, 2021; pp. 1–87. [Google Scholar] [CrossRef]
- Bussotti, S.; Terlizzi, A.; Fraschetti, S.; Belmonte, G.; Boero, F. Spatial and temporal variability of sessile benthos in shallow Mediterranean marine caves. Mar. Ecol. Prog. Ser. 2006, 325, 109–119. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Dimitriadis, C.; Arvanitidis, C.; Voultsiadou, E. Taxonomic and functional surrogates of sessile benthic diversity in Mediterranean marine caves. PLoS ONE 2017, 12, e0183707. [Google Scholar] [CrossRef] [PubMed]
- Dimarchopoulou, D.; Gerovasileiou, V.; Voultsiadou, E. Spatial variability of sessile benthos in a semi-submerged marine cave of a remote Aegean Island (eastern Mediterranean Sea). Reg. Stud. Mar. Sci. 2018, 17, 102–111. [Google Scholar] [CrossRef]
- Sempere-Valverde, J.; Sabino Lorenzo, Á.; Espinosa, F.; Gerovasileiou, V.; Sánchez-Tocino, L.; Navarro Barranco, C. Taxonomic and morphological descriptors reveal high benthic temporal variability in a Mediterranean marine submerged cave over a decade. Hydrobiologia 2019, 839, 177–194. [Google Scholar] [CrossRef]
- Digenis, M.; Arvanitidis, C.; Dailianis, T.; Gerovasileiou, V. Comparative study of marine cave communities in a protected area of the South-Eastern Aegean Sea, Greece. J. Mar. Sci. Eng. 2022, 10, 660. [Google Scholar] [CrossRef]
- Derrien, M.; Chevaldonné, P.; Pérez, T. Patterns of benthic diversity in marine underwater caves of the Marseille Region (France, North-Western Mediterranean Sea). Mediterr. Mar. Sci. 2024, 25, 666–681. [Google Scholar] [CrossRef]
- Ledoyer, M. Note sur la faune vagile des grottes sous-marines obscures. Rapp. Comm. Int. Mer Médit. 1965, 18, 121–124. [Google Scholar]
- Ledoyer, M. Écologie de la faune vagile des biotopes méditerranéens accessibles en scaphandre autonome. I—Introduction: Données analytiques sur les biotopes de substrat dur. Rec. Trav. Sta. Mar. Endoume 1966, 40, 103–149. [Google Scholar]
- Bussotti, S.; Denitto, F.; Guidetti, P.; Belmonte, G. Fish assemblages in shallow marine caves of the Salento Peninsula (Southern Apulia, SE Italy). Mar. Ecol. 2002, 23, 11–20. [Google Scholar] [CrossRef]
- Bussotti, S.; Guidetti, P.; Belmonte, G. Distribution patterns of the cardinal fish, Apogon imberbis, in shallow marine caves in southern Apulia (SE Italy). Ital. J. Zool. 2003, 70, 153–157. [Google Scholar] [CrossRef]
- Denitto, F.; Moscatello, S.; Belmonte, G. Occurrence and distribution pattern of Palaemon spp. shrimps in a shallow submarine cave environment: A study case in South-eastern Italy. Mar. Ecol. 2009, 30, 416–424. [Google Scholar] [CrossRef]
- Rastorgueff, P.A.; Harmelin-Vivien, M.; Richard, P.; Chevaldonné, P. Feeding strategies and resource partitioning mitigate the effects of oligotrophy for marine cave mysids. Mar. Ecol. Prog. Ser. 2011, 440, 163–176. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Chintiroglou, C.; Vafidis, D.; Koutsoubas, D.; Sini, M.; Dailianis, T.; Issaris, Y.; Akritopoulou, E.; Dimarchopoulou, D.; Voutsiadou, E. Census of biodiversity in marine caves of the eastern Mediterranean Sea. Mediterr. Mar. Sci. 2015, 16, 245–265. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Ganias, K.; Dailianis, T.; Voultsiadou, E. Occurrence of some rarely reported fish species in eastern Mediterranean marine caves. Cah. Biol. Mar. 2015, 56, 381–387. [Google Scholar]
- De La Torre, L.P.; Navarro-Barranco, C.; Gofas, S. Malacofauna from soft bottoms in the Cerro Gordo marine cave (Alboran Sea): Biodiversity and spatial distribution. Medit. Mar. Sci. 2020, 21, 684–704. [Google Scholar] [CrossRef]
- Di Franco, D.; Jimenez, C.; Albano, P.G. Unexpected high molluscan diversity in a submarine cave in the Eastern Mediterranean. Mar. Biodivers. 2021, 51, 85. [Google Scholar] [CrossRef]
- Ragkousis, M.; Digenis, M.; Kovačić, M.; Katsanevakis, S.; Gerovasileiou, V. Rarely reported cryptobenthic fish in marine caves of the eastern Mediterranean Sea. J. Mar. Sci. Eng. 2021, 9, 557. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Gerovasileiou, V.; Morri, C.; Froglia, C. Distribution and Ecology of Decapod Crustaceans in Mediterranean Marine Caves: A Review. Diversity 2022, 14, 176. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Martínez, A.; Sempere-Valverde, J.; Chebaane, S.; Digenis, M.; Plaitis, W.; Voultsiadou, E.; Gerovasileiou, V. Amphipods in Mediterranean marine and anchialine caves: New data and overview of existing knowledge. Diversity 2023, 15, 1180. [Google Scholar] [CrossRef]
- Kovačić, M.; Gerovasileiou, V.; Patzner, R.A. Fishes in marine caves. Fishes 2024, 9, 243. [Google Scholar] [CrossRef]
- Tsagarakis, K.; Agius Darmanin, S.; Al Mabruk, S.A.; Auriemma, R.; Azzurro, E.; Badouvas, N.; Bakiu, R.; Bariche, M.; Battaglia, P.; Betti, F.; et al. New records of rare species in the Mediterranean Sea. Medit. Mar. Sci. 2021, 22, 627–652. [Google Scholar] [CrossRef]
- Montesanto, F.; Albano, M.; Ayaş, D.; Betti, F.; Capillo, G.; Çinar, M.E.; Corsini-Foka, M.; Crocetta, F.; Dağlı, E.; D’Iglio, C.; et al. New records of rare species in the Mediterranean Sea. Medit. Mar. Sci. 2022, 23, 968–994. [Google Scholar] [CrossRef]
- Digenis, M.; Akyol, O.; Benoit, L.; Biel-Cabanelas, M.; Çamlik, Ö.Y.; Charalampous, K.; Chatzispyrou, A.; Crocetta, F.; Deval, M.C.; Di Capua, I.; et al. New records of rarely reported species in the Mediterranean Sea. Mediterr. Mar. Sci. 2024, 25, 84–115. [Google Scholar] [CrossRef]
- Bussotti, S.; Guidetti, P. Do Mediterranean fish assemblages associated with marine caves and rocky cliffs differ? Estuar. Coast. Shelf Sci. 2009, 81, 65–73. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Guerra-García, J.M.; Sánchez-Tocino, L.; García-Gómez, J.C. Soft-bottom crustacean assemblages in Mediterranean marine caves: The cave of Cerro Gordo (Granada, Spain) as case study. Helgol. Mar. Res. 2012, 66, 567–576. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Guerra-García, J.M.; Sánchez-Tocino, L.; García-Gómez, J.C. Mobile epifaunal community in marine caves in comparison to open habitats. Aquat. Biol. 2014, 20, 101–109. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Guerra-García, J.M.; Sánchez-Tocino, L.; Ros, M.; Florido, M.; García-Gómez, J.C. Colonization and successional patterns of the mobile epifaunal community along an environmental gradient in a marine cave. Mar. Ecol. Prog. Ser. 2015, 521, 105–115. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Guerra-García, J.M.; Sánchez-Tocino, L.; Florido, M.; García-Gómez, J.C. Amphipod community associated with invertebrate hosts in a Mediterranean marine cave. Mar. Biodivers. 2016, 46, 105–112. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Coll, M.; Piroddi, C.; Steenbeek, J.; Ben Rais-Lasram, F.; Zenetos, A.; Cardoso, A.C. Invading the Mediterranean Sea: Biodiversity patterns shaped by human activities. Front. Mar. Sci. 2014, 1, 32. [Google Scholar] [CrossRef]
- Carlot, J.; Galobart, C.; Gómez-Gras, D.; Santamaría, J.; Golo, R.; Sini, M.; Cebrian, E.; Gerovasileiou, V.; Ponti, M.; Turicchia, E.; et al. Vulnerability of benthic trait diversity across the Mediterranean Sea following mass mortality events. Nat. Commun. 2025, 16, 1571. [Google Scholar] [CrossRef]
- Rastorgueff, P.A.; Bellan-Santini, D.; Bianchi, C.N.; Bussotti, S.; Chevaldonné, P.; Guidetti, P.; Harmelin, J.G.; Montefalcone, M.; Morri, C.; Perez, T. An ecosystem-based approach to evaluate the ecological quality of Mediterranean undersea caves. Ecol. Indic. 2015, 54, 137–152. [Google Scholar] [CrossRef]
- Nepote, E.; Bianchi, C.N.; Morri, C.; Ferrari, M.; Montefalcone, M. Impact of a harbour construction on the benthic community of two shallow marine caves. Mar. Pollut. Bull. 2017, 114, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Montefalcone, M.; De Falco, G.; Nepote, E.; Canessa, M.; Bertolino, M.; Bavestrello, G.; Morri, C.; Bianchi, C.N. Thirty year ecosystem trajectories in a submerged marine cave under changing pressure regime. Mar. Environ. Res. 2018, 137, 98–110. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Voultsiadou, E.; Issaris, Y.; Zenetos, A. Alien biodiversity in Mediterranean marine caves. Mar. Ecol. 2016, 37, 239–256. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Bancila, R.I.; Katsanevakis, S.; Zenetos, A. Introduced species in Mediterranean marine caves: An increasing but neglected threat. Mediterr. Mar. Sci. 2022, 23, 995–1005. [Google Scholar] [CrossRef]
- Nicolosi, G.; Gerovasileiou, V. Towards invasion ecology for subterranean ecosystems. Biodivers. Conserv. 2024, 33, 1561–1569. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Trygonis, V.; Sini, M.; Koutsoubas, D.; Voultsiadou, E. Three-dimensional mapping of marine caves using a handheld echosounder. Mar. Ecol. Prog. Ser. 2013, 486, 13–22. [Google Scholar] [CrossRef]
- Riedl, R. Biologie der Meereshohlen; Paul Parey: Hamburg, Germany; Berlin, Germany, 1966; p. 636. [Google Scholar]
- Harmelin-Vivien, M.L.; Harmelin, J.G.; Chauvet, C.; Duval, C.; Galzin, R.; Lejeune, P.; Barnabé, G.; Blanc, F.; Chevalier, R.; Duclerc, J.; et al. Evaluation visuelle des peuplements et populations de poissons méthodes et problèmes. Rev. Ecol. Terre Vie 1985, 40, 467–539. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Voultsiadou, E. Marine caves of the Mediterranean Sea: A sponge biodiversity reservoir within a biodiversity hotspot. PLoS ONE 2012, 7, e39873. [Google Scholar] [CrossRef]
- Stamouli, C.; Gerovasileiou, V.; Voultsiadou, E. Sponge community patterns in mesophotic and deep-sea habitats in the Aegean and Ionian seas. J. Mar. Sci. Eng. 2023, 11, 2204. [Google Scholar] [CrossRef]
- Clarke, K.; Warwick, R.A. Further Biodiversity Index Applicable to Species Lists: Variation in Taxonomic Distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Mead, A. Review of the development of multidimensional scaling methods. J. R. Stat. Soc. Ser. D 1992, 41, 27–39. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; pp. 1–192. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Martí, R.; Uriz, M.J.; Ballesteros, E.; Turón, X. Benthic assemblages in two Mediterranean caves: Species diversity and coverage as a function of abiotic parameters and geographic distance. J. Mar. Biol. Assoc. U.K. 2004, 84, 557–572. [Google Scholar] [CrossRef]
- Riedl, R. Die Hydroiden des Golfes von Neapel und ihr Anteil an der Fauna unterseeischer Höhlen. Ergebnisse der Österreichischen Tyrrhenia-Expedition 1952. Teil 16. Pubbl. Stn Zool. Napoli. 1959, 30, 589–755. [Google Scholar]
- Morri, C.; Bianchi, C.N. Zonazione biologica. In Grotte Marine: Cinquant’Anni di Ricerca in Italia; Cicogna, F., Ed.; Ministero dell’Ambiente e della Tutela del Territorio: Rome, Italy, 2003; pp. 257–265. [Google Scholar]
- Bussotti, S.; Di Franco, A.; Francour, P.; Guidetti, P. Fish assemblages of Mediterranean marine caves. PLoS ONE 2015, 10, e0122632. [Google Scholar] [CrossRef]
- Bussotti, S.; Di Franco, A.; Pey, A.; Vieux-Ingrassia, J.V.; Planes, S.; Guidetti, P. Distribution patterns of marine cave fishes and the potential role of the cardinal fish Apogon imberbis (Linnaeus, 1758) for cave ecosystem functioning in the western Mediterranean. Aquat. Living Resour. 2017, 30, 15. [Google Scholar] [CrossRef][Green Version]
- Kovačić, M.; Glavičić, I.; Paliska, D.; Valić, Z. A first qualitative and quantitative study of marine cave fish assemblages of intracave cavities. Est. Coast. Shelf Sci. 2021, 263, 107624. [Google Scholar] [CrossRef]
- Iliffe, T.M.; Kornicker, L.S. Worldwide diving discoveries of living fossil animals from the depths of anchialine and marine caves. Smithson. Contrib. Mar. Sci. 2009, 38, 269–280. [Google Scholar] [CrossRef]
- Moldovan, O.T. An overview on the aquatic cave fauna. In Cave Ecology; Springer: Cham, Switzerland, 2018; pp. 173–194. [Google Scholar] [CrossRef]
- Iliffe, T.M. Crevicular dispersal of marine cave faunas. Mem. Biospeol. 1990, 17, 93–96. [Google Scholar]
- Öztürk, B. (Ed.) Marine Caves of the Eastern Mediterranean Sea. In Biodiversity, Threats and Conservation; Turkish Marine Research Foundation (TUDAV) Publication No: 53: Istanbul, Turkey, 2019; p. 258. [Google Scholar]
- Tsiamis, K.; Fernández-Álvarez, F.Á.; Balistreri, P.; Bariche, M.; Carden-Noad, S.; Corsini-Foka, M.; Crocetta, F.; Davidov, B.; Dimitriadis, C.; Dragičević, B.; et al. New Mediterranean Biodiversity Records (July 2015). Medit. Mar. Sci. 2015, 16, 472–488. [Google Scholar] [CrossRef]
- Kampouris, T.E.; Exadactylos, A. On the finding of Brachycarpus biunguiculatus (Lucas, 1846) from Sifnos Island, Cyclades, Aegean Sea. Rare or overlooked? Spixiana 2023, 46, 27–29. [Google Scholar]
- Noreña, C.; Marquina, D.; Perez, J.; Almon, B. First records of Cotylea (Polycladida, Platyhelminthes) for the Atlantic coast of the Iberian Peninsula. ZooKeys 2014, 404, 1–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lombardo, A.; Marletta, G. The nudibranchs (Gastropoda Heterobranchia) of the central-eastern coast of Sicily, II: Suborder Doridina. Biodivers. J. 2022, 13, 297–320. [Google Scholar] [CrossRef]
- Ouerghi, A.; Gerovasileiou, V.; Bianchi, C.N. Mediterranean marine caves: A synthesis of current knowledge and the Mediterranean Action Plan for the conservation of ‘dark habitats’. In Marine Caves of the Eastern Mediterranean Sea. Biodiversity, Threats and Conservation; Öztürk, B., Ed.; Turkish Marine Research Foundation (TUDAV): Istanbul, Turkey, 2019; pp. 1–13. [Google Scholar]
- Katsanevakis, S.; Zenetos, A.; Corsini-Foka, M.; Tsiamis, K. Biological Invasions in the Aegean Sea: Temporal Trends, Pathways, and Impacts. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Zenetos, A.; Doğan, A.; Bakir, A.K.; Chatzigeorgiou, G.; Corsini-Foka, M.; Dağli, E.; Evangelopoulos, A.; Meriç, E.; Stoumboudi, M.; Taşkin, E.; et al. Non-Indigenous Species (NIS) Know No Geopolitical Borders—An Update of NIS in the Aegean Sea. Diversity 2024, 17, 12. [Google Scholar] [CrossRef]
- Galil, B.S. Carupa tenuipes Dana, 1851: An indo-pacific swimming crab new to the Mediterranean (Decapoda, Brachyura, Portunidae). Crustaceana 2004, 77, 249–251. [Google Scholar]
- Yokes, B.; Galil, B.S. New records of alien decapods (Crustacea) from the Mediterranean coast of Turkey, with a description of a new palaemonid species. Zoosystema 2006, 28, 747–755. [Google Scholar]
- Galil, B.S.; Douek, J.; Gevili, R.; Goren, M.; Yudkovsky, Y.; Paz, G.; Rinekvich, B. The resurrection of Charybdis (Gonioinfradens) giardi (Nobili, 1905), newly recorded from the SE Mediterranean Sea. Zootaxa 2018, 4370, 580–590. [Google Scholar] [CrossRef]
- Orfanidis, S.; Alvito, A.; Azzurro, E.; Badreddine, A.L.I.; Souissi, J.B.; Chamorro, M.; Crocetta, F.; Dalyan, C.; Fortič, A.; Galanti, L.; et al. “New Alien Mediterranean Biodiversity Records” (March 2021). Mediterr. Mar. Sci. 2021, 22, 180–198. [Google Scholar] [CrossRef]
- Digenis, M.; Ragkousis, M.; Vasileiadou, K.; Gerovasileiou, V.; Katsanevakis, S. New records of the Indo-Pacific shrimp Urocaridella pulchella Yokes & Galil, 2006 from the Eastern Mediterranean Sea. Bioinvasions Rec. 2021, 10, 295–303. [Google Scholar] [CrossRef]
- Christidis, G.; Mucciolo, S.; Desiderato, A.; Ammar, I.A.; Antit, M. New records of introduced species in the Mediterranean (August 2024). Mediterr. Mar. Sci. 2024, 25, 453–479. [Google Scholar] [CrossRef]
- Bussotti, S.; Di Franco, A.; Bianchi, C.N.; Chevaldonné, P.; Egea, L.; Fanelli, E.; Lejeusne, C.; Musco, L.; Navarro-Barranco, C.; Pey, A.; et al. Fish mitigate trophic depletion in marine cave ecosystems. Sci. Rep. 2018, 8, 9193. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Sini, M.; Ragkousis, M.; Zenetos, A.; Katsanevakis, S. Cumulative Negative Impacts of Invasive Alien Species on Marine Ecosystems of the Aegean Sea. Biology 2023, 12, 933. [Google Scholar] [CrossRef]
- Öndes, F.; Özden, U.; Alan, V.; Irmak, E.; Güçlüsoy, H. Comparative feeding habits of the invasive non-indigenous devil firefish Pterois miles and the indigenous scorpionfishes Scorpaena porcus, Scorpaena scrofa, and Scorpaena notata on the southwest coast of Türkiye, eastern Mediterranean. Mediterr. Mar. Sci. 2024, 25, 753–767. [Google Scholar] [CrossRef]
- Koilakos, S.M.; Georgatis, I.; Leonardos, I. Feeding Strategies and Biological Traits of the Lessepsian Migrant Pterois miles (Bennett, 1828) in the Messenian Gulf, SW Greece. Fishes 2024, 9, 380. [Google Scholar] [CrossRef]
- Agnetta, D.; Bonaviri, C.; Badalamenti, F.; Scianna, C.; Vizzini, S.; Gianguzza, P. Functional traits of two co-occurring sea urchins across a barren/forest patch system. J. Sea Res. 2013, 76, 170–177. [Google Scholar] [CrossRef]
- Mariani, S.; Pinedo, S.; Jordana, E.; Cefalì, M.E.; Torras, X.; Bagur Bendito, M.; Verdura, J.; Ballesteros, E. Grazing in the dark: A behavioural adjustment in a population of the black sea urchin Arbacia lixula. Ecol. Evol. 2023, 13, e10428. [Google Scholar] [CrossRef]
- Guidetti, P.; Fraschetti, S.; Terlizzi, A.; Boero, F. Distribution patterns of sea urchins and barrens in shallow Mediterranean rocky reefs impacted by the illegal fishery of the rock-boring mollusc Lithophaga lithophaga. Mar. Biol. 2003, 143, 1135–1142. [Google Scholar] [CrossRef]
- Guidetti, P. Consumers of sea urchins, Paracentrotus lividus and Arbacia lixula, in shallow Mediterranean rocky reefs. Helgol. Mar. Res. 2004, 58, 110–116. [Google Scholar] [CrossRef]
- Guidetti, P.; Mori, M. Morpho-functional defences of Mediterranean sea urchins, Paracentrotus lividus and Arbacia lixula, against fish predators. Mar. Biol. 2005, 147, 797–802. [Google Scholar] [CrossRef]
- Bianchi, C.N. Flora e fauna: Lineamenti generali e prospettive. In Grotte Marine: Cinquant’Anni di Ricerca in Italia; Cicogna, F., Ed.; Ministero dell’Ambiente e della Tutela del Territorio: Rome, Italy, 2003; pp. 137–146. [Google Scholar]
- Topaloğlu, B. A Preliminary study on the macrobenthic organismal cover in an underwater cave in Gökçeada Island (North Aegean Sea, Turkey). In Proceedings of the 2nd Mediterranean Symposium on the Conservation of Dark Habitats, Antalya, Turkey, 17 January 2019; pp. 59–63. [Google Scholar]
- Airoldi, L.; Cinelli, F. Variability of fluxes of particulate material in a submarine cave with chemolithoautotrophic inputs of organic carbon. Mar. Ecol. Prog. Ser. 1996, 139, 205–217. [Google Scholar] [CrossRef][Green Version]
- Thanopoulou, Z.; Sini, M.; Vatikiotis, K.; Katsoupis, C.; Dimitrakopoulos, P.G.; Katsanevakis, S. How many fish? Comparison of two underwater visual sampling methods for monitoring fish communities. PeerJ 2018, 6, e5066. [Google Scholar] [CrossRef]
- Lanza-Arroyo, P.; Sempere-Valverde, J.; Digenis, M.; Remón, J.M.; Moreno, D.; Barrajón, A.; Linde, A.; Arroyo, M.C.; Fernández-Casado, M.; Mallofret, E.; et al. Baseline for marine cave monitoring strategies in the Alboran Sea using modified Cave Ecosystem-Based Quality Index (CavEBQI). Mar. Pollut. Bull. 2024, 209, 117065. [Google Scholar] [CrossRef]
- Streftaris, N.; Zenetos, A. Alien marine species in the Mediterranean-the 100 ‘Worst Invasives’ and their impact. Mediterr. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef]
- Digenis, M.; Ragkousis, M.; Katsanevakis, S.; Gerovasileiou, V. Alien and rarely reported mobile species in marine caves of the Aegean Sea, Greece. In Proceedings of the 4th International Congress on Applied Ichthyology, Oceanography & Aquatic Environment, Mytilene, Greece, 4–6 November 2021; pp. 558–559. [Google Scholar]
- Katsanevakis, S.; Tsirintanis, K.; Sini, M.; Gerovasileiou, V.; Koukourouvli, N. Aliens in the Aegean–a sea under siege (ALAS). RIO 2020, 6, e53057. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).