1. Introduction
The size of fish at any point in time is determined by its history of growth, which varies by species [
1] and environmental conditions [
2,
3]. Fish size is known to influence behavior, physical performance, thermal tolerance, and survival [
4,
5,
6,
7]. Variation in individual juvenile salmonid responses, such as growth, to environmental variability can reduce extirpation risk to populations [
8]; however, the factors driving variation remain poorly understood. Within species or life history stages, juvenile salmonid body size can be shaped by stream temperatures, density dependence, and food availability [
9,
10]. These factors often interact, leading to substantial spatial variation in body size among streams. This variability may enhance local diversity and population resilience, forming a portfolio effect that buffers salmonid populations against environmental changes [
9,
11].
Understanding the drivers of body size variation is increasingly important in the context of stream and habitat alterations caused by both the legacy of forest harvest and climate change. Arismendi et al. [
12] documented a 6–13% decline in coastal cutthroat trout body size over thirty years in Oregon, which they partially attributed to both climate change and the legacy forest harvest influences. Historical riparian logging has degraded stream habitat [
13,
14], and these impacts are likely to persist—or even intensify—in the near future [
15,
16], potentially influencing salmonid body size. Additionally, climate change may influence body size by increasing stream temperatures and reducing the size of streams [
17,
18]. A deeper understanding of how environmental indicators influence fish growth and body size is essential for guiding effective management and restoration efforts, predicting ecological responses to changing conditions, and supporting the long-term viability of salmonid populations.
Juvenile salmonid body size plays a pivotal role in shaping life history trajectories and survival, including the timing of migration. For instance, Peven et al. [
19] suggested that body size thresholds may trigger anadromous salmonid migrations. Later work has shown that such threshold size can vary based on other factors such as growth, age-class, and reproductive state and that individuals may follow different developmental pathways to reach similar stages [
20,
21,
22,
23,
24]. The possibility of migration thresholds combined with variation in growth rate among streams may help to explain variations in the age of smolts and the duration of freshwater residency among salmonids that spend a portion of their life histories in freshwater before migrating to the ocean (e.g., coho salmon
Oncorhynchus kisutch, steelhead
O. mykiss, coastal cutthroat trout
O. clarkii, stream-type Chinook salmon
O. tshawytscha) [
25]. For example, Hall et al. [
26] documented steelhead migration across different ages, lengths, and seasons (i.e., age-0 fall; <86 mm, age-1 spring and fall; 87–132 mm, age-2 spring and fall; >132 mm, and age-3 spring and fall), with only fish older than age-0 surviving to return as adults. Similarly, Roni et al. [
7] found that coho salmon had successful adult returns from both fall and spring migrations but larger individuals were more likely to survive and migrate in the spring. These studies reveal a nuanced process wherein juvenile salmon migrate at various ages, lengths, or seasons. While the “bigger-is-better” hypothesis has generally been theorized for ocean survival [
27,
28], research has uncovered contradictions, with studies on ocean survival yielding mixed results [
28,
29,
30,
31]. Although some studies support the “bigger is better” hypothesis [
7,
32], a diverse range of body sizes across a landscape may facilitate a bet-hedging strategy (an evolutionary strategy where organisms diversify traits to increase long-term population viability) [
11].
Stream temperature is often thought to be one of the primary indicators influencing body size of juvenile salmonids. Metabolic theory outlines how temperature influences organism size through biological metabolism [
33], with direct implications for cold-water ectothermic salmonids. Coho salmon, for instance, achieve optimal growth within an average weekly temperature range of 12–17 °C [
1,
34,
35]. However, greater variation in growth among fish within the same species has been found at lower temperatures within the range than at higher temperatures. For example, Lusardi et al. [
1] reported a sixfold increase in juvenile coho salmon growth at 16.6 °C compared to 13 °C and found that growth remained high at their highest studied temperature of 18.1 °C. The ability of salmonids to maintain high levels of growth in the upper end of their temperature growth range has been attributed to the consumption of higher amounts of prey [
1]. However, if stream temperatures exceed optimal ranges, fish growth will slow down or stop, resulting in reduced body size [
1,
36]. This variability in optimal stream temperature for growth supports the notion that adaptation and acclimation lead to varying ranges of maximum growth among salmonid populations [
1].
Density is another well-documented driver of juvenile salmonid body size [
37,
38,
39]. In general, juvenile salmonid body size decreases with increased densities, often following a negative power curve [
40,
41,
42]. Imer et al. [
41] theorized that the density responses observed at relatively low densities may be due to exploitative competition for stream drift. Furthermore, Amundsen [
43] found that intraspecific competition for food led to increased food intake and a subsequent improvement in growth rate at lower densities. In a meta-analysis, Grant and Imre [
37] reported that increased abundance significantly reduced growth in 15 of the 19 stream populations studied, suggesting widespread density-dependent regulation of body size. Walters et al. [
39] proposed several mechanisms for the relationship between density and growth including limited rearing habitat, relatively consistent spawning locations, and the trade-off juvenile fish must make between foraging and avoiding predators. However, density effects may shift from impacting body size at lower densities to increasing mortality and migration at higher densities [
37].
Stream physical characteristics, including habitat complexity and riparian vegetation, influence prey type, and abundance [
44,
45,
46,
47]. In general, greater habitat heterogeneity has been linked to richer and denser macroinvertebrate communities—key prey for salmonids [
48,
49,
50]. Moreover, Wipfli [
51] emphasized the influence of riparian vegetation on aquatic food webs and suggested that diverse and dense plant communities may foster more diverse and productive terrestrial insect communities—an important component of salmonid diets. Furthermore, physical habitat structure influences carrying capacity and may mediate the influences of density dependence [
46,
52,
53]. To some degree, all these factors and likely others influence fish growth and ultimately the body size of fish.
Despite insights from laboratory and modeling studies [
54,
55], there remains a limited understanding of how these indicators interact to influence juvenile salmonid body size under natural field conditions. Field studies are essential to assess the real-world implications of environmental variation and restoration efforts. In this study, we evaluated juvenile salmonid body size across a range of sites on Washington’s Olympic Peninsula, USA. Our objective was to determine the relative importance of stream temperature, density dependence, catchment size, and physical habitat (e.g., instream wood key piece density and % boulders) on salmonid body size. We hypothesized that stream temperature would have the strongest influence on body size, with streams closer to the upper limits of a species’ optimal growth range supporting larger fish. Specifically, we predicted that stream temperature metrics (daily temperature range, degree days) would explain more variation in body size than salmonid density (density dependence), watershed area (catchment size), or physical habitat (e.g., the density of key pieces of instream wood). We conclude by discussing the implications of our findings for climate change adaptation, habitat restoration, and riparian management strategies.
2. Material and Methods
2.1. Study Area
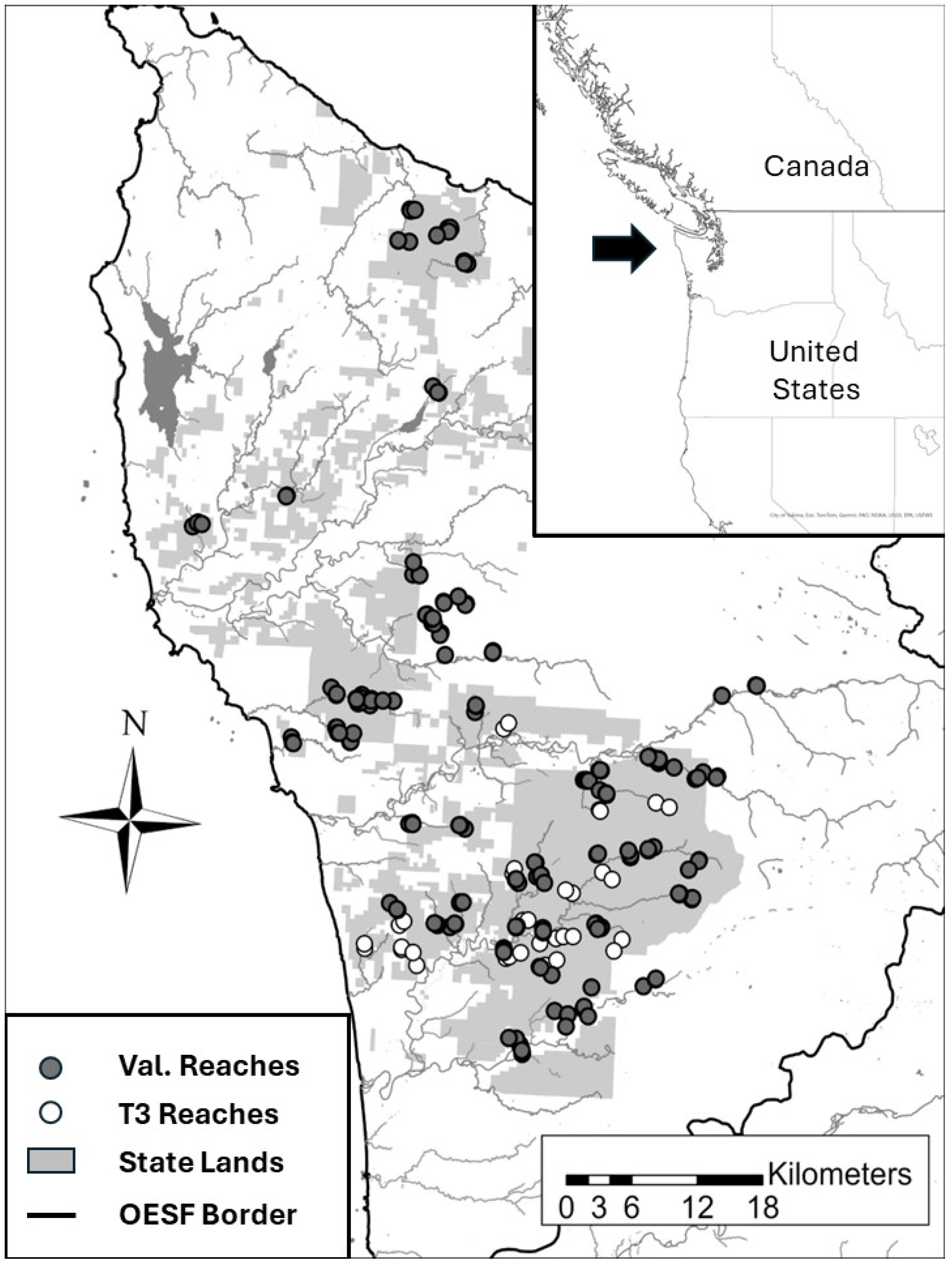
The Washington Department of Natural Resources’ (WDNR) Olympic Experimental State Forest (OESF) on Washington’s western Olympic Peninsula has been designated as a place for both research/monitoring and commodity production (primarily timber harvest). The OESF encompasses approximately 110,000 hectares of state lands within boundaries extending along the crest of the Olympic Mountains, following the watersheds of West Twin Creek and Lake Crescent to the east, the Strait of Juan de Fuca to the north, the Pacific Ocean to the west, and the Quinault River Watershed to the south (
Figure 1). Elevations within the OESF vary from sea level to 1155 m, and the region is characterized by a coastal rainforest climate with substantial precipitation, ranging from 203 to 355 cm annually, with the majority occurring during the winter months. The catchments in this study are forested with a mosaic of standages, the majority of which are second-growth conifer forests, though they also contain third-growth and some stand that have never been harvested.
The OESF contains over 4300 km of streams including portions of several major rivers such as the Queets, Clearwater, Hoh, Bogachiel, Calawah, Sol Duc, Dickey, Hoko, and Clallam. Based on the long-term monitoring of 50 of the small streams in this study, the average 7-day average daily maximum temperature was 14.4 °C; the highest average 7-day average daily maximum temperature for any of these monitored streams was 16.4 °C [
56]. While only two salmonid species (i.e., Lake Ozette sockeye salmon
O. nerka and bull trout
Salvelinus confluentus) are listed under the Endangered Species Act, salmonid populations in the region are largely reduced from historical levels [
57,
58,
59]. The small streams that are the focus of this study (stream order 1st–3rd) [
60] typically contain some combination of juvenile coho salmon, rainbow trout/steelhead (
O. mykiss), coastal cutthroat trout, lampreys (
Lampetra and Entosphenus spp.), and/or sculpins (
Cottus spp.). Juvenile coho salmon typically spend one year in freshwater, while coastal cutthroat trout and steelhead can spend anywhere from a year to a lifetime in freshwater [
61]. However, larger fish (>150 mm) are relatively rare in these small streams.
2.2. Monitoring Programs
Data from three monitoring efforts were used for this analysis. Fish data were collected through the WDNR’s Validation Monitoring and the T3 Watershed Experiment. Habitat variables and water temperature data were obtained from the WDNR’s Status and Trends program, Validation Monitoring, and the T3 Watershed Experiment. The Status and Trends and Validation Monitoring programs operate within the same reaches and complement each other. The T3 Watershed Experiment follows a similar protocol, combining the approaches used in the Status and Trends and Validation Monitoring programs.
The Status and Trends and Validation Monitoring catchments (n = 44) were selected using a stratified random design [
62]. Validation Monitoring samples a single stream reach near the outlet of each catchment. The program samples 20 catchments annually and samples the remaining 24 catchments on a two-year rotation (12 each in even and odd years), as per Martens [
63]. This sampling regimen is ongoing; however, data in this analysis were collected from 2016 to 2022. In contrast, the T3 Watershed Experiment sampled two stream reaches within each of 16 catchments annually from 2019 to 2022. As a result, all streams have been sampled at least three times.
2.3. Fish Surveys and Sampling Data Alignment
Fish sampling took place from mid-July to mid-October, a timeframe carefully selected to coincide with the period when age-0 spring spawning salmonids (i.e., steelhead and coastal cutthroat trout; hereafter referred to as age-0 trout) could be safely sampled, while avoiding complications caused by the accumulation of leaves in the block nets during the fall. Juvenile fish surveys were conducted using a multiple-pass removal electrofishing technique. In the T3 Watershed Experiment catchments, sample reaches were 100 m in length, while, in the Validation Monitoring catchments, sample reaches ranged from 100 to 120 m. Prior to sampling, seine nets were strategically positioned at the top and bottom of each reach to prevent fish movement. Once a reach was effectively blocked, a Smith–Root model 24b backpack electrofisher (Smith- Root, Vancouver, WA, USA) was employed to collect fish moving from the downstream net to the upstream net and then turning around and fishing downstream to the bottom net. Typically, electrofishing operated at a frequency of 60 hertz with a 25 percent duty cycle and voltage levels ranging from 300 to 600 volts. Fish sampling employed a variable-pass approach, varying between three to six passes, as determined by the Connolly [
64] charts and utilized as described in Martens and Connolly [
65].
Following electrofishing, all salmonids underwent anesthesia with MS-222, followed by identification and measured for fork length (from the fish nose to the fork in the tail) and weighed, before being released back into the stream. Field crews could not reliably determine the identity of juvenile coastal cutthroat trout and steelhead under 80 mm (young of the year) at this stage of their development, so they were combined under the designation “age-0 trout”, while all other fish were identified to species in the field. However, it should be noted that most of these fish were coastal cutthroat trout, as steelhead occurred at lower densities and in fewer streams within the study area. Due to the limited body size range in age-1 or older coastal cutthroat trout, variations between streams, and limited numbers of fish within each stream, all coastal cutthroat trout over 80 mm were combined into a single group for this analysis: age-1 or older coastal cutthroat trout.
After each field season, the length frequencies of age-0 trout were compared with those of age-1 or older coastal cutthroat trout and plotted together. An experienced reviewer then visually confirmed or adjusted the dividing point in fork length (initially 80 mm) between age-0 trout and age-1 or older coastal cutthroat. The dividing point was determined by identifying the low point in the bimodal distribution of small and larger fish. For each survey (i.e., each year × site combination), average fork length was calculated for each species (and age-class in the case of juvenile trout). Any sites with fewer than three fish per species or age-class per year were removed from further analysis. This three-fish threshold was a compromise that sought to reduce the influence of outlier fish lengths on average length at sites with very low densities while recognizing the value of data from low-fish-density sites that have been found to be important for identifying density dependence [
40,
41].
The total number of surveys having at least three fish were 84 for coho salmon, 140 for age-0 trout, and 154 for age-1 or older coastal cutthroat trout.
2.4. Habitat Data Collection
Water temperature was monitored year-round at 60-min intervals throughout the study at each of the stream sample reaches using Tidbit v2 temperature data loggers (HOBO® Data Loggers, Bourne, MA, USA). Average daily temperature range (from 1 June through 31 August) was calculated for each reach in each year. Water degree days were summed from 1 January to 31 July for each reach in each year.
Immediately following each fish survey, habitat units (e.g., pools, riffles, runs) within each of the sample reaches were delineated based on the methodology of Bisson et al. [
66] and Pleus et al. [
67]. The surface area of each habitat unit was calculated based on length and the average of three wetted-width measurements. The mean and maximum water depth of each habitat unit was measured to the nearest cm. Within each sample reach, key pieces of instream wood (defined as pieces > 45 cm diameter at the midpoint and >2 m long) were counted.
In each sample reach, five to six equally spaced cross-sections were established. At each cross-section, stream bankfull width was measured, and streambed substrate particle size was measured using a gravelometer at a minimum of 20 equally spaced sample locations. For each reach, median particle size was calculated, as was the percentage of particles classified as boulders (251 to 3999 mm diameter).
Canopy shade was measured using hemispherical photos taken at stream-center at each of the five or size cross sections. Photos were analyzed using Hemisfer software version 2.2 [
68] to calculate the percentage of shaded pixels, defined as those for which vegetation obstructed open sky, using an 82.7-degree field of view (the equivalent view of a spherical densiometer) [
69].
Catchment area was calculated using GIS (ArcMap version 10.6, ESRI, Redlands, CA, USA).
2.5. Data Analysis
Juvenile coho salmon (the vast majority of coho salmon were age-0 fish, with only an occasional age-1 fish collected), age-0 trout, and age-1 or older coastal cutthroat trout were used for this analysis because they were the most common species/age-classes found in the study area. Variables hypothesized to influence the average body size (fork length) of these salmonids were organized into four potential indicators: density, temperature, catchment size, and physical habitat. Variables were selected based on data availability, the probability of detecting a response based on relevant research, and the desire to look at a range of different types of variables. Salmonid density (fish per square meter of stream surface area) was used as the metric for the density indicator (Density;
Table 1). Steam temperature (Temperature) metrics included cumulative degree days (January through July) and average daily temperature range in the summer. Beyond the common metric of degree days for stream temperature, the range of daily stream variation was added since daily stream variation has been found to influence salmonids [
70] and may be important for determining body size. We used catchment area as a metric to represent catchment size. Finaly, physical habitat (Habitat) metrics were percent pool area, average stream depth (excluding pools), boulders (as a percentage of total substrate), the density of key pieces of instream wood (pieces over 2 m length and >45 cm diameter), and percent canopy shade.
Initially, all potential variables were analyzed using Pearson correlations in the program SigmaPlot (Version 13.0, Systat Software, Inc., San Jose, CA, USA). When any two potential variables showed a correlation coefficient above 0.70, one of the variables was removed from the analysis. Bankfull width and catchment area, average substrate size and percent boulders, salmonid density (per m
2) and salmonid biomass (per m
2), and degree days and elevation were found to be highly correlated. From these pairs, we opted to use catchment area, percent boulders, salmonid density, and degree days in our analysis because these variables were deemed to be more informative after initial examination. All models included the variable Julian day of the year and year. Julian day was added to account for any fish growth over time between sampling events over the summer field season (July-mid-October). Year was added to account for interannual variation in body size that was not explained by other variables (e.g., interannual variation in degree days). All variables except year were standardized between 0 and 1 in the program R (R Foundation for Statistical Computing, Vienna, Austria) using the package scales (version 1.4.0) [
71].
The data underwent analysis using linear mixed-effect models within the R package, lme4 (version 1.1-37) [
72]. Sixteen models for each of the three species/age classes (coho salmon, age-0 trout, and age-1 or older coastal cutthroat trout) were constructed using combinations of variables based on the four indicators (Density, Temperature, Catchment, and Habitat) along with a global model and a null model. When an indicator had multiple metrics (for example, degree days and daily stream range for the Temperature indicator), all metrics were included in the model. Additionally, site names were incorporated as a random effect in all models since sites were sampled between three and seven times. The global model encompassed all four indicators and included all 11 variables.
Assessments of the linear model assumptions for the global models were conducted to ensure compliance with key assumptions. Normality was assessed using the R package performance (version 0.15.0) [
73], and the homogeneity of variance was tested using Levene’s Test in the R package car (3.1-3) [
74].
Model ranking was then conducted using Akiaki’s Information Criterion (AICc) corrected for small sample sizes with the R package AICcmodavg (version 2.3-4) [
75]. Models were ranked based on Delta AICc with models within 2 delta AICc units considered to have substantial support compared to the top model [
76]. Since the top models in our analysis garnered most of the weight (0.96–0.59) and no other model was within 2 Delta AICc units of a top model (
Table 2,
Table 3 and
Table 4), the contributions of the fixed-effect variables from the top models were additionally assessed through hierarchical partitioning using the R package glmmhp (version 0.1-8) [
77] rather than using model averaging. Additionally, the direction of the relationship of the fixed effects were determined through 95% confidence intervals with variables considered significant if the entire confidence interval was positive or negative.
3. Results
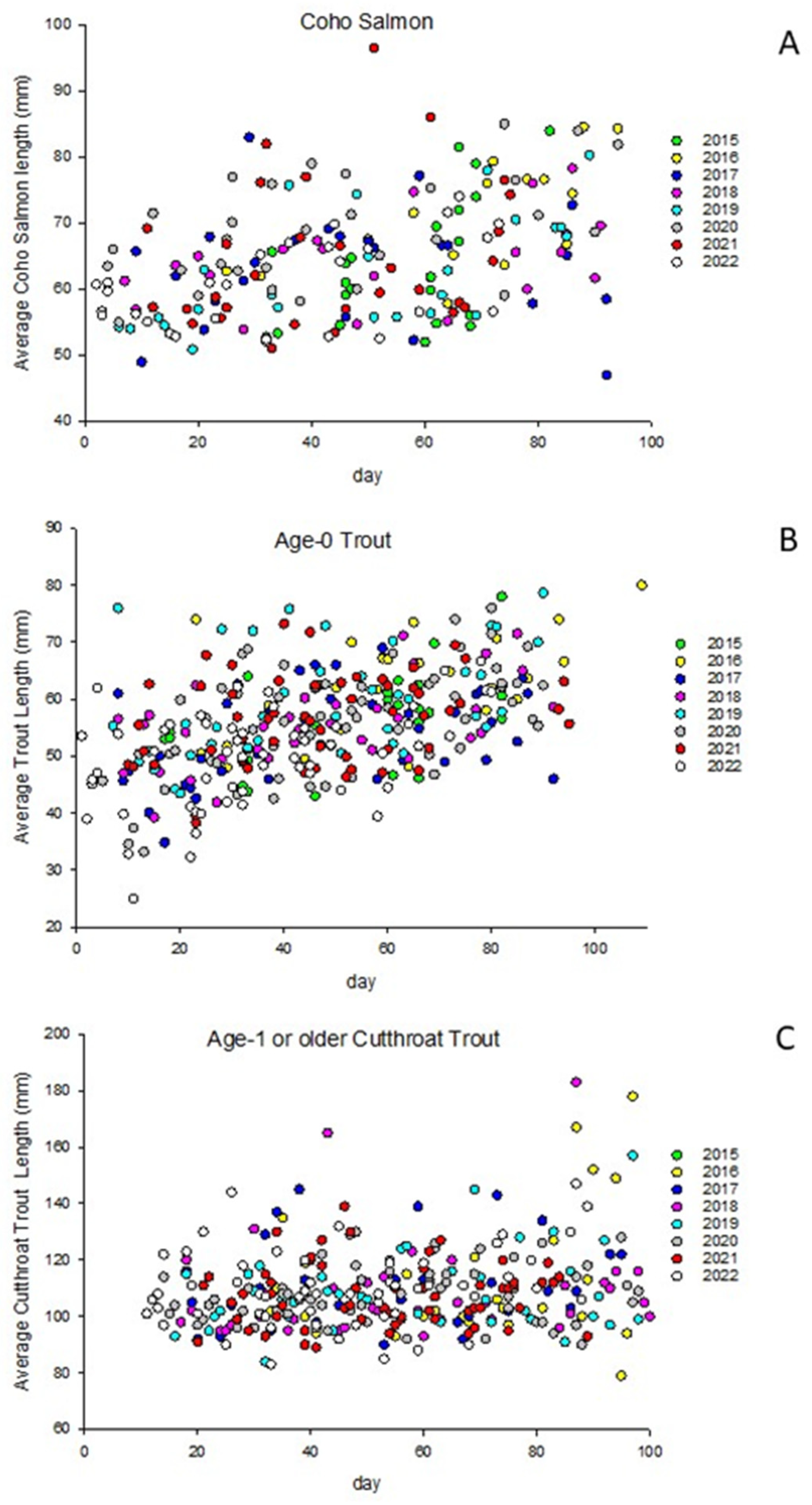
Juvenile coho salmon average body size ranged from 47 mm to 97 mm among sites, with an overall mean of 65 mm and standard deviation of 8 mm. The average body size was 59 mm in July and 72 mm in October (
Figure 2). For age-0 trout, average body size ranged from 25 mm to 80 mm among sites, with an overall mean of 56 mm and standard deviation of 9 mm. Age-0 trout had mean body sizes of 49 mm in July and 64 mm in October. Age-1 or older coastal cutthroat trout had an average body size ranging from 84 mm to 152 mm among sites, with a mean of 106 mm and standard deviation of 11 mm. In July, age-1 or older coastal cutthroat trout averaged 103 mm, increasing slightly to 107 mm in October.
The global model (Density + Temperature + Catchment + Habitat) emerged as the top model for coho salmon, age-0 trout, and age-1 or older coastal cutthroat trout. Specifically, for coho salmon, the top model (R
2m = 0.4581, R
2c = 0.5859) was 3.6 times more parsimonious than the next closest model (Density + Temperature + Habitat;
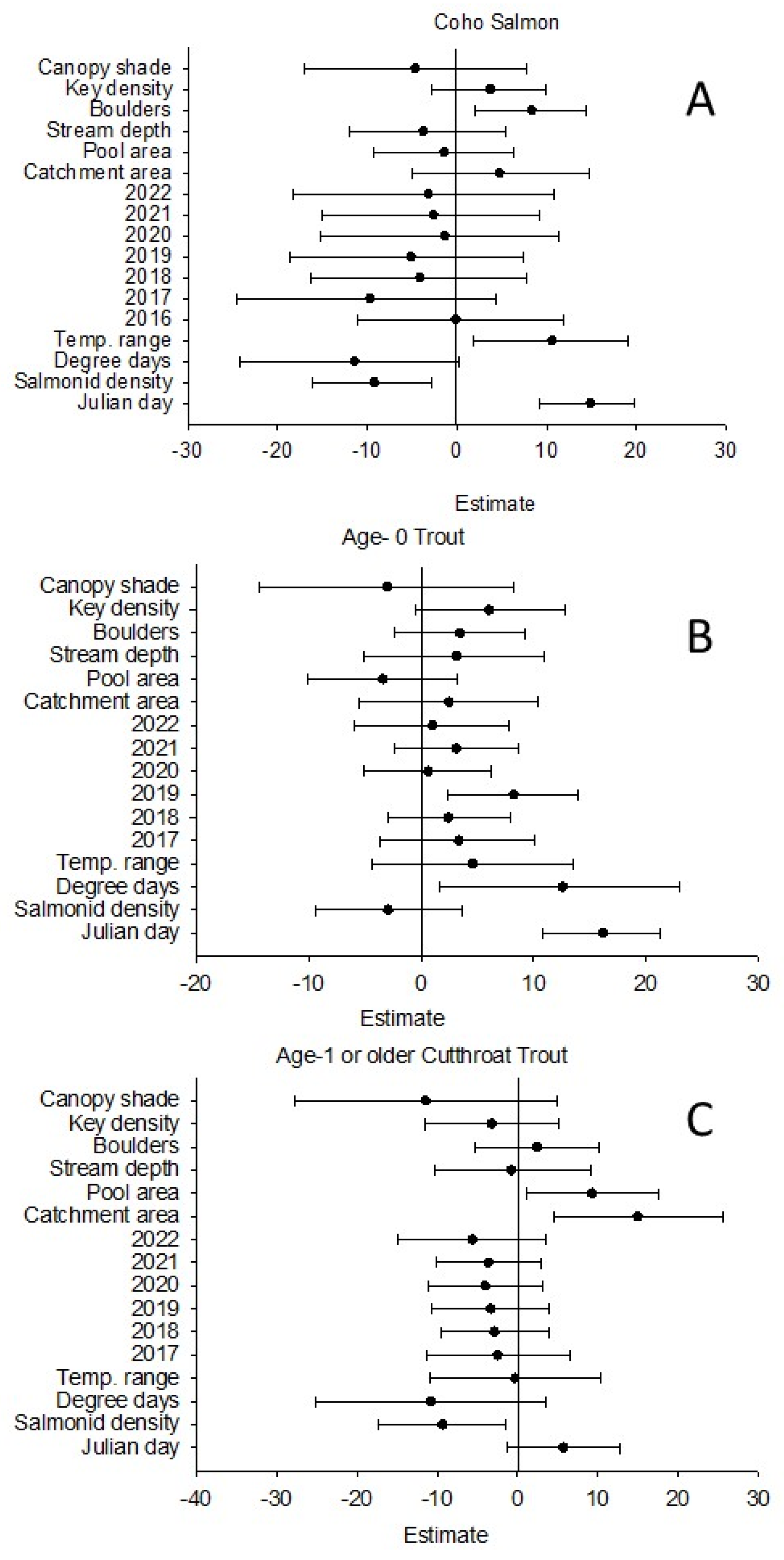
Table 2). In the top model, Julian day of the year (β = 14.87, 95% CI = 9.20 to 19.84), salmonid density (β = −9.25, 95% CI = −16.12 to −2.79), daily mean temperature range (β = 10.62, 95% CI = 1.85 to 19.12), and boulders (β = 8.33, 95% CI = 2.02 to 14.41) exhibited significant relationships with average coho salmon body size;
Figure 3;
Appendix A Table A1).
Table 2.
Model selection results for models of average juvenile coho salmon body size in OESF streams from 2015 to 2022. Variables within each indicator were: Density = salmonid density; Temperature = degree days, and daily stream temperature range; Catchment = catchment area; and Habitat = pool area, stream depth, boulders, key piece density, and canopy shade. All models also included year and Julian day of the year as a fixed effect and site name as a random effect. K = the number of parameters, WT = weight, and LL = log-likelihood of the model.
Table 2.
Model selection results for models of average juvenile coho salmon body size in OESF streams from 2015 to 2022. Variables within each indicator were: Density = salmonid density; Temperature = degree days, and daily stream temperature range; Catchment = catchment area; and Habitat = pool area, stream depth, boulders, key piece density, and canopy shade. All models also included year and Julian day of the year as a fixed effect and site name as a random effect. K = the number of parameters, WT = weight, and LL = log-likelihood of the model.
| Model | K | AICc | Delta AICc | WT | LL |
|---|
| Coho Salmon | | | | | |
|---|
| Density + Temperature + Catchment + Habitat | 20 | 499.65 | 0 | 0.75 | −223.16 |
| Density + Temperature + Habitat | 19 | 502.21 | 2.56 | 0.21 | −226.17 |
| Temperature + Catchment + Habitat | 19 | 506.16 | 6.51 | 0.03 | −228.14 |
| Density + Catchment + Habitat | 18 | 509.34 | 9.69 | 0.01 | −231.41 |
| Temperature + Habitat | 18 | 509.37 | 9.72 | 0.01 | −231.43 |
| Density + Habitat | 17 | 511.85 | 12.20 | 0.00 | −234.29 |
| Catchment + Habitat | 17 | 513.34 | 13.70 | 0.00 | −235.04 |
| Habitat | 16 | 516.39 | 16.74 | 0.00 | −238.13 |
| Density + Temperature + Catchment | 15 | 517.49 | 17.84 | 0.00 | −240.22 |
| Density + Temperature | 14 | 520.92 | 21.27 | 0.00 | −243.41 |
| Temperature + Catchment | 14 | 524.72 | 25.07 | 0.00 | −245.32 |
| Density + Catchment | 13 | 532.37 | 32.72 | 0.00 | −250.59 |
| Temperature | 12 | 533.33 | 33.68 | 0.00 | −252.47 |
| Catchment | 12 | 535.67 | 36.02 | 0.00 | −253.64 |
| Density | 12 | 536.07 | 36.42 | 0.00 | −253.84 |
| Null | 3 | 576.12 | 76.47 | 0.00 | −284.91 |
For age-0 trout, the top model (R
2m = 0.4117, R
2c = 0.5968) was 3.3 times more parsimonious than the next closest model (Temperature + Catchment + Habitat;
Table 3). Within the top model, Julian day of the year (β = 16.12, 95% CI = 10.80 to 21.23) and degree days (β = 12.38. 95% CI = 1.58 to 23.04) had significant positive relationships with average age-0 trout body size (
Figure 3;
Appendix A Table A2).
Table 3.
Model selection results for models of average age-0 trout body size in OESF streams from 2015 to 2022. Variables within each indicator were: Density = salmonid density; Temperature = degree days, and daily stream temperature range; Catchment = catchment area; and Habitat = pool area, stream depth, boulders, key piece density, and canopy shade. All models also included year and Julian day of the year as a fixed effect and site name as a random effect. K = the number of parameters, WT = weight, and LL = log-likelihood of the model.
Table 3.
Model selection results for models of average age-0 trout body size in OESF streams from 2015 to 2022. Variables within each indicator were: Density = salmonid density; Temperature = degree days, and daily stream temperature range; Catchment = catchment area; and Habitat = pool area, stream depth, boulders, key piece density, and canopy shade. All models also included year and Julian day of the year as a fixed effect and site name as a random effect. K = the number of parameters, WT = weight, and LL = log-likelihood of the model.
| Model | K | AICc | Delta AICc | WT | LL |
|---|
| Age-0 Trout | | | | | |
|---|
| Density + Temperature + Catchment + Habitat | 19 | 907.94 | 0 | 0.59 | −431.80 |
| Temperature + Catchment + Habitat | 18 | 910.30 | 2.36 | 0.18 | −434.32 |
| Density + Temperature + Habitat | 18 | 910.34 | 2.39 | 0.18 | −434.34 |
| Temperature + Habitat | 17 | 912.81 | 4.87 | 0.05 | −436.90 |
| Density + Catchment + Habitat | 17 | 919.06 | 11.12 | 0 | −440.02 |
| Catchment + Habitat | 16 | 921.52 | 13.58 | 0 | −442.55 |
| Density + Habitat | 16 | 923.16 | 15.21 | 0 | −443.37 |
| Habitat | 15 | 925.86 | 17.92 | 0 | −445.99 |
| Density + Temperature + Catchment | 14 | 926.93 | 18.99 | 0 | −447.78 |
| Density + Temperature | 13 | 929.70 | 21.76 | 0 | −450.41 |
| Temperature + Catchment | 13 | 929.92 | 21.98 | 0 | −450.52 |
| Density + Catchment | 12 | 936.38 | 28.44 | 0 | −454.96 |
| Temperature | 11 | 938.86 | 30.92 | 0 | −457.40 |
| Catchment | 11 | 939.29 | 31.35 | 0 | −457.61 |
| Density | 11 | 941.28 | 33.34 | 0 | −458.61 |
| Null | 3 | 1011.89 | 103.95 | 0 | −502.86 |
The top model for age-1 or older coastal cutthroat trout (R
2m = 0.2407, R
2c = 0.5188) was 30.3 times more parsimonious than the next closest model (Temperature + Catchment + Habitat;
Table 4). Within the top model, catchment area (β = 14.97, 95% CI 4.43 to 25.53) and pool area (β = 9.26, 95% CI 1.05 to 17.56) had significant positive relationships with average body size (
Figure 3;
Appendix A Table A3).
Table 4.
Model selection results for models of average age-1 or older coastal cutthroat trout body size in OESF streams from 2015 to 2022. Variables within each indicator were: Density = salmonid density; Temperature = degree days, and daily stream temperature range; Catchment = catchment area; and Habitat = pool area, stream depth, boulders, key piece density, and canopy shade. All models also included year and Julian day of the year as a fixed effect and site name as a random effect. K = the number of parameters, WT = weight, and LL = log-likelihood of the model.
Table 4.
Model selection results for models of average age-1 or older coastal cutthroat trout body size in OESF streams from 2015 to 2022. Variables within each indicator were: Density = salmonid density; Temperature = degree days, and daily stream temperature range; Catchment = catchment area; and Habitat = pool area, stream depth, boulders, key piece density, and canopy shade. All models also included year and Julian day of the year as a fixed effect and site name as a random effect. K = the number of parameters, WT = weight, and LL = log-likelihood of the model.
| Model | K | AICc | Delta AICc | WT | LL |
|---|
| Age-1 or older Coastal Cutthroat Trout | | | | | |
|---|
| Density + Temperature + Catchment + Habitat | 19 | 1055.41 | 0 | 0.96 | −505.83 |
| Temperature + Catchment + Habitat | 18 | 1062.23 | 6.82 | 0.03 | −510.54 |
| Density + Temperature + Habitat | 18 | 1065.01 | 9.60 | 0.01 | −511.94 |
| Temperature + Habitat | 17 | 1071.72 | 16.31 | 0 | −516.58 |
| Density + Temperature + Catchment | 14 | 1074.33 | 18.92 | 0 | −521.63 |
| Temperature + Catchment | 13 | 1080.00 | 24.59 | 0 | −525.68 |
| Density + Catchment + Habitat | 13 | 1088.76 | 33.34 | 0 | −525.13 |
| Density + Temperature | 13 | 1096.49 | 35.28 | 0 | −531.03 |
| Catchment + Habitat | 16 | 1094.31 | 38.90 | 0 | −529.17 |
| Density + Habitat | 16 | 1096.49 | 41.08 | 0 | −530.26 |
| Temperature | 11 | 1100.32 | 44.90 | 0 | −538.21 |
| Habitat | 15 | 1102.06 | 46.65 | 0 | −534.29 |
| Density + Catchment | 12 | 1108.03 | 52.62 | 0 | −540.91 |
| Catchment | 11 | 1112.66 | 57.26 | 0 | −544.40 |
| Density | 11 | 1123.64 | 68.22 | 0 | −549.89 |
| Null | 3 | 1148.14 | 92.73 | 0 | −570.99 |
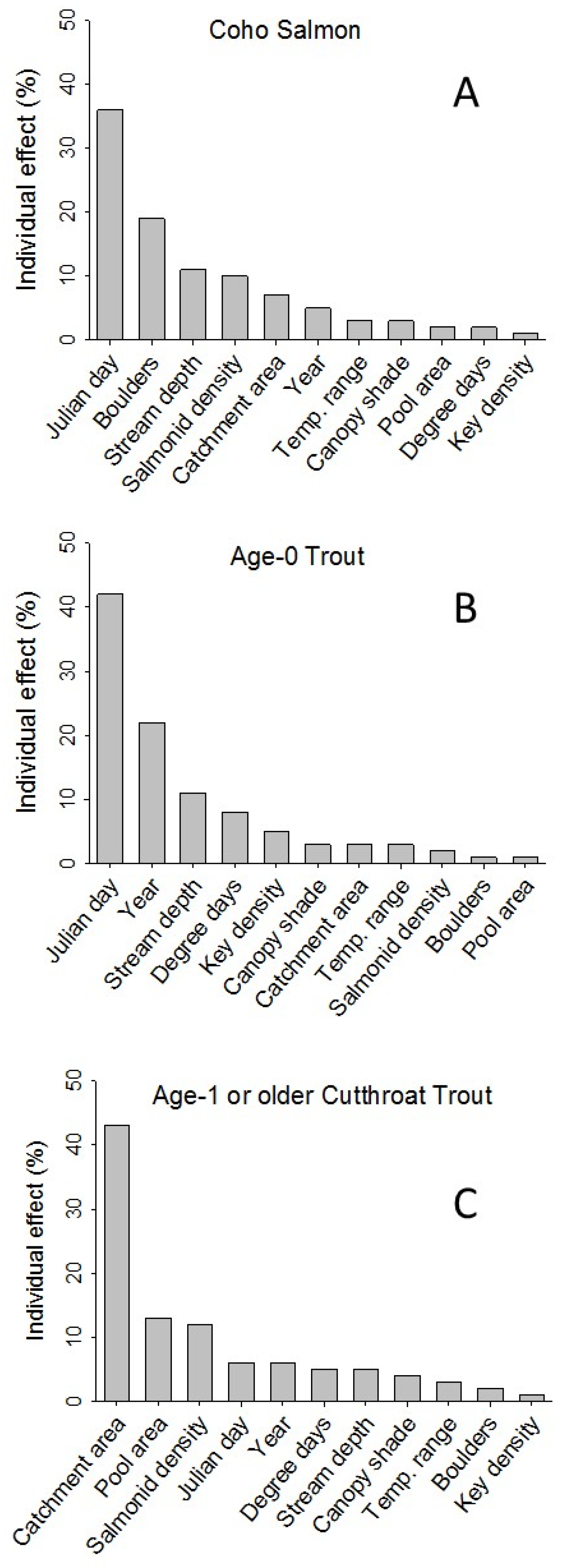
Julian day of the year emerged as the most important variable in models of average body size at sites in both the coho salmon and age-0 trout top models, with over 35% of individual contributions, but was among the least important variables for age-1 or older coastal cutthroat trout, only contributing 6.2% (
Figure 4). Coho salmon had five variables (Julian day of the year = 35.7%, boulders = 19.1%, stream depth = 11.0%, salmonid density = 9.8%, and catchment area = 6.8%) collectively making up over 82% of the total individual contributions. Age-0 trout exhibited six variables (Julian day of the year = 42.1%, year = 22.0%, stream depth = 10.5%, degree days = 7.6%, and Key piece density = 5.3%) which constituted 88% of the total individual contributions. Age-1 or older coastal cutthroat trout had five predictors (catchment area = 43.0%, pool area = 13.0%, salmonid density = 11.8%, Julian day of the year = 6.2%, and year = 6.1%), comprising 80% of the total individual contributions.