Stunted Versus Normally Growing Fish: Adapted to Different Niches
Abstract
1. Introduction
2. Method
3. Why Do Fish Stunt?
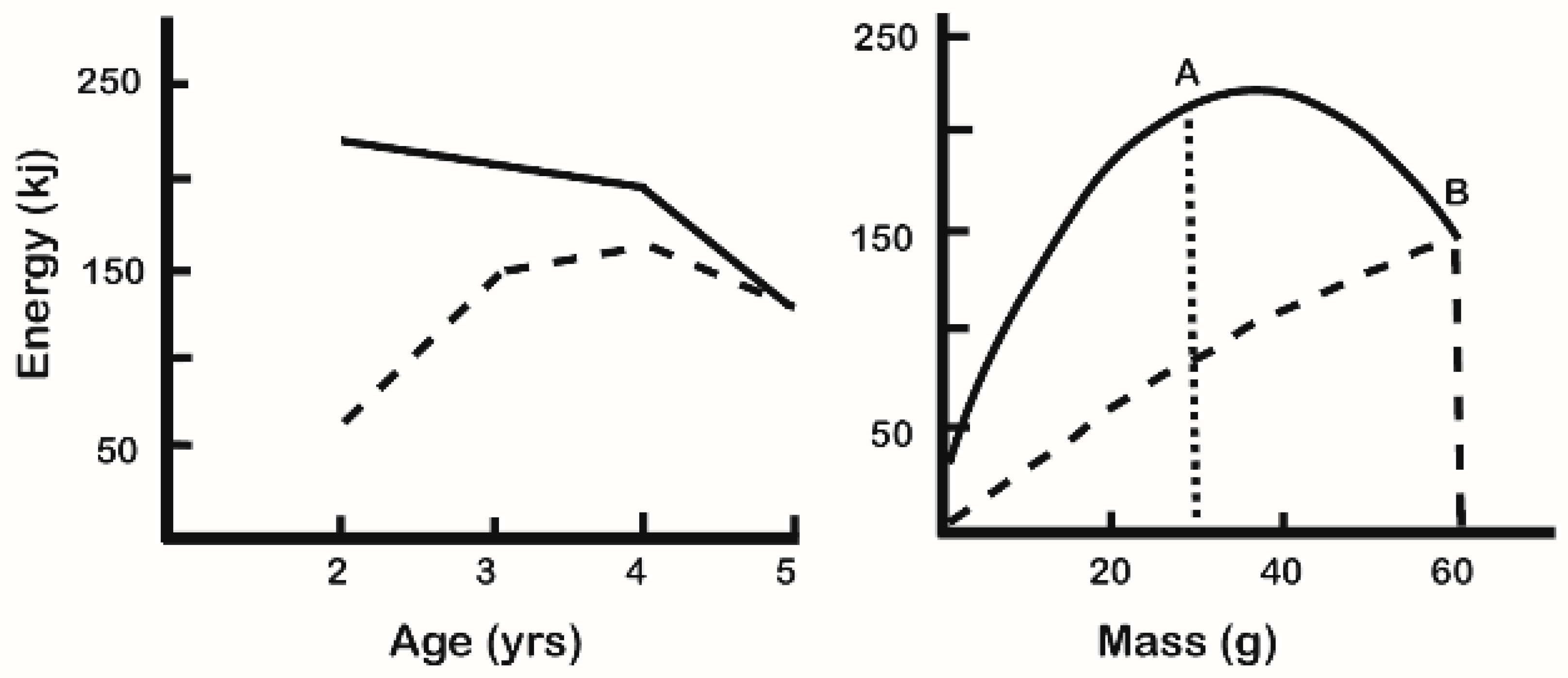
4. Energy Budget, Growth, and Food Intake
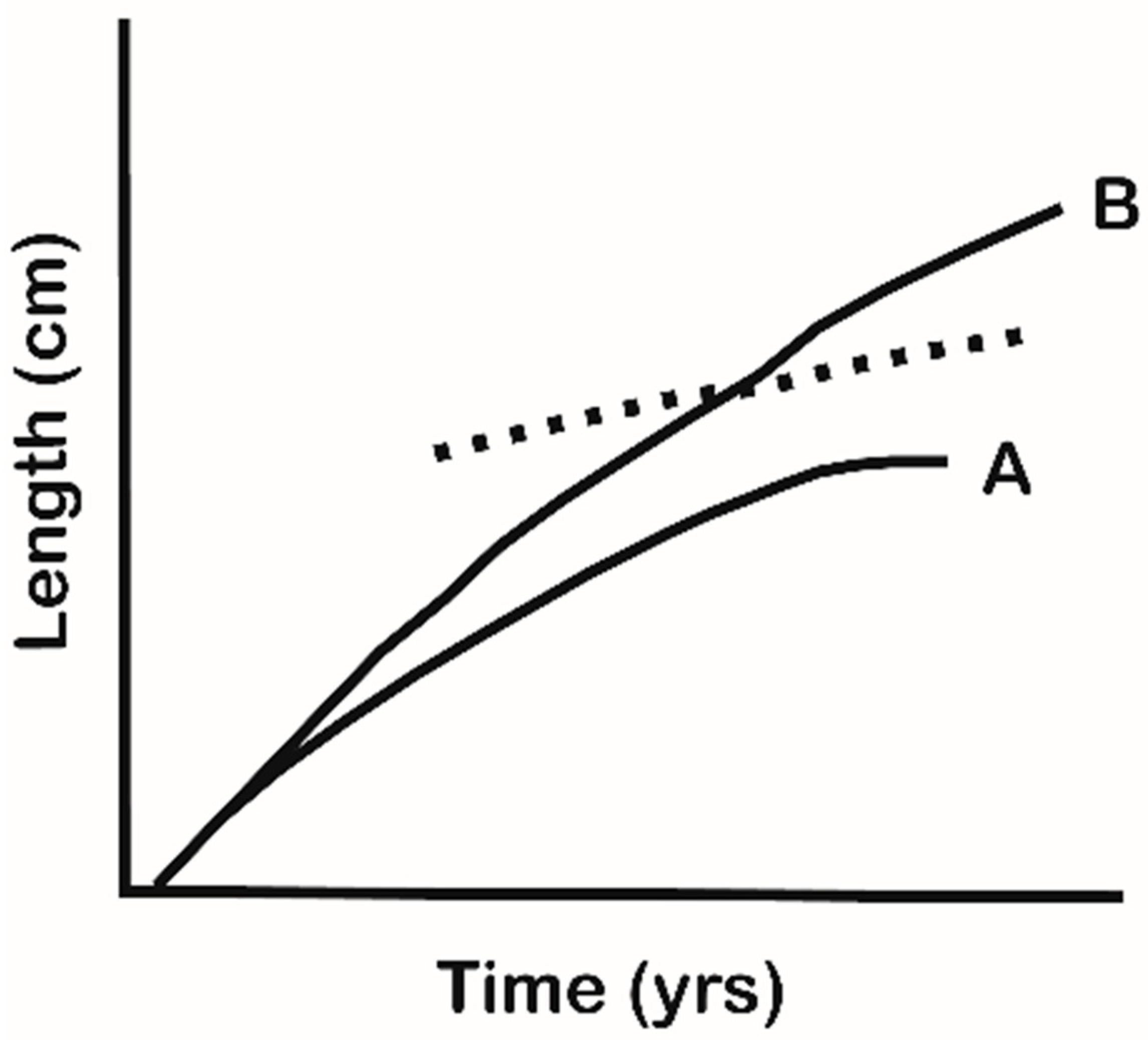
5. Morphology and Habitat
6. Life History
7. Discussion
8. Further Perspective
9. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alm, G. Investigations on the growth etc. by different forms of trout. Medd. Unders. Førsøksanst. Søtvf. 1939, 15, 1–93. [Google Scholar]
- Alm, G. Reasons for the occurrence of stunted fish populations-with special regard to the perch. Medd. Unders. Førsøksanst. Søtvf. 1946, 25, 1–146. [Google Scholar]
- Beckman, W.C. Further studies on the increased growth rate of the rock bass Ambloplites rupestris (Rafinesque), following reduction in density of the population. Trans. Am. Fish. Soc. 1943, 72, 72–78. [Google Scholar] [CrossRef]
- Dahl, K. Studier and Experiments on Trout and Trout Waters; Centraltrykkeriet: Christiania, Norway, 1917. (In Norwegian) [Google Scholar]
- Deedler, C.L. A contribution to the knowledge of the stunted growth of perch (Perca fluviatilis L.) in Holland. Hydrobiologia 1951, 3, 357–378. [Google Scholar] [CrossRef]
- Swingle, H.S. Experiments with combinations of largemouth black bass, bluegills, and minnows in ponds. Trans. Am. Fish. Soc. 1946, 76, 46–62. [Google Scholar] [CrossRef]
- Weatherley, A.H. Ecology of fish growth. Nature 1966, 212, 1321–1324. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Primicerio, R.; Smalås, A.; Henriksen, E.H.; Knudsen, R.; Kristoffersen, R.; Klemetsen, A. Long-term ecological studies in northern lakes—Challenges, experiences, and accomplishments. Limnol. Oceanogr. 2019, 64, 11–21. [Google Scholar] [CrossRef]
- Babu, P.P.S.; Anuraj, A.; Loka, J.; Praveen, N.D.; Rao, P.S.; Shilta, M.T.; Anikuttan, K.K.; Jayakumar, R.; Nazar, A.K.A.; Boby, I.; et al. Impact of duration of stunting on compensatory growth and biometrics of snubnose pompano, Trachinotus blochii (Lacepede, 1801) in low saline conditions. Thalass. Int. J. Mar. Sci. 2022, 38, 1301–1310. [Google Scholar] [CrossRef]
- Denechaud, C.; Smoliński, S.; Geffen, A.J.; Godiksen, J.A.; Campana, S.E. A century of fish growth in relation to climate change, population dynamics and exploitation. Glob. Change Biol. 2020, 26, 5661–5678. [Google Scholar] [CrossRef]
- Lingam, S.S.; Sawant, P.B.; Chadha, N.K. Duration of stunting impacts compensatory growth and carcass quality of farmed milkfish, Chanos chanos (Forsskal, 1775) under field conditions. Sci. Rep. 2019, 9, 16747. [Google Scholar] [CrossRef]
- Neuenfeldt, S.; Bartolino, V.; Orio, A.; Andersen, K.; Andersen, N.G.; Niiranen, S.; Bergström, U.; Kulatska, N.; Casini, M. Feeding and growth of Atlantic cod (Gadus morhua L.) in the eastern Baltic Sea under environmental change. ICES J. Mar. Sci. 2020, 77, 624–632. [Google Scholar] [CrossRef]
- Stearns, S.C. The evolutionary significance of phenotypic plasticity. BioScience 1989, 39, 436–445. [Google Scholar] [CrossRef]
- Forsman, A. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 2015, 115, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Gomulkiewicz, R.; Stinchcombe, J.R. Phenotypic plasticity made simple, but not too simple. Am. J. Bot. 2022, 109, 1519–1524. [Google Scholar] [CrossRef]
- Jonsson, B.; Hindar, K. Reproductive strategy of dwarf and normal Arctic charr (Salvelinus alpinus) from Vangsvatnet Lake, western Norway. Can. J. Fish. Aquat. Sci. 1982, 39, 1404–1413. [Google Scholar] [CrossRef]
- Hindar, K.; Jonsson, B. Habitat and food segregation of dwarf and normal Arctic charr (Salvelinus alpinus) from Vangsvatnet Lake, western Norway. Can. J. Fish. Aquat. Sci. 1982, 39, 1030–1045. [Google Scholar] [CrossRef]
- Ylikarjula, J.; Heino, M.; Dickmann, U. Ecology and adaptation of stunted growth in fish. Evol. Ecol. 1999, 13, 433–453. [Google Scholar] [CrossRef]
- Berg, O.K. The formation of non-anadromous populations of Atlantic salmon, Salmo salar L., in Europe. J. Fish Biol. 1985, 27, 805–815. [Google Scholar] [CrossRef]
- Forseth, T.; Ugedal, O.; Jonsson, B. The energy budget, niche shift, reproduction and growth in a population of Arctic charr, Salvelinus alpinus. J. Anim. Ecol. 1994, 63, 116–126. [Google Scholar] [CrossRef]
- Huusko, A.; Mäki-Petäys, A.; Stickler, M.; Mykrä, H. Fish can shrink under harsh living conditions. Funct. Ecol. 2011, 25, 628–635. [Google Scholar] [CrossRef]
- Letcher, B.H.; Nislow, K.H.; O’Donnell, M.J.; Hayden, M.J.; Dubreuil, T.L. Negative growth in body mass of trout and salmon in a small stream network. Can. J. Fish. Aquat. Sci. 2025, 82, 1–14. [Google Scholar] [CrossRef]
- Leggett, W.C.; Power, G. Differences between two populations of landlocked Atlantic salmon (Salmo salar) in Newfoundland. J. Fish. Res. Board Can. 1969, 26, 1585–1596. [Google Scholar] [CrossRef]
- Davidsen, J.G.; Eikås, L.; Hedger, R.D.; Thorstad, E.B.; Rønning, L.; Sjursen, A.D.; Berg, O.K.; Bremset, G.; Karlsson, S.; Sundt-Hansen, L.E. Migration and habitat use of the landlocked riverine Atlantic salmon Salmo salar småblank. Hydrobiologia 2020, 847, 2295–2306. [Google Scholar] [CrossRef]
- Aday, D.D. Exploring stunted body size: Where have we been, what do we know, and where do we go? Am. Fish. Soc. Symp. 2008, 62, 349–367. [Google Scholar]
- Lorenzen, K.; Enberg, K. Density-dependent growth as a key mechanism in the regulation of fish populations: Evidence from among-population comparisons. Proc. Biol. Sci. 2002, 269, 49–54. [Google Scholar] [CrossRef]
- Zimmermann, F.; Ricard, D.; Heino, M. Density regulation in Northeast Atlantic fish populations: Density dependence is stronger in recruitment than in somatic growth. J. Anim. Ecol. 2018, 78, 1763–1778. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.M.; Vollins, S.F.; Nannini, M.A.; Wahl, D.H. Competition during early ontogeny: Effects of native and invasive planktivores on the growth, survival, and habitat use of bluegill. Freshw. Biol. 2019, 64, 697–707. [Google Scholar] [CrossRef]
- Croll, J.C.; van Kooten, T.; de Roos, A.M. The consequences of density-dependent individual growth for sustainable harvesting and management of fish stocks. Fish Fish. 2023, 24, 427–438. [Google Scholar] [CrossRef]
- Lobon-Cervia, J. Density-dependent growth in stream-living brown trout Salmo trutta L. Funct. Ecol. 2007, 21, 117–124. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Knudsen, R.; Klemetsen, A. Intraspecific competition and density dependence of food competition and growth in Arctic charr. J. Anim. Ecol. 2007, 76, 149–158. [Google Scholar] [CrossRef]
- Huss, M.; Persson, L.; Borcherding, J.; Heermann, L. Timing of the diet shift from zooplankton to macroinvertebrates and size at maturity determine whether normally piscivorous fish can persist in otherwise fishless lakes. Freshw. Biol. 2013, 58, 1416–1424. [Google Scholar] [CrossRef]
- Cantin, A.; Post, J.R. Habitat availability and ontogenetic shifts alter bottlenecks in size-structured fish populations. Ecology 2018, 99, 1644–1659. [Google Scholar] [CrossRef] [PubMed]
- Matte, J.M.O.; Grant, J.W.A.; Fraser, D.J. Meta-analysis reveals that density dependent growth, survival and their trade-off vary systematically among habitats and taxa. Can. J. Zool. 2025, 103, 1–15. [Google Scholar] [CrossRef]
- Zuh, C.K.; Abodi, S.M.; Campion, B.B. Comparative assessment of age, growth and food habit of the black-chinned tilapia, Sarotherodon melanotheron (Rüppell, 1852), from a closed and open lagoon, Ghana. Fish. Aquat. Sci. 2019, 22, 31. [Google Scholar] [CrossRef]
- Rindorf, A.; van Deurs, M.; Howell, D.; Andonegi, E.; Berger, A.; Bogstad, B.; Cadigan, N.; Elvarsson, B.Þ.; Hintzen, N.; Roland, M.S.; et al. Strength and consistency of density dependence in marine fish productivity. Fish Fish. 2022, 231, 812–828. [Google Scholar] [CrossRef]
- Schram, E.; van der Heul, J.W.; Kamstra, A.; Verdegem, M.C.J. Stocking density dependent growth of Dover sole (Solea solea). Aquaculture 2006, 252, 339–347. [Google Scholar] [CrossRef]
- Agrawal, A.A. Phenotypic plasticity in the interactions and evolution of species. Science 2001, 294, 321–326. [Google Scholar] [CrossRef]
- Heath, D.D.; Roff, D.A. The role of trophic bottlenecks in stunting: A field test of an allocation model of growth and reproduction in yellow perch, Perca flavescens. Environ. Biol. Fish. 1996, 45, 53–63. [Google Scholar] [CrossRef]
- Blouzdis, C.E.; Ivan, L.N.; Pothoven, S.A.; Roswell, C.R.; Foley, C.J.; Höök, T.O. A trophic bottleneck?: The ecological role of trout-perch Percopsis omiscomaycus in Saginaw Bay, Lake Huron. J. Appl. Ichthyol. 2013, 29, 416–424. [Google Scholar] [CrossRef]
- Jakobsen, P.J.; Johnsen, G.H.; Larsson, P. Effect of predation risk on the feeding ecology, habitat us and abundance of lacustrine threespine stickleback (Gasterosteus aculeatus). Can. J. Fish. Aquat. Sci. 1988, 45, 426–431. [Google Scholar] [CrossRef]
- Damsgård, B.; Ugedal, O. The influence of predation risk on habitat selection and food intake by Arctic charr, Salvelinus alpinus (L.). Ecol. Freshw. Fish 1997, 6, 95–101. [Google Scholar] [CrossRef]
- Bordeau, P.E.; Johansson, F. Predator-induced morphological defenses as by-products of prey behavior: A review and prospectus. Oikos 2012, 121, 1175–1190. [Google Scholar] [CrossRef]
- Bell, A.M.; Dingemanse, N.J.; Hankison, S.J.; Langenhof, M.B.W.; Rollins, K. Early exposure to non-lethal predation risk by size-selective predators increases somatic growth and decreases size at adulthood of three-spined sticklebacks. J. Evol. Biol. 2011, 24, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Gosch, N.J.C.; Pierce, L.L.; Pope, K.L. The effect of predation on stunted and nonstunted white perch. Ecol. Freshw. Fish 2010, 19, 401–407. [Google Scholar] [CrossRef]
- Czorlich, Y.; Aykanat, T.; Erkinaro, J.; Orell, P.; Primmer, C.R. Rapid evolution in salmon life history induced by direct and indirect effects of fishing. Science 2022, 376, 420–423. [Google Scholar] [CrossRef]
- Sneider, J.C.; Lockwood, R.N. Use of walleye stocking, antimycin treatments, and catch-and-release angling regulations to increase growth and length of stunted bluegill populations in Michigan lakes. N. Am. J. Fish. Manag. 2002, 22, 1041–1052. [Google Scholar] [CrossRef]
- de Roos, A.M.; Schellekens, T.; van Kooten, T.; Persson, L. Stage-specific predator species help each other to persist while competing for a single prey. Proc. Natl. Acad. Sci. USA 2008, 105, 13930–13935. [Google Scholar] [CrossRef]
- Boerlijst, M.C.; de Roos, A.M. Competition and facilitation between a disease and a predator in a stunted prey population. PLoS ONE 2015, 10, e0132251. [Google Scholar] [CrossRef]
- Chizinski, C.J.; Pope, K.L.; Wilde, G.R.; Strauss, R.E. Implications of stunting on morphology of freshwater fishes. J. Fish Biol. 2010, 76, 564–579. [Google Scholar] [CrossRef]
- Mustafa, S.A.; Al-Rudainy, A.J.; Salman, N.M. Effect of environmental pollutants on fish health: An overview. Egypt. J. Aquat. Res. 2024, 50, 225–233. [Google Scholar] [CrossRef]
- Bolle, L.J.; Hoek, R.; Pennock, I.; Poiesz, S.S.H.; van Beusekom, J.E.E.; van der Veer, H.; Wille, J.I.J.; Tulp, I. Evidence for reduced growth in resident fish species in the era of de-eutrophication in a coastal area in NW Europe. Mar. Environ. Res. 2021, 169, 105364. [Google Scholar] [CrossRef]
- Neuheimer, A.; Thresher, R.; Lyle, J.; Semmens, J.M. Tolerance limit for fish growth exceeded by warming waters. Nat. Clim. Chang. 2011, 1, 110–113. [Google Scholar] [CrossRef]
- Kolding, J.; Haug, L.; Stefansson, S. Effect of ambient oxygen on growth and reproduction in Nile tilapia (Oreochromis niloticus). Can. J. Fish. Aquat. Sci. 2008, 65, 1413–1424. [Google Scholar] [CrossRef]
- Thorarensen, H.; Gústavsson, A.; Gunnarsson, S.; Árnason, J.; Steinarsson, A.; Björnsdóttir, R.; Imsland, A.K.D. The effect of oxygen saturation on the growth and food conversion of juvenile Atlantic cod Gadus morhua L. Aquaculture 2017, 475, 24–28. [Google Scholar] [CrossRef]
- Mackett, D.B.; Tam, W.H.; Fryer, V.N. Histological changes in insulin-immunoreactive pancreatic beta-cells, and suppression of insulin-secretion and somatotropic activity in brook trout (Salvelinus fontinalis) maintained on reduced food-intake or exposed to acidic environment. Fish Physiol. Biochem. 1992, 10, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Brogowski, Z.; Siewert, H.; Keplinger, D. Feeding and growth responses of bluegill fish (Lepomis macrochirus) at various pH levels. Pol. J. Environ. Stud. 2005, 14, 517–519. [Google Scholar]
- Schlichting, C.D.; Wund, M.A. Phenotypic plasticity and epigenetic marking: An assessment of evidence for epigenetic accommodation. Evolution 2014, 68, 656–672. [Google Scholar] [CrossRef]
- Vogt, G. Facilitation of environmental adaptation by epigenetic phenotype variation: Insights from clonal, invasive, polyploid, and domesticated animals. Environ. Epigen. 2017, 3, dvx002. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Metcalfe, N.B. How important is hidden phenotypic plasticity arising from alternative but converging developmental trajectories, and what limits it? J. Exp. Biol. 2024, 227 (Suppl. 1), jeb246010. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Butler, A.E.; Jamialahmadi, T.; Sahebkar, A. Molecular mechanisms linking stress and insulin resistance. Excli. J. 2022, 21, 317–334. [Google Scholar] [PubMed]
- Duan, C.M.; Plisetskaya, E.M.; Dickhoff, W.W. Expression of insulin-like growth-factor in normally and abnormally developing coho salmon (Oncorhynchus kisuch). Endocrinology 1995, 136, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Canosa, L.F.; Bertucci, J.I. The effect of environmental stressors on growth in fish and its endocrine control. Front. Endicrinol. 2023, 14, 1109461. [Google Scholar] [CrossRef] [PubMed]
- Nardocci, G.; Navarro, C.; Cortés, P.P.; Imarai, M.; Montoya, M.; Valenzuela, B.; Jara, P.; Acuña-Castillo, C.; Fernandez, R. Neuroendocrine mechanisms for immune system regulation during stress in fish. Fish Shellf. Immun. 2014, 40, 531–538. [Google Scholar] [CrossRef]
- Patel, D.M.; Brinchmann, M.F.; Hansen, A.; Iversen, M.H. Changes in the skin proteome and signs of allostatic overload Type 2, chronic stress, in response to repeated overcrowding of lumpfish (Cyclopterus lumpus L.). Front. Mar. Sci. 2022, 9, 891451. [Google Scholar] [CrossRef]
- Janhunen, M.; Peuhkuri, N.; Piironen, J. A comparison of growth patterns between a stunted and two large predatory Arctic charr populations under identical hatchery conditions. Environ. Biol. Fish. 2010, 87, 113–121. [Google Scholar] [CrossRef]
- Canale, C.J.; Henry, P.Y. Adaptive phenotypic plasticity and resilience of vertebrates to increasing climatic instability. Clim. Res. 2010, 43, 135–147. [Google Scholar] [CrossRef]
- Khare, S.B.; Holt, R.D.; Scheiner, S.M. The genetics of phenotypic plasticity. XVIII. Developmental limits restrict adaptive plasticity. Evolution 2024, 78, 1761–1773. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Naiel, A.E.; Said, M.B.; Alnajeebi, A.M.; Nasr, F.A.; Al-Doaiss, A.A.; Mahasneh, Z.M.H.; Noreldin, A.E. Environmental epigenetics: Exploring phenotypic plasticity and transgenerational adaptation in fish. Environ. Res. 2024, 252, 118799. [Google Scholar] [CrossRef]
- Chukwuma, C. Epigenetics and its essence in understanding human growth, development and disease. J. Med. Dis. 2022, 8, 165–172. [Google Scholar]
- Kanherkar, R.R.; Bhatia-Dey, N.; Csoka, A.B. Epigenetics across the human lifespan. Front. Cell Dev. Biol. 2014, 2, 49. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C. Growth disorders caused by variants in epigenetic regulators; progress and prospects. Front. Endocrinol. 2024, 15, 1327378. [Google Scholar] [CrossRef] [PubMed]
- Oplinger, R.W.; Wahl, D.H. Egg characteristics and larval growth of bluegill from stunted and non-stunted populations. J. Freshw. Ecol. 2015, 30, 299–309. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Hansen, M.M. Knock-on effects of environmental influences during embryonic development of ectothermic vertebrates. Q. Rev. Biol. 2022, 97, 95–139. [Google Scholar] [CrossRef]
- Martell, D.J.; Kieffer, D.J.; Trippel, E.A. Effects of temperature during early life history on embryonic and larval development and growth in haddock. J. Fish Biol. 2005, 66, 1558–1575. [Google Scholar] [CrossRef]
- Korwin-Kossakowski, M. The influence of temperature during the embryonic period on larval growth and development in carp, Cyprinus carpio L. and grass carp, Ctenopharyngodon idella (Val.): Theoretical and practical aspects. Arch. Pol. Fish. 2008, 16, 231–314. [Google Scholar] [CrossRef]
- Finstad, A.G.; Jonsson, B. Effect of incubation temperature on growth performance in Atlantic salmon. Mar. Ecol. Progr. Ser. 2012, 454, 75–82. [Google Scholar] [CrossRef]
- Steinbacher, P.; Wanzenböck, J.; Brandauer, M.; Holper, R.; Landertshammer, J.; Mayr, M.; Platzl, C.; Stoiber, W. Thermal experience during embryogenesis contributes to the induction of dwarfism in whitefish Coregonus lavaretus. PLoS ONE 2017, 12, e0185384. [Google Scholar] [CrossRef]
- Carballo, C.; Firmino, J.; Anjos, L.; Santos, S.; Power, D.M.; Manchad, M. Short- and long-term effects on growth and expression patterns in response to incubation temperature in Senegalese sole. Aquaculture 2018, 495, 222–231. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Finstad, A.G. Linking embryonic temperature with adult reproductive investment. Mar. Ecol. Progr. Ser. 2014, 515, 217–226. [Google Scholar] [CrossRef]
- Almeida, L.Z.; Ludsin, S.A.; Faust, M.D.; Marschall, E.A. Lingering legacies: Past growth and parental experience influence somatic growth in a fish population. J. Anim. Ecol. 2024, 93, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Forseth, T.; Jonsson, B.; Neumann, R.; Ugedal, O. Radioisotope method for estimating food consumption by brown trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 1992, 49, 1328–1335. [Google Scholar] [CrossRef]
- Ugedal, O.; Jonsson, B.; Njåstad, O.; Næumann, R. Effects of temperature and body size on radiocaesium retention in brown trout, Salmo trutta. Freshw. Biol. 1992, 28, 165–171. [Google Scholar] [CrossRef]
- Jonsson, B.; Forseth, T.; Ugedal, T. Chernobyl radioactivity persists in fish. Nature 1999, 400, 417. [Google Scholar] [CrossRef]
- Rowan, D.J.; Rasmussen, J.B. Measuring the bioenergetic cost of fish activity in situ using a globally dispersed radiotracer (137Cs). Can. J. Fish. Aquat. Sci. 1996, 53, 734–745. [Google Scholar] [CrossRef][Green Version]
- Kennedy, B.P.; Klaue, B.; Blum, J.D.; Folt, C.L. Integrative measures of consumption rates in salmon: Expansion and application of a trace element approach. J. Appl. Ecol. 2004, 41, 1009–1020. [Google Scholar] [CrossRef]
- de Groot, V.A.; Trueman, C.; Bates, A.E. Incorporating otolith-isotope inferred field metabolic rate into conservation strategies. Cons. Physiol. 2024, 12, coae013. [Google Scholar] [CrossRef]
- Anacleto, P.; Figueiredo, C.; Baptista, M.; Maulvault, A.L.; Camacho, C.; Pousao-Ferreira, P.; Valente, L.M.P.; Marques, A.; Rosa, R. Fish energy budget under ocean warming and flame retardant exposure. Environ. Res. 2018, 164, 186–196. [Google Scholar] [CrossRef]
- Assis, Y.P.A.S.; de Assis Porto, L.; de Melo, N.F.A.C.; Palheta, G.D.A.; Luz, R.K.; Favero, G.C. Feeding restriction a feed management strategy in Colossoma macropomum juveniles under recirculating aquaculture system (RAS). Aquaculture 2020, 529, 735689. [Google Scholar] [CrossRef]
- Schultz, E.T.; Langford, T.E.; Conover, D.O. The covariance of routine and compensatory juvenile growth rates over a seasonality gradient in a coastal fish. Oecologia 2002, 133, 501–509. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Das, P.C.; Nanda, S.; Sahu, B.; Muduli, L. Compensatory growth response of Catla catla (Hamilton, 1822) juveniles, stunted with varied stocking density and photoperiod, in subsequent grow-out phase. Ind. J. Fish. 2021, 68, 49–55. [Google Scholar] [CrossRef]
- Wootton, R.J. Ecology of Teleost Fishes; Chapman & Hall: London, UK, 1990. [Google Scholar]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.E. Species packing and niche complementarity in three sunfishes. Am. Nat. 1977, 111, 553–578. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Thorpe, J.E. Anorexia and defended energy levels in over-wintering juvenile salmon. J. Anim. Ecol. 1992, 61, 175–181. [Google Scholar] [CrossRef]
- Nikolsky, G.V. Ecology of Fishes; Academic Press: London, UK, 1963. [Google Scholar]
- Hvas, M.; Kolrevic, J.; Noble, C.; Oppedahl, F.; Stien, L.H. Fasting and its implications for fish welfare in Atlantic salmon aquaculture. Rev. Aquacult. 2024, 16, 1308–1332. [Google Scholar] [CrossRef]
- Diana, J.S. Stunting in northern pike Esox lucius is caused by lack of suitable prey size, lack of thermal refuges in mid-summer overpopulations and competition. Trans. Am. Fish. Soc. 1987, 116, 612–617. [Google Scholar] [CrossRef]
- Venturelli, P.A.; Tonn, W.M. Diet and growth in the absence of prey fishes: Initial consequences in disturbance-prone lakes. Trans. Am. Fish. Soc. 2016, 135, 1512–1522. [Google Scholar] [CrossRef]
- Xie, B.; Huang, C.; Wang, Y.; Zhou, X.; Peng, G.; Tao, Y.; Huang, J.; Lin, X.; Huang, L. Trophic gauntlet effects on fisheries recovery: A case study in Sansha Bay, China. Ecosyst. Health Sustain. 2021, 7, 1965035. [Google Scholar] [CrossRef]
- Finstad, A.G.; Berg, O.K.; Langeland, A.; Lohrmann, A. Reproductive investment and energy allocation in an Alpine Arctic charr, Salvelinus alpinus, population. Environ. Biol. Fish. 2002, 65, 63–70. [Google Scholar] [CrossRef]
- Amundsen, P.A. Contrasting life-history strategies facilitated by cannibalism in a stunted Arctic charr population. Hydrobiologia 2016, 783, 11–19. [Google Scholar] [CrossRef]
- Sandlund, O.T.; Gunnarson, K.; Jónasson, P.M.; Jonsson, B.; Lindem, T.; Magnússon, K.P.; Malmquist, H.J.; Sigurjónsdóttir, H.; Skúlason, S.; Snorrason, S.S. The Arctic charr Salvelinus alpinus (L.) in Thingvallavatn. Oikos 1992, 64, 305–351. [Google Scholar] [CrossRef]
- Langeland, A.; L’Abée-Lund, J.H.; Jonsson, B.; Jonsson, N. Resource partitioning and niche shift in Arctic charr, Salvelinus alpinus and brown trout, Salmo trutta. J. Anim. Ecol. 1991, 60, 895–912. [Google Scholar] [CrossRef]
- L’Abée-Lund, J.H.; Langeland, A.L.; Jonsson, B. Spatial segregation by age and size in brown trout and Arctic charr: A trade-off between feeding possibilities and risk of predation. J. Anim. Ecol. 1993, 62, 160–168. [Google Scholar] [CrossRef]
- Holopainen, I.J.; Aho, J.; Vornanen, M.; Huuskonen, H. Phenotypic plasticity and predator effects on morphology and physiology of crucian carp in nature and in the laboratory. J. Fish Biol. 1997, 50, 781–798. [Google Scholar] [CrossRef]
- Hindar, K.; Jonsson, B. Ecological polymorphism in Arctic charr. Biol. J. Linn. Soc. 1993, 8, 63–74. [Google Scholar] [CrossRef]
- Snorrason, S.S.; Skulason, S.; Jonsson, B.; Malmquist, H.; Jonasson, P.M.; Sandlund, O.T.; Lindem, T. Trophic specialization in Arctic charr Salvelinus alpinus (Pisces: Salmonidae): Morphological divergence and ontogenetic shifts. Biol. J. Linn. Soc. 1994, 52, 1–18. [Google Scholar] [CrossRef]
- Sandlund, O.T.; Jonsson, B.; Malmquist, H.J.; Gydemo, R.; Lindem, T.; Snorrason, S.S.; Skùlason, S.; Jónasson, P.M. Habitat use by Arctic charr Salvelinus alpinus in lake Thingvallavatn, Iceland, as shown by gill net catches. Environ. Biol. Fish. 1987, 20, 263–274. [Google Scholar] [CrossRef]
- Malmquist, H.J.; Snorrason, S.S.; Skulason, S.; Jonsson, B.; Sandlund, O.T.; Jonasson, P.M. Diet differentiation in polymorphic arctic charr, Salvelinus alpinus (L.) in Thingvallavatn, Iceland. J. Anim. Ecol. 1992, 61, 21–35. [Google Scholar] [CrossRef]
- Svedäng, H. Genetic basis of life history variation of dwarf and normal Arctic charr, Salvelinus alpinus (L.) in Stora Rösjön, Central Sweden. J. Fish Biol. 1990, 36, 917–932. [Google Scholar] [CrossRef]
- Gardeno-Paz, M.V.; Huntingford, F.A.; Garrett, S.; Adams, C.E. A phenotypically magic trait promotes reproductive isolation in stickleback. Evol. Ecol. 2020, 34, 123–131. [Google Scholar] [CrossRef]
- Klemetsen, A. The charr problem revisited: Exceptional phenotypic plasticity promotes ecological speciation in postglacial lakes. Freshw. Rev. 2010, 3, 49–74. [Google Scholar] [CrossRef]
- Brönmark, C.; Miner, J.G. Predator-induced phenotypical change in body morphology in crucian carp. Science 1992, 258, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- Olsén, K.H.; Bonow, M. Crucian carp (Carassius carassius (L.)), an anonymous fish with great skills. Ichthyol. Res. 2023, 70, 313–331. [Google Scholar] [CrossRef]
- Brönmark, C.; Petterson, L.B. Chemical cues from piscivores induce a change in morphology in crucian carp. Oikos 1994, 70, 364–402. [Google Scholar] [CrossRef]
- Andersson, J.; Johansson, F.; Söderlund, T. Interactions between predator- and diet-induced phenotypic changes in body shape of crucian carp. Proc. Biol. Sci. 2005, 273, 431–437. [Google Scholar] [CrossRef]
- Arnett, H.A.; Kinnison, M.T. Predation-induced phenotypic plasticity of shape and behavior: Parallel and unique patterns across sexes and species. Curr. Zool. 2017, 63, 369–378. [Google Scholar]
- Meutthen, D.; Ferrari, M.C.O.; Lane, T.; Chivers, D.P. Predation risk induces age- and sex-specific morphological plastic responses in the fathead minnow Pimephales promelas. Sci. Rep. 2019, 9, 15378. [Google Scholar] [CrossRef]
- Yang, Y.; Axelrod, C.J.; Grant, E.; Earl, S.R.; Urquart, E.M.; Talbert, K.; Johnson, L.E.; Walker, Z.; Hsiao, K.; Stone, I.; et al. Evolutionary divergence of developmental plasticity and learning of mating tactics in Trinidadian guppies. J. Anim. Ecol. 2025, 94, 276–290. [Google Scholar] [CrossRef]
- Persson, L. Food consumption and competition between age classes in a perch Perca fluviatilis population in a shallow eutrophic lake. Oikos 1983, 40, 197–207. [Google Scholar] [CrossRef]
- Jonsson, N.; Næsje, T.F.; Jonsson, B.; Saksgård, R.; Sandlund, O.T. The influence of piscivory on life history traits of brown trout. J. Fish Biol. 1999, 55, 1129–1141. [Google Scholar] [CrossRef]
- Persson, A.; Brönmark, C. Foraging capacity and resource synchronization in an ontogenetic diet switcher, pikeperch (Stizostedion lucioperca). Ecology 2002, 83, 3014–3022. [Google Scholar] [CrossRef]
- Naeem, M.; Salam, A.; Ishtiaq, A. Length-weight relationships of wild and farmed Tor putitora from Pakistan. J. Appl. Ichthyol. 2011, 27, 1133–1134. [Google Scholar] [CrossRef]
- Shrestha, J. Coldwater fish and fisheries in Nepal. FAO Fish. Tech. Pap. 1999, 385, 13–40. [Google Scholar]
- Gatz, A.J. Community organization in fishes as indicated by morphological features. Ecology 1979, 60, 711–718. [Google Scholar] [CrossRef]
- Binning, S.A.; Chapman, L.J. Is intraspecific variation in diet and morphology related to environmental gradients? Exploring Liem’s paradox. Integr. Zool. 2010, 5, 241–255. [Google Scholar] [CrossRef]
- Shuai, F.; Yu, S.; Li, X. Habitat effects on intra-species variation in functional morphology: Evidence from freshwater fish. Ecol. Evol. 2018, 8, 10902–10913. [Google Scholar] [CrossRef]
- Davis, A.M.; Pusey, B.J.; Pearson, R.G. Trophic ecology of Terapontid fishes (Pisces: Terapontidae): The role of morphology and ontogeny. Mar. Freshw. Res. 2012, 63, 128–141. [Google Scholar] [CrossRef]
- Berchtoldi, A.E.; Colborne, S.F.; Longsstaffe, F.J.; Neff, B.D. Ecomorphological patterns linking morphology and diet across three populations of Pumpkinseed sunfish (Lepomis gibbosus). Can. J. Zool. 2015, 93, 289–297. [Google Scholar] [CrossRef]
- Wainwright, P.C. Ecomorphology: Experimental functional anatomy for ecological problems. Am. Zool. 1991, 31, 680–693. [Google Scholar] [CrossRef]
- Elliott, J.P.; Bellwood, D.R. Alimentary tract morphology and diet in three coral reef fish families. J. Fish Biol. 2003, 63, 1598–1609. [Google Scholar] [CrossRef]
- Sullam, K.E.; Dalton, C.M.; Russell, J.A.; Kilham, S.S.; El-Sabaawi, R.; German, D.P.; Flecker, A.S. Changes in digestive traits and body nutritional composition accommodate a trophic niche shift in Trinidadian guppies. Oecologia 2015, 177, 245–257. [Google Scholar] [CrossRef]
- McCormick, S.D. Evolution of the hormonal control of animal performance: Insights from the seaward migration of salmon. Integr. Comp. Biol. 2009, 49, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Ojima, D.; Iwata, M. Central administration of growth hormone-releasing hormone and corticotropin-releasing hormone stimulate downstream movement and thyroxine secretion in fall-smolting coho salmon (Oncorhynchus kisutch). Gen. Comp. Endocrin. 2010, 168, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Duarte, V.; Gaetano, P.; Striberny, A.; Hazlerigg, D.; Jørgensen, E.H.; Fuentes, J.; Campinho, M.A. Modulation of intestinal growth and differentiation by photoperiod and dietary treatment during smoltification in Atlantic salmon (Salmo salar L.). Aquaculture 2023, 566, 739164. [Google Scholar] [CrossRef]
- Yeda, T.; Iguchi, K.; Yamamoto, S.; Sakano, H.; Takasawa, T.; Katsura, K.; Abe, N.; Aawata, S.; Uchida, K. Prolactin and upstream migration of the amphidromous teleost, ayu Plecoglossus altivelis. Zool. Scr. 2014, 31, 507–514. [Google Scholar] [CrossRef][Green Version]
- Regish, A.M.; Ardren, W.R.; Staats, N.R.; Bouchard, H.; Withers, J.L.; Castro-Santos, T.; McCormick, S.D. Surface water with more natural temperatures promotes physiological and endocrine changes in landlocked Atlantic salmon smolts. Can. J. Fish. Aquat. Sci. 2021, 78, 775–786. [Google Scholar] [CrossRef]
- Axelrod, C.J.; Laberge, F.C.; Robinson, B.W. Isolating the effects of ontogenetic niche shift on brain size development using pumpkinseed sunfish ecotypes. Evol. Dev. 2020, 22, 312–322. [Google Scholar] [CrossRef]
- Salas, C.A.; Yopak, K.E.; Warrington, R.E.; Hart, N.S.; Potter, I.C.; Collin, S.P. Ontogenetic shifts in brain scaling reflect behavioral changes in the life cycle of the pouched lamprey Geotria australis. Front. Neurosci. 2015, 9, 251. [Google Scholar] [CrossRef]
- Lisney, T.; Wagner, H.J.; Collin, S.P. Ontogenetic shifts in the number of axons in the olfactory tract and optic nerve in two species of deep-sea grenadier fish (Gadiformes: Macrouridae: Coryphaenoides). Front. Ecol. Evol. 2018, 6, 168. [Google Scholar] [CrossRef]
- Økland, F.; Jonsson, B.; Jensen, A.J.; Hansen, L.P. Is there a threshold size regulating smolt size in brown trout and Atlantic salmon? J. Fish Biol. 1993, 42, 541–550. [Google Scholar] [CrossRef]
- Roff, D.A. Evolution of Life Histories: Theory and Analysis; Chapman & Hall: New York, NY, USA, 1992. [Google Scholar]
- Werner, E.E.; Gilliam, J.F. The ontogenetic niche and species interactions in size-structured populations. Ann. Rev. Ecol. Syst. 1984, 15, 393–425. [Google Scholar] [CrossRef]
- Fonseca, V.F.; Cabral, H.N. Are fish early growth and condition patterns related to life-history strategies? Rev. Fish Biol. Fish. 2007, 17, 545–564. [Google Scholar] [CrossRef]
- Jonsson, B.; Hindar, K.; Northcote, T.G. Optimal age at sexual maturity of sympatric and experimentally allopatric cutthroat trout and Dolly Varden charr. Oecologia 1984, 61, 319–325. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Partial migration: Niche shift versus sexual maturation in fishes. Rev. Fish Biol. Fish. 1993, 3, 348–365. [Google Scholar] [CrossRef]
- Jensen, A.L. Origin of the relation between K and Linf and synthesis of relations among life history parameters. Can. J. Fish. Aquat. Sci. 1997, 54, 987–989. [Google Scholar] [CrossRef]
- Rossignol, O.; Dodson, J.J.; Guderley, H. Relationship between metabolism, sex and reproductive tactics in young Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 159, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Froese, R.; Binohlan, C. Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish Biol. 2000, 56, 758–773. [Google Scholar] [CrossRef]
- Rien, T.A.; Beamesderfer, R.C. Accuracy and precision of white sturgeon age estimates from pectoral fin rays. Trans. Am. Fish. Soc. 1994, 123, 255–265. [Google Scholar] [CrossRef]
- Jonsson, N.; Hansen, L.P.; Jonsson, B. Variation in age, size and repeat spawning of adult Atlantic salmon in relation to river discharge. J. Anim. Ecol. 1991, 60, 937–947. [Google Scholar] [CrossRef]
- Rideout, R.M.; Rose, G.A.; Burton, M. Skipped spawning in female iteroparous fish. Fish Fish. 2005, 6, 50–72. [Google Scholar] [CrossRef]
- Bell, G. The costs of reproduction and their consequences. Am. Nat. 1980, 116, 45–76. [Google Scholar] [CrossRef]
- Reznick, D.N. Cost of reproduction: An evaluation of the empirical evidence. Oikos 1985, 44, 257–267. [Google Scholar] [CrossRef]
- Sneider, J. Energy balance and reproduction. Physiol. Behav. 2004, 81, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Ginther, S.C.; Cameron, H.; White, C.R.; Marshall, D.J. Metabolic loads and the cost of metazoan reproduction. Science 2024, 384, 763–767. [Google Scholar] [CrossRef]
- Jonsson, B. Life history patterns of freshwater resident and sea-run migrant brown trout in Norway. Trans. Am. Fish. Soc. 1985, 114, 182–194. [Google Scholar] [CrossRef]
- Lothian, A.J.; Rodger, J.; Wilkie, L.; Walters, M.; Miller, R.; Conroy, C.; Marshall, S.; MacKenzie, M.; Adams, C.E. Smolting in post-sexually mature male Atlantic salmon (Salmo salar L.) parr in the wild. Ecol. Freshw. Fish 2024, 33, e12755. [Google Scholar] [CrossRef]
- Rochet, M.J. A comparative approach to life history strategies and tactics among four orders of teleost fish. ICES J. Mar. Sci. 2000, 57, 228–239. [Google Scholar] [CrossRef]
- Scarnecchia, D.L.; Ryckman, L.F.; Lim, Y.; Power, G.J.; Schmitz, B.J.; Firehammer, J.A. Life-history and the costs of reproduction in Northern Great Plains paddlefish (Polyodon spathula) as a potential framework for other Acipenseriform fishes. Rev. Fish. Sci. 2007, 15, 211–263. [Google Scholar] [CrossRef]
- Kuparinen, A.; Hardie, D.C.; Hutchings, J.A. Evolutionary and ecological feedbacks of the survival cost of reproduction. Evol. Appl. 2011, 5, 245–255. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Fraser, N.H.C.; Burns, M.D. Food availability and the nocturnal vs. diurnal foraging trade-off in juvenile salmon. J. Anim. Ecol. 1999, 68, 371–381. [Google Scholar] [CrossRef]
- Jonsson, N.; Jonsson, B.; Hansen, L.P. Changes in proximate composition and estimates of energetic costs during upstream migration and spawning in Atlantic salmon Salmo salar. J. Anim. Ecol. 1997, 66, 425–436. [Google Scholar] [CrossRef]
- Glebe, B.D.; Leggett, W.C. Latitudinal differences in energy allocation and use during the freshwater migrations of American shad (Alosa sapidissima) during the spawning migration. Can. J. Fish. Aquat. Sci. 1981, 38, 806–820. [Google Scholar] [CrossRef]
- Daufresne, F.; FitzGerald, G.J.; Lachance, S. Age and size-related differences in reproductive success and reproductive costs in threespine sticklebacks (Gasterosteus aculeatus). Behav. Ecol. 1990, 1, 140–147. [Google Scholar] [CrossRef]
- Pauly, D. Female fish grow bigger: Let’s deal with it. Trends Ecol. Evol. 2018, 34, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, B. Life history and habitat use of Norwegian brown trout (Salmo trutta). Freshw. Biol. 1989, 21, 71–86. [Google Scholar] [CrossRef]
- Fleming, I.A. Reproductive strategies of Atlantic salmon: Ecology and evolution. Rev. Fish Biol. Fish. 1996, 6, 379–416. [Google Scholar] [CrossRef]
- Schaffer, W.M.; Elson, P.F. The adaptive significance of variations in life history among local populations of Atlantic salmon in North America. Ecology 1975, 56, 577–590. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Sexual size dimorphism in anadromous brown trout Salmo trutta. J. Fish Biol. 2015, 87, 187–193. [Google Scholar] [CrossRef]
- Gross, M.R. Alternative reproductive strategies and tactics: Diversity within sexes. Trends Ecol. Evol. 1996, 11, 92–98. [Google Scholar] [CrossRef]
- Babu, P.P.S.; Rao, P.S.; Prasad, J.K.; Sharma, R.; Rani, A.M.B.; Biju, I.F. Observations on impact of stunting on breeding performance of farmed rohu Labeo rohita (Hamilton, 1822). Ind. J. Fish. 2021, 68, 117–121. [Google Scholar]
- Jonsson, N.; Jonsson, B. Trade-off between egg size and numbers in brown trout. J. Fish Biol. 1999, 55, 767–783. [Google Scholar]
- Wood, C.C.; Foote, C.J. Evidence for sympatric genetic divergence of anadromous and nonanadromous morphs of sockeye salmon (Oncorhynchus nerka). Evolution 1996, 50, 1265–1279. [Google Scholar] [PubMed]
- Jonsson, B.; Jonsson, N. Polymorphism and speciation in Arctic charr. J. Fish Biol. 2001, 58, 605–638. [Google Scholar] [CrossRef]
- Parker, H.H.; Noonburg, E.G.; Nisbet, R.M. Models of alternative life-history strategies, population structure and potential speciation in salmonid fish stocks. J. Anim. Ecol. 2001, 70, 260–272. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Lipid energy reserves influence life-history decision of Atlantic salmon (Salmo salar) and brown trout (S. trutta) in fresh water. Ecol. Freshw. Fish 2005, 14, 296–301. [Google Scholar] [CrossRef]
- Aday, D.D.; Kush, C.M.; Wahl, D.H.; Phillip, D.P. The influence of stunted body size on the reproductive ecology of bluegill Lepomis macrochirus. Ecol. Freshw. Fish 2002, 11, 190–195. [Google Scholar] [CrossRef]
- Santiago, C.B.; Gonzales, A.C.; Aralar, E.V.; Arcilla, R.P. Effect of stunting of juvenile bighead carp Aristichthys nobilis (Richardson) on compensatory growth and reproduction. Aquacult. Res. 2004, 35, 836–841. [Google Scholar] [CrossRef]
- Blanck, A.; Tedesco, P.A.; Lamouroux, N. Relationships between life-history strategies of European freshwater fish species and their habitat preferences. Freshw. Biol. 2007, 52, 843–859. [Google Scholar] [CrossRef]
- Aykanat, T.; Debes, P.V.; Jansouz, S.; Gueguen, L.; House, A.H.; Roukolainen, A.; Erkinaro, J.; Prichard, V.L.; Primmer, C.R.; Bolstad, G.H. Large effect life-history genomic regions are associated with functional morphological traits in Atlantic salmon. G3 Genes Genomes Genet. 2025, 15, jkaf106. [Google Scholar] [CrossRef]
- Barson, N.J.; Aykanat, T.; Hindar, K.; Baranski, M.; Bolstad, G.H.; Fiske, P.; Jacq, C.; Jensen, A.J.; Johnston, S.E.; Karlsson, S.; et al. Sex dependent dominance at a single locus maintains variation in age at maturity in salmon. Nature 2015, 528, 405–408. [Google Scholar] [CrossRef]
- Pritchard, V.L.; Makinen, H.; Vaha, J.P.; Erkinaro, J.; Orell, P.; Primmer, C.R. Genomic signatures of fine-scale local selection in Atlantic salmon suggest involvement of sexual maturation, energy homeostasis and immune defence-related genes. Mol. Ecol. 2018, 27, 2560–2575. [Google Scholar] [CrossRef]
- Bangura, P.B.; Tiira, K.; Niemela, P.T.; Erkinaro, J.; Liljeström, P.; Toikkanen, A.; Primmer, C.R. Linking vgll3 genotype and aggressive behaviour in juvenile Atlantic salmon (Salmo salar). J. Fish Biol. 2022, 100, 1264–1271. [Google Scholar] [CrossRef]
- Prokkola, J.M.; Åsheim, E.R.; Morozov, S.; Bangura, P.; Erkinaro, J.; Ruokolainen, A.; Primmer, C.R.; Aykanat, T. Genetic coupling of life-history and aerobic performance in Atlantic salmon. Proc. R. Soc. B 2022, 289, 20212500. [Google Scholar] [CrossRef]
- Fraley, K.M.; Warburton, H.J.; Jellyman, P.G.; Kelly, D.; McIntosh, A.R. Do body mass and habitat factors predict trophic position in temperate stream fishes? Freshw. Sci. 2020, 39, 405–414. [Google Scholar] [CrossRef]
- Arim, M.; Abades, S.R.; Laufer, G.; Loureiro, M.; Marquet, P.A. Food web structure and body size: Trophic position and resource acquisition. Oikos 2010, 119, 147–153. [Google Scholar] [CrossRef]
- Ou, C.; Montaña, C.; Winemiller, K.O. Body size—Trophic position relationships among fishes of the lower Mekong basin. R. Soc. Open Sci. 2017, 4, 160645. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.M.; Brose, U.; Scheu, S.; Tiunov, A.V. Trophic position of consumers and size structure of food webs across aquatic and terrestrial ecosystems. Am. Nat. 2019, 194, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Kopf, R.K.; McPhan, L.; McInerney, P.J.; Zampatti, B.; Thiem, J.; Koster, W.; Butler, L.G.; Bond, N.; Thompson, R.M. Intraspecific body size determines isotopic trophic structure of a large river fish community. J. Anim. Ecol. 2025, 94, 1435–1448. [Google Scholar] [CrossRef]
- Hallbert, T.B.; Keeley, E.R. Allometric shifts in foraging site selection and area increase energy intake for Yellowstone cutthroat trout but are constrained by functional limits to prey capture. Trans. Am. Fish. Soc. 2024, 153, 660–673. [Google Scholar] [CrossRef]
- Osenberg, C.W.; Mittelbach, G.G. Effects on body size on the predator prey interaction between pumpkinseed sunfish and gastropods. Ecol. Monogr. 1989, 59, 405–432. [Google Scholar] [CrossRef]
- Olson, M.H.; Mittelbach, G.G.; Osenberg, C.W. Competition between predator and prey—Resource-based mechanisms and implications for stage-structured dynamics. Ecology 1995, 76, 1758–1771. [Google Scholar] [CrossRef]
- Matte, J.M.O.; Fraser, D.J.; Grant, J.W.A. Mechanisms of density dependence in juvenile salmonids: Prey depletion, interference competition, or energy expenditure? Ecosphere 2021, 12, e0367. [Google Scholar] [CrossRef]
- Pauly, D. Why do fish reach first maturity when they do? J. Fish Biol. 2022, 101, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Alm, G. Connection between maturity, size and age in fishes. Inst. Freshw. Res. Rep. 1959, 40, 4–145. [Google Scholar]
- Rowe, D.; Thorpe, J.E. Difference in growth between maturing and nonmaturing male Atlantic salmon, Salmo salar. J. Fish Biol. 1990, 36, 643–658. [Google Scholar] [CrossRef]
- Taylor, B.M.; Choat, J.H.; DeMartini, E.E.; Hoey, A.S.; Mershell, A.; Priest, M.S.; Rhodes, K.L.; Meekan, M.G. Demographic plasticity facilitates ecological and economic resilience in a commercially important reef fish. J. Anim. Ecol. 2019, 88, 1888–1900. [Google Scholar] [CrossRef]
- Melo, M.; Anderssson, E.; Fjelldal, P.G.; Bogerd, J.; França, L.R.; Taranger, G.L.; Schulz, R.W. Salinity and photoperiod modulate pubertal development in Atlantic salmon (Salmo salar). J. Endocrinol. 2014, 220, 319–332. [Google Scholar] [CrossRef]
- Good, C.; Davidson, J. A review of factors influencing maturation of Atlantic salmon, Salmo salar, with focus on water recirculation aquaculture system environments. World Aquacult. Soc. 2016, 47, 605–632. [Google Scholar] [CrossRef]
- Crouse, C.; Davidson, J.; Good, C. The effects of two water temperature regimes on Atlantic salmon (Salmo salar) growth performance and maturation in freshwater recirculating aquaculture systems. Aquaculture 2022, 553, 738063. [Google Scholar] [CrossRef]
- Schaffer, W.M. Optimal reproductive effort in fluctuating environments. Am. Nat. 1974, 108, 783–790. [Google Scholar] [CrossRef]
- Charnov, E.L. Gompertz mortality, natural selection, and the ‘shape of ageing’. Evol. Ecol. Res. 2014, 16, 435–439. [Google Scholar]
- Steiner, U.K.; Tuljapurkar, S.; Coulson, T. Generation time, net reproductive rate, and growth in stage-age structured populations. Am. Nat. 2014, 183, 771–783. [Google Scholar] [CrossRef]
- Forseth, T.; Næsje, T.F.; Jonsson, B.; Hårsaker, K. Juvenile migration in brown trout: A consequence of energetic state. J. Anim. Ecol. 1999, 68, 783–793. [Google Scholar] [CrossRef]
- Alioravainen, N.; Orell, P.; Erkinaro, J. Long-term trends in freshwater and marine growth patterns in three sub-Arctic Atlantic salmon populations. Fishes 2023, 8, 441. [Google Scholar] [CrossRef]
- Berglund, I.; Hansen, L.P.; Lundqvist, H.; Jonsson, B.; Eriksson, T.; Thorpe, J.E.; Eriksson, L.O. Effects of elevated winter temperature on seawater adaptability, sexual rematuration and downstream migratory behaviour in mature male Atlantic salmon parr (Salmo salar). Can. J. Fish. Aquat. Sci. 1991, 48, 1041–1047. [Google Scholar] [CrossRef]
- Nevoux, M.; Finstad, B.; Davidsen, J.G.; Finlay, R.; Josset, Q.; Poole, R.; Höjesjö, J.; Aarestrup, K.; Persson, L.; Tolvanen, O.; et al. Environmental influences of life history strategies in partial anadromous brown trout (Salmo trutta, Salmonidae). Fish Fish. 2019, 20, 1051–1082. [Google Scholar] [CrossRef]
- Jonsson, B.; Finstad, A.G.; Jonsson, N. Winter temperature and food quality affect age and size at maturity in ectotherms: An experimental test with Atlantic salmon. Can. J. Fish Aquat. Sci. 2012, 69, 1817–1826. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Finstad, A.G. Effects of temperature and food quality on age at maturity of ectotherms: An experimental test of Atlantic salmon. J. Anim. Ecol. 2013, 82, 201–210. [Google Scholar] [CrossRef]
- Åsheim, E.R.; Debes, P.V.; House, A.; Liljeström, P.; Niemelä, P.T.; Siren, J.; Erkinaro, J.; Primmer, C.R. Atlantic salmon (Salmo salar) age at maturity is strongly affected by temperature, population, and age-at-maturity genotype. Cons. Physiol. 2023, 11, coac86. [Google Scholar] [CrossRef]
- Killen, S.S.; Glazier, D.S.; Rezende, E.L.; Clark, T.D.; Atkinson, D.; Willener, A.S.T.; Halsey, L.G. Ecological influences and morphological correlates of resting and maximal metabolic rates across teleost fish species. Am. Nat. 2016, 187, 592–606. [Google Scholar] [CrossRef]
- Wang, S.; Bigman, J.S.; Dolvy, N.K. The metabolic pace of life histories across fishes. Proc. Biol. Sci. 2021, 288, 20210910. [Google Scholar] [CrossRef]
- Thorpe, J.E. Age at first maturity in Atlantic salmon, Salmo salar: Freshwater period influences and conflicts with smolting. Can. Spec. Publ. Fish. Aquat. Sci. 1986, 89, 7–14. [Google Scholar]
- Edwards, R.W. The relation of oxygen consumption to body size and to temperature in the larvae of Chironomus riparius Meigen. J. Exp. Biol. 1958, 35, 383–395. [Google Scholar] [CrossRef]
- Imsland, A.K. Sexual maturation in turbot (Scophthalmus maximus) is related to genotypic oxygen affinity: Experimental support for Pauly’s juvenile-to-adult transition hypothesis. ICES J. Mar. Sci. 1999, 56, 320–325. [Google Scholar] [CrossRef]
- Amarasinghe, U.S.; Pauly, D. The relationship between size at maturity and maximum size in cichlid populations corroborates the gill-oxygen limitation theory (GOLT). Asian Fish. Sci. 2021, 34, 14–22. [Google Scholar] [CrossRef]
- Czorlich, Y.; Aykanat, T.; Erkinaro, J.; Orell, P.; Primmer, C.R. Rapid sex specific evolution of age at maturity is shaped by genetic architecture in Atlantic salmon. Nat. Evol. Ecol. 2018, 2, 1800–1807. [Google Scholar] [CrossRef]
- Yuan, R.; Hascup, E.; Hascup, K.; Bartke, A. Relationships among development, growth, body size, reproduction, aging, and longevity—Trade-offs and pace-of-life. Biochemistry 2023, 88, 1692–1703. [Google Scholar]
- Mousseau, T.A.; Roff, D.A. Natural selection and the heritability of fitness components. Heredity 1987, 59, 181–197. [Google Scholar] [CrossRef]
- McFarlane, S.E.; Gorrell, J.C.; Coltman, D.W.; Humphries, M.M.; Boutin, S.; McAdam, A.G. Very low levels of direct additive genetic variance in fitness and fitness components in a red squirrel population. Ecol. Evol. 2014, 4, 1729–1738. [Google Scholar] [CrossRef]
- Aykanat, T.; Ozerov, M.; Vähä, J.P.; Orell, P.; Niemelä, E.; Erkinaro, J.; Primmer, C.R. Co-inheritance of age at maturity and iteroparity in the Atlantic salmon vgll3 genomic region. J. Evol. Biol. 2019, 32, 343–355. [Google Scholar] [CrossRef]
- House, H.; Debes, P.V.; Holopainen, M.; Käkelä, R.; Donner, I.; Frapin, M.; Ahi, E.P.; Kurko, J.; Ruhanen, H.; Primmer, C.R. Seasonal and genetic effects on lipid profiles of juvenile Atlantic salmon. Biochim. Biophys. Mol. Cell Biol. Lip. 2025, 1870, 159565. [Google Scholar] [CrossRef]
- Oplinger, R.W.; Wahl, D.H.; Philip, D.P. Parental influence on the size and age at maturity of bluegills. Trans. Am. Fish. Soc. 2013, 142, 1067–1074. [Google Scholar] [CrossRef]
- Chavanne, H.; Jansen, K.; Hofherr, J.; Contini, F.; Haffray, P.; Komen, H.; Nielsen, E.E.; Bargelloni, L. A comprehensive survey on selective breeding programs and seed market in the European aquaculture fish industry. Aquacult. Int. 2016, 24, 1287–1307. [Google Scholar] [CrossRef]
- Gjedrem, T.; Rye, M. Selection response in fish and shellfish: A review. Rev. Aquacult. 2018, 10, 168–179. [Google Scholar] [CrossRef]
- Devlin, R.H.; Leggatt, R.A.; Benfey, T.J. Genetic modification of growth in fish species used in aquaculture: Phenotypic and physiological responses. In Fish Physiology; Farrell, A.P., Brenner, C.J., Benfey, T.J., Eds.; Academic Press: New York, NY, USA, 2020; Volume 38, pp. 237–272. [Google Scholar]
- Walsh, M.R.; Munch, S.B.; Chiba, S.; Conover, D.O. Maladaptive changes in multiple traits caused by fishing: Impediments to population recovery. Ecol. Lett. 2006, 9, 142–148. [Google Scholar] [CrossRef]
- Jonsson, N.; Jonsson, N.; Hansen, L.P. Does climate during embryonic development influences parr growth and age of seaward migration in Atlantic salmon (Salmo salar) smolts? Can. J. Fish. Aquat. Sci. 2005, 62, 2502–2508. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Phenotypic plasticity and epigenetics of fish: Embryo temperature affects later developing traits. Aquat. Biol. 2019, 28, 21–32. [Google Scholar] [CrossRef]
- Murugananthkumar, R.; Sudhakumari, C.C. Understanding the impact of stress on teleostean reproduction. Aquacult. Fish. 2022, 7, 553–561. [Google Scholar] [CrossRef]
- Kho, J.; Ruzzante, D.E. The role of DNA methylation in facilitating life history trait diversity in fishes. Rev. Fish. Biol. Fish. 2024, 34, 1531–1566. [Google Scholar] [CrossRef]
- Lee, W.S.; Monaghan, P.; Metcalfe, N.B. The pattern of early growth trajectories affects adult breeding performance. Ecology 2012, 93, 902–912. [Google Scholar] [CrossRef]
- Kim, S.Y.; Metcalfe, N.B.; da Silva, A.; Velando, A. Thermal conditions during early life influence seasonal maternal strategies in the three-spined stickleback. BMC Ecol. 2017, 17, 34. [Google Scholar] [CrossRef]
- Ramsteijn, A.S.; Ndiaye, M.; Kalashikam, R.R.; Htet, M.K.; Dm, D.Y.; Augustine, L.F.; Zahra, N.L.; Djigal, A.; Yanti, D.; Angelin, T.C.; et al. Epigenetic studies in children at risk of stunting and their parents in India, Indonesia and Sene and Senegal: A UKRI GCRF Action Against Stunting Hub protocol paper. BMJ Pediatr. Open 2024, 8 (Suppl. 1), e001770. [Google Scholar] [CrossRef]
- Morán, P.; Pérez-Figueroa, A. Methylation changes associated with early maturation stages in the Atlantic salmon. BMC Genet. 2011, 12, 86. [Google Scholar] [CrossRef]
- Liu, S.; Jonsson, B.; Greenberg, L.; Hansen, M.M. Limited persistence of temperature-induced methylation compared to significant parental effects in juvenile brown trout Salmo trutta. Ecol. Evol. 2025; accepted. [Google Scholar]
- Aday, D.D.; Wahl, D.H.; Philipp, D.P. Assessing population-specific and environmental influences on bluegill life histories: A common garden approach. Ecology 2003, 84, 3370–3375. [Google Scholar] [CrossRef]
- Fischer-Rousseau, L.; Clautier, R.; Zelditch, M. Morphological integration and developmental progress during fish ontogeny in two contrasting habitats. Evol. Dev. 2009, 11, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Sibly, R.M.; Baker, J.; Grady, J.M.; Brown, J.H. Fundamental insights into ontogenetic growth from theory and fish. Proc. Natl. Acad. Sci. USA 2015, 112, 13934–13939. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhou, T.; Gao, D.Y. Genetic and epigenetic regulation of growth, reproduction, disease resistance and stress responses in aquaculture. Front. Genet. 2022, 13, 994471. [Google Scholar] [CrossRef]
| Ecological Term | Explanation |
|---|---|
| Adaptation | (1) Characteristics of an organism that evolved as a consequence of natural selection in its evolutionary past and which result in a close match with features of the environment and/or constrain the organism to life in a narrow range of environments. (2) Changes in form and/or behaviour of an organism as a response to environmental stimuli. |
| Allostatic overload | The body’s stress response system being overwhelmed by prolonged or excessive stress. |
| Anadromous fish | Fish that find a major part of their food in salt water but breed in fresh water. |
| Assortative mating | Reproducing with phenotypically similar individuals. |
| Compensatory growth | Accelerated growth after a period of environmentally induced growth depression. |
| Conspecific | Of the same species. |
| Density-dependent growth | The rate at which individuals grow (or decline) is influenced by the population’s density. |
| Density-dependent mortality | The death rate is influenced by the density of the population. |
| Developmental trajectory | The course of development (growth and phenotypic change) that an individual follows over time. |
| DNA methylation | A genetic change where a methyl group (CH3) is added to a DNA molecule without altering the underlying DNA sequence. This process is a type of epigenetic modification that typically acts to repress gene transcription by turning genes off. |
| Energy budget | How obtained energy from food is used. |
| Environmental stressor | Variables in the surroundings that can cause negative impacts on the well-being or survival of organisms. |
| Epibenthic | Living on or near the bottom substratum. |
| Epigenetic | Heritable changes in gene expression that occur without altering the DNA sequence itself. |
| Exploitative competition | Competition in which any adverse effect on an organism is caused by a reduction in resource levels caused by its competitors. |
| Hypothalamic–pituitary–adrenal axis | A neuroendocrine pathway between the hypothalamus, pituitary gland, and adrenal glands that regulate stress responses of organisms. |
| Interference competition | Competition where organisms actively prevent others from accessing resources through physical interaction or aggressive behaviour. |
| Interspecific competition | Competition between individuals of different species. |
| Intraspecific competition | Competition between individuals of the same species. |
| Iteroparity | Repeated production of offspring at intervals during the life span. |
| Lentic | Living in still fresh water. |
| Limnetic | Living in the upper layers of a freshwater lake or pond away from the shore and the bottom. |
| Lotic | Living in running water. |
| Net reproductive rate | The average number of daughters a cohort of females produce given lifetime fertility and mortality rates. |
| Parental effect | The influence a parent has on its offspring’s phenotype in addition to the direct genetic effect. |
| Omnivorous | Feeding on both plants and animals. |
| Ontogenetic | Occurring during an organism’s development |
| Phenotypic plasticity | The ability of a single genotype to express itself in diverse ways in different environments. |
| Polymorphism | The occurrence of several phenotypes among the members of a population influenced by genetic differences. |
| Polyphenism | The occurrence of several phenotypes that is not a result of genetic differences but environmental conditions. |
| Speciation | Spitting of a phyletic line and multiplication of species. |
| Sympatric speciation | Speciation without geographic isolation. |
| Sympatry | The presence of two or more populations in the same environment so they can potentially interact. |
| Trophic bottleneck | The availability of a specific food resource, or prey type, limits the growth and survival of a predator population, and thereby restricts the energy flow at a particular trophic level within an ecosystem. |
| Character | Stunted Compared with Non-Stunted Conspecifics |
|---|---|
| Relative head size | Larger |
| Relative length of mid-body | Shorter |
| Secondary sexual characters | Less pronounced |
| Fecundity | Lower |
| Absolute reproductive effort | Smaller |
| Feeding habitat | Juvenile like |
| Camouflage colours | Juvenile like |
| Adult size | Smaller |
| Growth rate | Lower |
| Age at maturity | Younger |
| Length of life span | Shorter |
| Movements | Less migratory |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonsson, B. Stunted Versus Normally Growing Fish: Adapted to Different Niches. Fishes 2025, 10, 376. https://doi.org/10.3390/fishes10080376
Jonsson B. Stunted Versus Normally Growing Fish: Adapted to Different Niches. Fishes. 2025; 10(8):376. https://doi.org/10.3390/fishes10080376
Chicago/Turabian StyleJonsson, Bror. 2025. "Stunted Versus Normally Growing Fish: Adapted to Different Niches" Fishes 10, no. 8: 376. https://doi.org/10.3390/fishes10080376
APA StyleJonsson, B. (2025). Stunted Versus Normally Growing Fish: Adapted to Different Niches. Fishes, 10(8), 376. https://doi.org/10.3390/fishes10080376






