Study on the Reproductive Group Behavior of Schizothorax wangchiachii Based on Acoustic Telemetry
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Methods
2.2.1. Acoustic Tags and Receivers
2.2.2. Experimental Fish and Tagging Methods
2.2.3. Experimental Process Record
2.3. Data Processing and Analysis
2.3.1. Trajectory Data Preprocessing
2.3.2. Individual Trajectory Similarity Analysis
2.3.3. Fish Schooling Behavior Characteristics Analysis
- (1)
- Activity Feature Extraction and Active State Identification
- (2)
- Wavelet Transform and Dominant Period Extraction
- (3)
- Quantification of Active Time and Aggregation States
3. Results
3.1. Trajectory Similarity Clustering Results
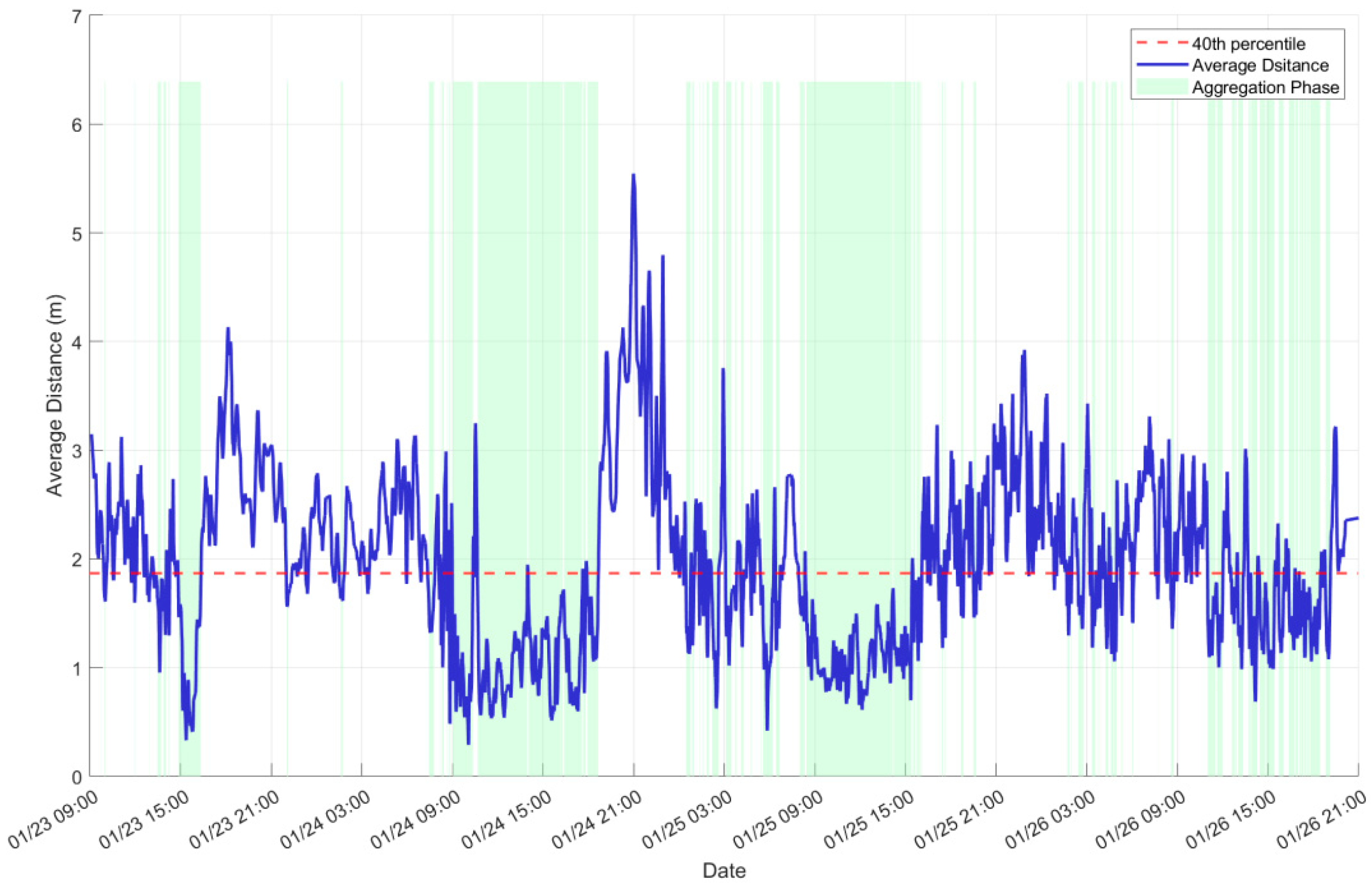
3.2. Periodicity of Aggregation Behavior
3.3. Behavioral Activity Characteristics of the Fish Group
4. Discussion
4.1. Effects of Tagging on the Normal Activity of Schizothorax wangchiachii
4.2. Fish School Structure and Subgroup Dynamics
4.3. Reproductive Behavior of S. wangchiachii
4.4. Schooling and Activity Rhythms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitcher, T.J.; Parrish, J.K. Functions of shoaling behaviour in teleosts. In The Behaviour of Teleost Fishes; Springer: Dordrecht, The Netherlands, 1993; pp. 294–337. [Google Scholar] [CrossRef]
- Danylchuk, A.J.; Adams, A.J.; Horodysky, A.Z.; Suski, C.D.; Ward-Paige, C.A.; Brooks, E.J.; Cooke, S.J. Ecological and management implications of gear interactions for Nassau grouper spawning aggregations. Rev. Fish Biol. Fish. 2011, 21, 311–325. [Google Scholar] [CrossRef]
- Shi, L.; Ye, S.W.; Zhu, H.; Ji, X.; Wang, J.C.; Liu, C.A.; Liu, X.G. The spatial-temporal distribution of fish in lake usingacoustic tagging and tracking method. Acta Hydrobiol. Sin. 2022, 46, 611–620. [Google Scholar]
- Wang, C.Y.; Wei, Q.W.; Du, H.; Zhang, H.; Liu, Z.G. Applications of ultrasonic telemetry in aquatic animal ecology. Chin. J. Ecol. 2010, 29, 2286–2292. [Google Scholar]
- Cooke, S.J.; Wagner, G.N. Training, Experience, and Opinions of Researchers Who Use Surgical Techniques to Implant Telemetry Devices into Fish. Fisheries 2004, 29, 10–18. [Google Scholar] [CrossRef]
- Welch, D.W.; Ward, B.R.; Batten, S.D. Early ocean survival and marine movements of hatchery and wild steelhead trout (Oncorhynchus mykiss) determined by an acoustic array: Queen Charlotte Strait, British Columbia. Deep. Sea Res. Part Ⅱ Top. Stud. Oceanogr. 2004, 51, 897–909. [Google Scholar] [CrossRef]
- Hinch, S.G.; Standen, E.M.; Healey, M.C.; Farrell, A.P. Swimming patterns and behaviour of upriver-migrating adult pink (Oncorhynchus gorbuscha) and sockeye (O. nerka) salmon as assessed by EMG telemetry in the Fraser River, British Columbia, Canada. In Aquatic Telemetry; Springer: Dordrecht, The Netherlands, 2002; pp. 147–160. [Google Scholar] [CrossRef]
- Sulak, K.J.; Randall, M.T.; Edwards, R.E.; Summers, T.M.; Luke, K.E.; Smith, W.T.; Norem, A.D.; Harden, W.M.; Lukens, R.H.; Parauka, F.; et al. Defining winter trophic habitat of juvenile Gulf Sturgeon in the Suwannee and Apalachicola rivermouth estuaries, acoustic telemetry investigations. J. Appl. Ichthyol. 2009, 25, 505–515. [Google Scholar] [CrossRef]
- Yano, A.; Ogura, M.; Sato, A.; Sakaki, Y.; Ban, M.; Nagasawa, K. Development of Ultrasonic Telemetry Technique for Investigating the Magnetic of Salmonids. Fish. Sci. 1996, 62, 698–704. [Google Scholar] [CrossRef][Green Version]
- Ogura, M.; Ishida, Y. Homing behavior and vertical movements of four species of Pacific salmon (Oncorhynchus spp.) in the central Bering Sea. Can. J. Fish. Aquat. Sci. 1995, 52, 532–540. [Google Scholar] [CrossRef]
- Cazelles, B.; Chavez, M.; Berteaux, D.; Ménard, F.; Vik, J.O.; Jenouvrier, S.; Stenseth, N.C. Wavelet analysis of ecological time series. Oecologia 2008, 156, 287–304. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Wang, S.Y.; Leclair, O.U.; Danilova, N.P. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001, 903, 263–268. [Google Scholar] [CrossRef]
- Sakoe, H.; Chiba, S. Dynamic Programming Algorithm Optimization for Spoken Word Recognition. In Readings in Speech Recognition; Elsevier: Amsterdam, The Netherlands, 1990; pp. 159–165. [Google Scholar] [CrossRef]
- Nathan, R.; Getz, W.M.; Revilla, E.; Holyoak, M.; Kadmon, R.; Saltz, D.; Smouse, P.E. A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. USA 2008, 105, 19052–19059. [Google Scholar] [CrossRef]
- Gallarda, B.W.; Sharpee, T.O.; Pfaff, S.L.; Alaynick, W.A. Defining rhythmic locomotor burst patterns using a continuous wavelet transform. Ann. N. Y. Acad. Sci. 2010, 1198, 133–139. [Google Scholar] [CrossRef]
- Leise, T.L. Wavelet analysis of circadian and ultradian behavioral rhythms. J. Circadian Rhythm. 2013, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, M.; Kollios, G.; Gunopulos, D. Discovering similar multidimensional trajectories. In Proceedings of the 18th International Conference on Data Engineering, San Jose, CA, USA, 26 February–1 March 2002. [Google Scholar] [CrossRef]
- Chen, L.; Özsu, M.T.; Oria, V. Robust and fast similarity search for moving object trajectories. In Proceedings of the 2005 ACM SIGMOD International Conference on Management of Data. SIGMOD/PODS05: International Conference on Management of Data and Symposium on Principles Database and Systems, Baltimore, MD, USA, 14 June 2005. [Google Scholar] [CrossRef]
- Dodge, S.; Weibel, R.; Lautenschütz, A.-K. Towards a taxonomy of movement patterns. Inf. Vis. 2008, 7, 240–252. [Google Scholar] [CrossRef]
- Wu, X.W. Monograph on Cyprinidae Fishes of China; Shanghai Scientific and Technical Press: Shanghai, China, 1982. [Google Scholar]
- Yan, W.B. Studies on Reproductive Behavioural Ecology of Schizothorax wangchiachii; Shanghai Ocean University: Shanghai, China, 2016. [Google Scholar]
- Rong, Y.F.; Ban, X.; Zhou, Y.H.; Liu, W.C.; Qi, H.F.; Yang, J.X.; Zhang, H.; Zhou, W.G.; Yu, L.X.; Du, H. The spawning habitat characteristics of Gymnocypris przewalskii and its identification by the UAV in tributary of Qinghai Lake—Taking Quanji River as an example. Acta Ecol. Sin. 2022, 42, 9371–9382. [Google Scholar] [CrossRef]
- Qing, J.; Cheng, B.X.; Yan, X.; Yang, S.R.; Zhu, D.Z. Discussion on key technical problems of fish habitat ecological restoration in Heishui River. In Proceedings of the 2019 Annual Science and Technology Conference of the Chinese Society for Environmental Sciences—Sub-forum on Technological Innovation and Application in Environmental Engineering, Chengdu, China, 18–19 November 2019; Shanghai Investigation, Design & Research Institute Co., Ltd.: Shanghai, China; China Three Gorges Construction Management Co., Ltd.: Wuhan, China; Volume 3, pp. 154–157. [Google Scholar] [CrossRef]
- Teng, H.; Tian, H.-W.; Liu, H.-W.; Cheng, B.-X.; Yang, S.-R.; Liu, S.-P.; Chen, D.-Q.; Duan, X.-B. Fish resources status in Heishui River, a tributary of the lower reaches of Jinsha River. Chin. J. Ecol. 2021, 40, 1499–1511. [Google Scholar]
- Yang, Z.; Zhang, P.; Tang, H.Y.; Gong, Y.; Dong, C.; Chen, X.J.; Zhao, N. The formation of habitat suitability curves for Coreius guichenoti (Sauvage & Dabry de Thiersant, 1874) of the lower Jinsha River. Ecol. Sci. 2017, 36, 129–137. [Google Scholar] [CrossRef]
- Guillemette, M.; Woakes, A.J.; Flagstad, A.; Butler, P.J. Effects of Data-Loggers Implanted for a Full Year in Female Common Eiders. Condor 2002, 104, 448–452. [Google Scholar] [CrossRef]
- Bridger, C.J.; Booth, R.K. The Effects of Biotelemetry Transmitter Presence and Attachment Procedures on Fish Physiology and Behavior. Rev. Fish. Sci. 2003, 11, 13–34. [Google Scholar] [CrossRef]
- Luo, H.W.; Duan, X.B.; Liu, S.P.; Chen, D.Q. Effects of transmitter’s surgical implantation on fish: Research progress. Chin. J. Appl. Ecol. 2013, 24, 1160–1168. [Google Scholar] [CrossRef]
- Snyder, J.P. Professional Paper. In Map Projections: A Working Manual; U.S. Government Printing Office: Washington, DC, USA, 1987. [Google Scholar] [CrossRef]
- Pebesma, E. Simple Features for R: Standardized Support for Spatial Vector Data. R J. 2018, 10, 439. [Google Scholar] [CrossRef]
- Berndt, D.J.; Clifford, J. Using dynamic time warping to find patterns in time series. In Proceedings of the AAAI Workshop on Knowledge Discovery in Databases, Seattle, WA, USA, 31 July 1994; pp. 359–370. [Google Scholar]
- Hadi, A.S.; Kaufman, L.; Rousseeuw, P.J. Finding Groups in Data: An Introduction to Cluster Analysis. Technometrics 1992, 34, 111. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Couzin, I.D.; Krause, J.; Franks, N.R.; Levin, S.A. Effective leadership and decision-making in animal groups on the move. Nature 2005, 433, 513–516. [Google Scholar] [CrossRef]
- Addison, P.S. The illustrated wavelet transform handbook: Introductory theory and applications in science, engineering, medicine and finance, 2nd edition. Contemp. Phys. 2017, 59, 100. [Google Scholar] [CrossRef]
- Bode, N.W.F.; Faria, J.J.; Franks, D.W.; Krause, J.; Wood, A.J. How perceived threat increases synchronization in collectively moving animal groups. Proc. R. Soc. B Biol. Sci. 2010, 277, 3065–3070. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G.P. A Practical Guide to Wavelet Analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Vicsek, T.; Zafeiris, A. Collective motion. Phys. Rep. 2012, 517, 71–140. [Google Scholar] [CrossRef]
- Bousquet, C.A.H.; Sumpter, D.J.T.; Manser, M.B. Moving calls: A vocal mechanism underlying quorum decisions in cohesive groups. Proc. R. Soc. B Biol. Sci. 2010, 278, 1482–1488. [Google Scholar] [CrossRef]
- Krause, J.; Ruxton, G.D.; James, R. Important topics in group living. In Social Behaviour: Genes, Ecology and Evolution; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Ward, A.J.W.; Webster, M.M. Sociality: The Behaviour of Group-Living Animals; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Miyagi, A.; Amakasu, K.; Abe, K.; Imaizumi, T.; Miyamoto, Y.; Kakihara, T. Change of source level of pinger due to implanting into fish. Nippon Suisan Gakkaishi 2011, 77, 791–798. [Google Scholar] [CrossRef][Green Version]
- McCovey, B.W., Jr. Klamath river green sturgeon acoustic tagging and biotelemetry monitoring 2010. In Final Technical Report; Yurok Tribal Fisheries Program, Klamath River Division: Hoopa, CA, USA, 2011; pp. 1–15. [Google Scholar]
- Luo, H.; Duan, X.; Liu, S.; Chen, D. Effects of surgically implanted dummy ultrasonic transmitters on physiological response of bighead carp Hypophthalmichthys nobilis. Fish Physiol. Biochem. 2014, 40, 1521–1532. [Google Scholar] [CrossRef]
- Grothues, T.M. A review of acoustic telemetry technology and a perspective on its diversification relative to coastal tracking arrays. In Tagging and Tracking of Marine Animals with Electronic Devices; Nielsen, J.L., Arrizabalaga, H., Fragoso, N., Hobday, A., Lutcavage, M., Sibert, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 77–90. [Google Scholar]
- Zhou, Y.; Wang, J.; Qian, W.; Cao, D.; Zhang, Z.; Liu, L. Review of fish schooling behavior study. J. Shanghai Ocean. Univ. 2013, 22, 734–743. [Google Scholar]
- Partridge, B.L. Internal dynamics and the interrelations of fish schools. J. Comp. Physiol. A Neuroethol. 1981, 144, 313–325. [Google Scholar] [CrossRef]
- Parrish, J.K.; Viscido, S.V.; Grünbaum, D. Self-organized fish schools: An examination of emergent properties. Biol. Bull. 2002, 202, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Mamei, M.; Menezes, R.; Tolksdorf, R.; Zambonelli, F. Case studies for self-organization in computer science. J. Syst. Archit. 2006, 52, 443–460. [Google Scholar] [CrossRef]
- Couzin, I.D.; Krause, J. Self-organization and collective behavior in vertebrates. Adv. Study Behav. 2003, 32, 10–1016. [Google Scholar] [CrossRef]
- Gautrais, J.; Jost, C.; Theraulaz, G. Key behavioural factors in a self-organised fish school model. Ann. Zool. Fenn. 2008, 45, 415–428. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, Q. Study on the reproductive ecology biology of Sichuan cleft fish V: Reproductive groups and reproductive habits. J. Bijie Teach. Coll. 1997, 1, 1–5. [Google Scholar]
- Yan, W.; Zhu, T.; Wu, X.; Yang, D.; Chen, L. An observation of spawning behavior of Schizothorax wangchiachii. Freshw. Fish. 2017, 47, 9–15. [Google Scholar] [CrossRef]
- Ma, B.S.; Xie, C.X.; Huo, B.; Yang, X.F. Research progress on the biology of Schizothoracinae fishes. Jiangxi Fish. Sci. Technol. 2011, 128, 36–40. [Google Scholar]
- Zhou, X.J.; Xie, C.X.; Huo, B.; Duan, Y.J.; Yang, X. Reproductive biology of Schizothorax waltoni (Cyprinidae: Schizothoracinae) in the Yarlung Zangbo River in Tibet, China. Environ. Biol. Fishes 2015, 98, 597–609. [Google Scholar] [CrossRef]
- State Intellectual Property Office of China. Artificial Breeding Method for Schizothorax. China Patent Gazette CN1561704A, 2005. Granted on 1 July 2009. [Google Scholar]
- Reebs, S.G. Plasticity of diel and circadian activity rhythms in fishes. Rev. Fish Biol. Fish. 2002, 12, 349–371. [Google Scholar] [CrossRef]
- Pitcher, T.J.; Parrish, J.K. Functions of shoaling behaviour in teleosts. In The Behaviour of Teleost Fishes, 2nd ed.; Pitcher, T.J., Ed.; Chapman & Hall: London, UK, 1993; pp. 363–439. [Google Scholar]
- Boujard, T.; Leatherland, J.F. Circadian rhythms and feeding time in fishes. Environ. Biol. Fishes 1992, 35, 109–131. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Li, S.C.; Li, D.J. Ecology and Biology of Fishes on the Qinghai-Tibet Plateau; China Agriculture Press: Beijing, China, 2008. [Google Scholar]
- Jobling, M. Environmental Biology of Fishes; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Blaxter, J.H.S. The effect of temperature on larval fishes. Neth. J. Zool. 1991, 42, 336–357. [Google Scholar] [CrossRef]
- Zheng, Q.Y. Diel Activity Rhythms and Activity Intensity of Three Endemic Sinocyclocheilus Species in China. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2017. [Google Scholar]
- Krause, J.; Ruxton, G.D. Living in Groups; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
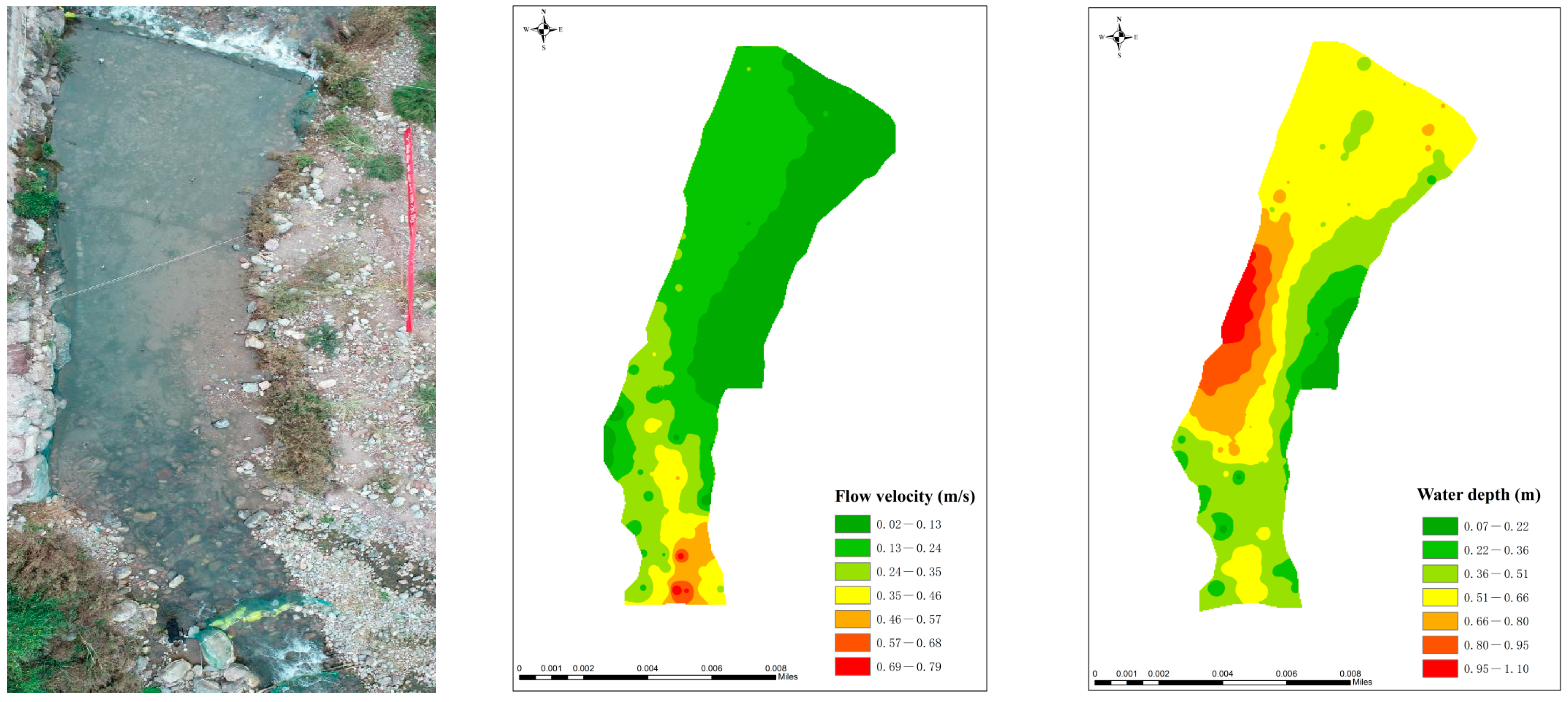





| No. | Tagging Number | Total Length (mm) | Body Length (mm) | Body Weight (g) | Gender |
|---|---|---|---|---|---|
| 1 | 7003.27 | 420 | 350 | 750 | Male ♂ |
| 2 | 7059.23 | 500 | 420 | 1100 | Male ♂ |
| 3 | 7101.20 | 490 | 395 | 1000 | Male ♂ |
| 4 | 7143.17 | 450 | 380 | 750 | Male ♂ |
| 5 | 7171.01 | 440 | 370 | 750 | Male ♂ |
| 6 | 7185.02 | 500 | 410 | 1150 | Male ♂ |
| 7 | 7199.03 | 470 | 380 | 1250 | Female ♀ |
| 8 | 7269.08 | 480 | 380 | 1000 | Female ♀ |
| 9 | 7339.14 | 480 | 410 | 950 | Female ♀ |
| 10 | 7353.15 | 498 | 420 | 1150 | Female ♀ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Hu, F.; Li, W.; Su, W.; Zhu, J.; Jiang, W. Study on the Reproductive Group Behavior of Schizothorax wangchiachii Based on Acoustic Telemetry. Fishes 2025, 10, 362. https://doi.org/10.3390/fishes10070362
Li B, Hu F, Li W, Su W, Zhu J, Jiang W. Study on the Reproductive Group Behavior of Schizothorax wangchiachii Based on Acoustic Telemetry. Fishes. 2025; 10(7):362. https://doi.org/10.3390/fishes10070362
Chicago/Turabian StyleLi, Bo, Fanxu Hu, Wenjing Li, Wei Su, Jiazhi Zhu, and Wei Jiang. 2025. "Study on the Reproductive Group Behavior of Schizothorax wangchiachii Based on Acoustic Telemetry" Fishes 10, no. 7: 362. https://doi.org/10.3390/fishes10070362
APA StyleLi, B., Hu, F., Li, W., Su, W., Zhu, J., & Jiang, W. (2025). Study on the Reproductive Group Behavior of Schizothorax wangchiachii Based on Acoustic Telemetry. Fishes, 10(7), 362. https://doi.org/10.3390/fishes10070362






