Eat First, Fight Later: Competitive Advantage of an Invasive Cichlid over a Native Competitor for Food Resources
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Acquisition and Holding Conditions
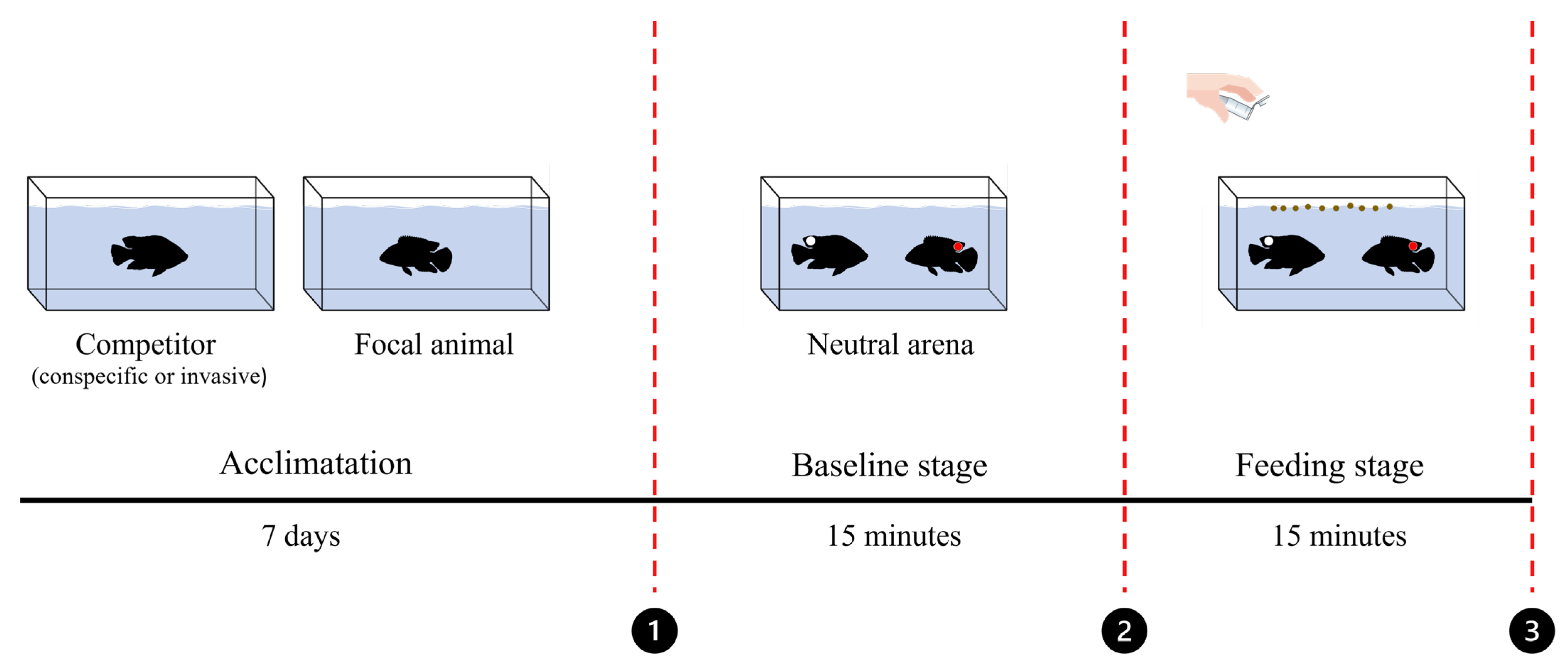
2.2. Experimental Design
2.3. Procedures
2.4. Behavioral Analysis
2.5. Statistical Analysis
3. Results
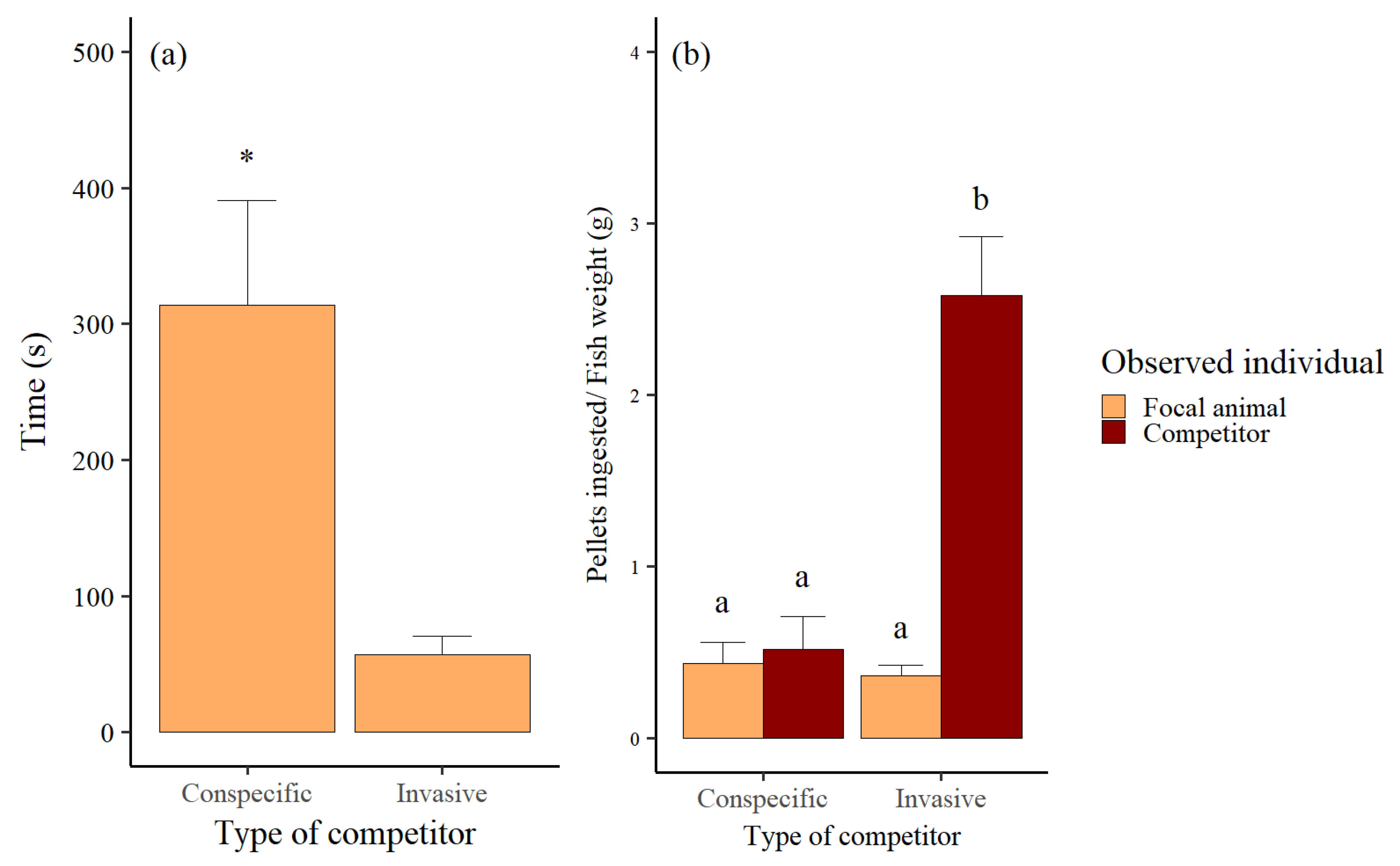
3.1. Aggressive Behaviors
3.2. Feeding Behaviors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowry, E.; Rollinson, E.J.; Laybourn, A.J.; Scott, T.E.; Aiello-Lammens, M.E.; Gray, S.M.; Mickley, J.; Gurevitch, J. Biological invasions: A field synopsis, systematic review, and database of the literature. Ecol. Evol. 2013, 3, 182–196. [Google Scholar] [CrossRef] [PubMed]
- David, P.; Thébault, E.; Anneville, O.; Duyck, P.-F.; Chapuis, E.; Loeuille, N. Impacts of Invasive Species on Food Webs. Adv. Ecol. Res. 2017, 56, 1–60. [Google Scholar] [CrossRef]
- Bradley, B.A.; Laginhas, B.B.; Whitlock, R.; Allen, J.M.; Bates, A.E.; Bernatchez, G.; Diez, J.M.; Early, R.; Lenoir, J.; Vilà, M.; et al. Disentangling the abundance–impact relationship for invasive species. Proc. Natl. Acad. Sci. USA 2019, 116, 9919–9924. [Google Scholar] [CrossRef]
- Le Louarn, M.; Couillens, B.; Deschamps-Cottin, M.; Clergeau, P. Interference competition between an invasive parakeet and native bird species at feeding sites. J. Ethol. 2016, 34, 291–298. [Google Scholar] [CrossRef]
- Song, Y.; Yang, X.; Zhang, H.; Zhang, D.; He, W.; Wyckhuys, K.A.G.; Wu, K. Interference competition and predation between invasive and native herbivores in maize. J. Pest Sci. 2021, 94, 1053–1063. [Google Scholar] [CrossRef]
- Meyerson, L.A.; Mooney, H.A. Invasive alien species in an era of globalization. Front. Ecol. Environ. 2007, 5, 199–208. [Google Scholar] [CrossRef]
- Richter-Boix, A.; Garriga, N.; Montori, A.; Franch, M.; Sebastián, O.S.; Villero, D.; Llorente, G.A. Effects of the non-native amphibian species Discoglossus pictus on the recipient amphibian community: Niche overlap, competition and community organization. Biol. Invasions 2013, 15, 799–815. [Google Scholar] [CrossRef]
- Zengeya, T.A.; Booth, A.J.; Chimimba, C.T. Broad niche overlap between invasive nile tilapia Oreochromis niloticus and indigenous congenerics in Southern Africa: Should we be concerned? Entropy 2015, 17, 4959–4973. [Google Scholar] [CrossRef]
- Yalçın Özdilek, Ş.; Partal, N.; Jones, R.I. An invasive species, Carassius gibelio, alters the native fish community through trophic niche competition. Aquat. Sci. 2019, 81, 29. [Google Scholar] [CrossRef]
- Britton, J.R.; Ruiz-Navarro, A.; Verreycken, H.; Amat-Trigo, F. Trophic consequences of introduced species: Comparative impacts of increased interspecific versus intraspecific competitive interactions. Funct. Ecol. 2018, 32, 486–495. [Google Scholar] [CrossRef]
- Almela, V.D.; South, J.; Britton, J.R. Predicting the competitive interactions and trophic niche consequences of a globally invasive fish with threatened native species. J. Anim. Ecol. 2021, 90, 2651–2662. [Google Scholar] [CrossRef] [PubMed]
- Gallien, L.; Carboni, M. The community ecology of invasive species: Where are we and what’s next? Ecography 2017, 40, 335–352. [Google Scholar] [CrossRef]
- Bøhn, T.; Amundsen, P.A.; Sparrow, A. Competitive exclusion after invasion? Biol. Invasions 2008, 10, 359–368. [Google Scholar] [CrossRef]
- Moran, E.V.; Alexander, J.M. Evolutionary responses to global change: Lessons from invasive species. Ecol. Lett. 2014, 17, 637–649. [Google Scholar] [CrossRef]
- Catford, J.A.; Bode, M.; Tilman, D. Introduced species that overcome life history tradeoffs can cause native extinctions. Nat. Commun. 2018, 9, 2131. [Google Scholar] [CrossRef]
- Grether, G.F.; Peiman, K.S.; Tobias, J.A.; Robinson, B.W. Causes and Consequences of Behavioral Interference Between Species. Trends Ecol. Evol. 2017, 32, 760–772. [Google Scholar] [CrossRef]
- Warren, R.J.; King, J.R.; Bradford, M.A. Disentangling resource acquisition from interspecific behavioral aggression to understand the ecological dominance of a common, widespread temperate forest ant. Insectes Soc. 2020, 67, 179–187. [Google Scholar] [CrossRef]
- Watz, J.; Nyqvist, D. Interspecific competition among terrestrial slugs. J. Molluscan Stud. 2022, 88, eyac007. [Google Scholar] [CrossRef]
- Damas-Moreira, I.; Riley, J.L.; Carretero, M.A.; Harris, D.J.; Whiting, M.J. Getting ahead: Exploitative competition by an invasive lizard. Behav. Ecol. Sociobiol. 2020, 74, 117. [Google Scholar] [CrossRef]
- White, E.M.; Wilson, J.C.; Clarke, A.R. Biotic indirect effects: A neglected concept in invasion biology. Divers. Distrib. 2006, 12, 443–455. [Google Scholar] [CrossRef]
- Vanni, M.J.; Lampert, W. Food quality effects on life history traits and fitness in the generalist herbivore Daphnia. Oecologia 1992, 92, 48–57. [Google Scholar] [CrossRef]
- Grunicke, F.; Wagner, A.; von Elert, E.; Weitere, M.; Berendonk, T. Riparian detritus vs. stream detritus: Food quality determines fitness of juveniles of the highly endangered freshwater pearl mussels (Margaritifera margaritifera). Hydrobiologia 2023, 850, 729–746. [Google Scholar] [CrossRef]
- Harrison, X.A.; Blount, J.D.; Inger, R.; Norris, D.R.; Bearhop, S. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 2011, 80, 4–18. [Google Scholar] [CrossRef]
- Barry, K.L. You Are What You Eat: Food Limitation Affects Reproductive Fitness in a Sexually Cannibalistic Praying Mantid. PLoS ONE 2013, 8, e78164. [Google Scholar] [CrossRef] [PubMed]
- Stahlschmidt, Z.R.; Rollinson, N.; Acker, M.; Adamo, S.A. Are all eggs created equal? Food availability and the fitness trade-off between reproduction and immunity. Funct. Ecol. 2013, 27, 800–806. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Bshary, R. Expanding the concept of social behavior to interspecific interactions. Ethology 2021, 127, 758–773. [Google Scholar] [CrossRef]
- Hudina, S.; Hock, K.; Žganec, K. The role of aggression in range expansion and biological invasions. Curr. Zool. 2014, 60, 401–409. [Google Scholar] [CrossRef]
- Arnott, G.; Elwood, R.W. Information gathering and decision making about resource value in animal contests. Anim. Behav. 2008, 76, 529–542. [Google Scholar] [CrossRef]
- Goubault, M.; Cortesero, A.M.; Poinsot, D.; Wajnberg, E.; Boivin, G. Does host value influence female aggressiveness, contest outcome and fitness gain in parasitoids? Ethology 2007, 113, 334–343. [Google Scholar] [CrossRef]
- Ancona, S.; Drummond, H.; Zaldívar-Rae, J. Male whiptail lizards adjust energetically costly mate guarding to male-male competition and female reproductive value. Anim. Behav. 2010, 79, 75–82. [Google Scholar] [CrossRef]
- Magellan, K.; Kaiser, H. The function of aggression in the swordtail, Xiphophorus helleri: Resource defence. J. Ethol. 2010, 28, 239–244. [Google Scholar] [CrossRef]
- Sacchi, R.; Coladonato, A.J.; Battaiola, M.; Pasquariello, C.; Buratti, S.; Matellini, C.; Mangiacotti, M.; Scali, S.; Zuffi, M.A.L. Subjective resource value affects aggressive behavior independently of resource-holding-potential and color morphs in male common wall lizard. J. Ethol. 2021, 39, 179–189. [Google Scholar] [CrossRef]
- Archundia, M.; Arce, E. Fighting behaviour in native fish: The Mexican mojarra (Cichlasoma istlanum) wins when confronted with the non-native convict cichlid fish (Amatitlania nigrofasciata). J. Ethol. 2019, 37, 67–73. [Google Scholar] [CrossRef]
- Franco, M.; Arce, E. Invasive Cichlids Display Higher Aggression During Nest Defence Compared to the Native Mexican Mojarra. Ecol. Freshw. Fish 2004, 34, e12815. [Google Scholar] [CrossRef]
- Johnsson, J.I.; Akerman, A. Watch and learn: Preview of the fighting ability of opponents alters contest behaviour in rainbow trout. Anim. Behav. 1998, 56, 771–776. [Google Scholar] [CrossRef]
- Elias, D.O.; Kasumovic, M.M.; Punzalan, D.; Andrade, M.C.; Mason, A.C. Assessment during aggressive contests between male jumping spiders. Anim. Behav. 2008, 76, 901–910. [Google Scholar] [CrossRef]
- Arnott, G.; Elwood, R.W. Assessment of fighting ability in animal contests. Anim. Behav. 2009, 77, 991–1004. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Reddon, A.R.; Ligocki, I.Y.; Hellmann, J.K.; Garvy, K.A.; Marsh-Rollo, S.E.; Hamilton, I.M.; Balshine, S. Motivation but not body size influences territorial contest dynamics in a wild cichlid fish. Anim. Behav. 2015, 107, 19–29. [Google Scholar] [CrossRef]
- Peiman, K.S.; Robinson, B.W. Ecology and evolution of resource-related heterospecific aggression. Q. Rev. Biol. 2010, 85, 133–158. [Google Scholar] [CrossRef]
- Grether, G.F.; Losin, N.; Anderson, C.N.; Okamoto, K. The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biol. Rev. 2009, 84, 617–635. [Google Scholar] [CrossRef]
- Grether, G.F.; Anderson, C.N.; Drury, J.P.; Kirschel, A.N.G.; Losin, N.; Okamoto, K.; Peiman, K.S. The evolutionary consequences of interspecific aggression. Ann. N. Y. Acad. Sci. 2013, 1289, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.M.S.; Clark, D.L.; Herrel, A.; Losos, J.B. Recent biological invasion shapes species recognition and aggressive behaviour in a native species: A behavioural experiment using robots in the field. J. Anim. Ecol. 2020, 89, 1604–1614. [Google Scholar] [CrossRef]
- Champneys, T.; Genner, M.J.; Ioannou, C.C. Invasive Nile tilapia dominates a threatened indigenous tilapia in competition over shelter. Hydrobiologia 2021, 848, 3747–3762. [Google Scholar] [CrossRef]
- Bush, J.M.; Ellison, M.; Simberloff, D. Impacts of an invasive species (Anolis sagrei) on social and spatial behaviours of a native congener (Anolis carolinensis). Anim. Behav. 2022, 183, 177–188. [Google Scholar] [CrossRef]
- Grant, J.W.A.; Guha, R.T. Spatial clumping of food increases its monopolization and defense by convict cichlids, Cichlasoma nigrofasciatum. Behav. Ecol. 1993, 4, 293–296. [Google Scholar] [CrossRef]
- Grant, J.W.A. Whether or not to defend? The influence of resource distribution. Mar. Behav. Physiol. 1993, 23, 137–153. [Google Scholar] [CrossRef]
- McCallum, E.S.; Gulas, S.T.; Balshine, S. Accurate resource assessment requires experience in a territorial fish. Anim. Behav. 2017, 123, 249–257. [Google Scholar] [CrossRef]
- Grand, T.C.; Grant, J.W.A. Spatial predictability of food influences its monopolization and defence by juvenile convict cichlids. Anim. Behav. 1994, 47, 91–100. [Google Scholar] [CrossRef]
- Desjardins, J.K.; Hofmann, H.A.; Fernald, R.D. Social Context Influences Aggressive and Courtship Behavior in a Cichlid Fish. PLoS ONE 2012, 7, e32781. [Google Scholar] [CrossRef]
- Satoh, S.; Nishida, Y.; Saeki, T.; Kawasaka, K.; Kohda, M.; Awata, S. The functional role of sibling aggression and “best of a bad job” strategies in cichlid juveniles. Behav. Ecol. 2021, 32, 488–499. [Google Scholar] [CrossRef]
- Nelson, K.A.; Collins, S.F.; Sass, G.G.; Wahl, D.H. A response-surface examination of competition and facilitation between native and invasive juvenile fishes. Funct. Ecol. 2017, 31, 2147–2166. [Google Scholar] [CrossRef]
- Faria, L.; Cuthbert, R.N.; Dickey, J.; Jeschke, J.M.; Ricciardi, A.; Dick, J.T.A.; Vitule, J.R.S. Non-native species have higher consumption rates than their native counterparts. Biol. Rev. 2025. [Google Scholar] [CrossRef]
- Argolo, L.A.; López-Fernández, H.; Batalha-Filho, H.; Affonso, P.R.A.d.M. Unraveling the systematics and evolution of the Geophagus brasiliensis (Cichliformes: Cichlidae) species complex. Mol. Phylogenet. Evol. 2020, 150, 106855. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture. In Sustainability in Action; FAO: Rome, Italy, 2020; pp. 1–244. [Google Scholar] [CrossRef]
- Zambrano, L.; Martínez-Meyer, E.; Menezes, N.; Peterson, A.T. Invasive potential of common carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus) in American freshwater systems. Can. J. Fish. Aquat. Sci. 2006, 63, 1903–1910. [Google Scholar] [CrossRef]
- Njiru, M.; Okeyo-Owuor, J.B.; Muchiri, M.; Cowx, I.G. Shifts in the Food of Nile Tilapia, Oreochromis niloticus (L.) in Lake Victoria, Kenya. Afr. J. Ecol. 2004, 42, 163–170. [Google Scholar] [CrossRef]
- Atwood, H.L.; Tomasso, J.R.; Webb, K.; Gatlin, D.M. Low-temperature tolerance of Nile tilapia, Oreochromis niloticus: Effects of environmental and dietary factors. Aquac. Res. 2003, 34, 241–251. [Google Scholar] [CrossRef]
- Peña-Mendoza, B.; Gómez-Márquez, J.L.; Salgado-Ugarte, I.H.; Ramírez-Noguera, D. Reproductive Biology of Oreochromis niloticus (Perciformes: Cichlidae) at Emiliano Zapata Dam, Morelos, Mexico. Rev. Biol. Trop. 2005, 53, 515–522. [Google Scholar] [CrossRef]
- Getabu, A. Growth parameters and total mortality in Oreochromis niloticus (Linnaeus) from Nyanza Gulf, Lake Victoria. Hydrobiologia 1992, 232, 91–97. [Google Scholar] [CrossRef]
- Martin, C.W.; Valentine, M.M.; Valentine, J.F. Competitive interactions between invasive Nile tilapia and native fish: The potential for altered trophic exchange and modification of food webs. PLoS ONE 2010, 5, e14395. [Google Scholar] [CrossRef]
- Linde, A.R.; Izquierdo, J.I.; Moreira, J.C.; Garcia-Vazquez, E. Invasive tilapia juveniles are associated with degraded river habitats. Aquat. Conserv. 2008, 18, 891–895. [Google Scholar] [CrossRef]
- Kadry, V.O.; Barreto, R.E. Environmental enrichment reduces aggression of pearl cichlid, Geophagus brasiliensis, during resident-intruder interactions. Neotrop. Icthyol. 2010, 8, 329–332. [Google Scholar] [CrossRef]
- Sanches, F.H.C.; Miyai, C.A.; Costa, T.M.; Christofoletti, R.A.; Volpato, G.L.; Barreto, R.E. Aggressiveness overcomes body-size effects in fights staged between invasive and native fish species with overlapping niches. PLoS ONE 2012, 7, e29746. [Google Scholar] [CrossRef]
- Hsu, Y.; Earley, R.L.; Wolf, L.L. Modulation of aggressive behaviour by fighting experience: Mechanisms and contest outcomes. Biol. Rev. 2006, 81, 33–74. [Google Scholar] [CrossRef]
- Moretz, J.A. Aggression and RHP in the Northern Swordtail Fish, Xiphophorus cortez: The Relationship Between Size and Contest Dynamics in Male–Male Competition. Ethology 2003, 109, 995–1008. [Google Scholar] [CrossRef]
- Reddon, A.R.; Voisin, M.R.; Menon, N.; Marsh-Rollo, S.E.; Wong, M.Y.; Balshine, S. Rules of engagement for resource contests in a social fish. Anim. Behav. 2011, 82, 93–99. [Google Scholar] [CrossRef]
- Alvarenga, C.M.D.; Volpato, G.L. Agonistic profile and metabolism in alevins of the Nile tilapia. Physiol. Behav. 1995, 57, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Earley, R.L.; Edwards, J.T.; Aseem, O.; Felton, K.; Blumer, L.S.; Karom, M.; Grober, M.S. Social interactions tune aggression and stress responsiveness in a territorial cichlid fish (Archocentrus nigrofasciatus). Physiol. Behav. 2006, 88, 353–363. [Google Scholar] [CrossRef]
- Torrezani, C.S.; Pinho-Neto, C.F.; Miyai, C.A.; Sanches, F.H.C.; Barreto, R.E. Structural enrichment reduces aggression in Tilapia rendalli. Mar. Freshw. Behav. Physiol. 2013, 46, 183–190. [Google Scholar] [CrossRef]
- Volpato, G.L.; Bovi, T.S.; de Freitas, R.H.A.; da Silva, D.F.; Delicio, H.C.; Giaquinto, P.C.; Barreto, R.E. Red Light Stimulates Feeding Motivation in Fish but Does Not Improve Growth. PLoS ONE 2013, 8, e59134. [Google Scholar] [CrossRef]
- Arvigo, A.L.; Miyai, C.A.; Sanches, F.H.C.; Barreto, R.E.; Costa, T.M. Combined effects of predator odor and alarm substance on behavioral and physiological responses of the pearl cichlid. Physiol. Behav. 2019, 206, 259–263. [Google Scholar] [CrossRef]
- Pekár, S.; Brabec, M. Generalized estimating equations: A pragmatic and flexible approach to the marginal GLM modelling of correlated data in the behavioural sciences. Ethology 2018, 124, 86–93. [Google Scholar] [CrossRef]
- Halekoh, U.; Højsgaard, S.; Yan, J. The R Package Geepack for Generalized Estimating Equations. J. Stat. Softw. 2006, 15, 1–11. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Leese, J.M.; Blatt, T. Sex differences in how territory quality affects aggression in convict cichlids. Integr. Organ Biol. 2021, 3, obab028. [Google Scholar] [CrossRef]
- Drescher, J.; Feldhaar, H.; Blüthgen, N. Interspecific Aggression and Resource Monopolization of the Invasive Ant Anoplolepis gracilipes in Malaysian Borneo. Biotropica 2011, 43, 93–99. [Google Scholar] [CrossRef]
- Grabowska, J.; Błońska, D.; Kati, S.; Nagy, S.A.; Kakareko, T.; Kobak, J.; Antal, L. Competitive interactions for food resources between the invasive Amur sleeper (Perccottus glenii) and threatened European mudminnow (Umbra krameri). Aquat. Conserv. 2019, 29, 2231–2239. [Google Scholar] [CrossRef]
- Le Bourlot, V.; Tully, T.; Claessen, D. Interference versus Exploitative Competition in the Regulation of Size-Structured Populations. Am. Nat. 2014, 184, 609–623. [Google Scholar] [CrossRef]
- Zaviezo, T.; Soares, A.O.; Grez, A.A. Interspecific exploitative competition between Harmonia axyridis and other coccinellids is stronger than intraspecific competition. Biol. Control 2019, 131, 62–68. [Google Scholar] [CrossRef]
- Ode, P.J.; Vyas, D.K.; Harvey, J.A. Extrinsic Inter- and Intraspecific Competition in Parasitoid Wasps. Annu. Rev. Entomol. 2022, 67, 305–328. [Google Scholar] [CrossRef]
- Griffen, B.D.; Altman, I.; Bess, B.M.; Hurley, J.; Penfield, A. The role of foraging in the success of invasive Asian shore crabs in New England. Biol. Invasions 2012, 14, 2545–2558. [Google Scholar] [CrossRef]
- Nagelkerke, L.A.J.; van Onselen, E.; Van Kessel, N.; Leuven, R.S.E.W. Functional feeding traits as predictors of invasive success of alien freshwater fish species using a food-fish model. PLoS ONE 2018, 13, e0197636. [Google Scholar] [CrossRef]
- Castro, N.; Ros, A.F.H.; Becker, K.; Oliveira, R.F. Metabolic costs of aggressive behaviour in the Siamese fighting fish, Betta splendens. Aggress. Behav. 2006, 32, 474–480. [Google Scholar] [CrossRef]
- Ros, A.F.H.; Becker, K.; Oliveira, R.F. Aggressive behaviour and energy metabolism in a cichlid fish, Oreochromis mossambicus. Physiol. Behav. 2006, 89, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A. Scramble in behaviour and ecology. Philos. Trans. R. Soc. B 2000, 355, 1637–1645. [Google Scholar] [CrossRef]
- Karplus, I.; Zion, B.; Rosenfeld, L.; Grinshpun, Y.; Slosman, T.; Goshen, Z.; Barki, A. Social facilitation of learning in mixed-species schools of common carp Cyprinus carpio L. and Nile tilapia Oreochromis niloticus (L.). J. Fish Biol. 2007, 71, 1023–1034. [Google Scholar] [CrossRef]
- Ogura, Y.; Matsushima, T. Social facilitation revisited: Increase in foraging efforts and synchronization of running in domestic chicks. Front. Neurosci. 2011, 5, 11069. [Google Scholar] [CrossRef]
- Schrandt, M.N.; Powers, S.P. Facilitation and dominance in a schooling predator: Foraging behavior of Florida Pompano, Trachinotus carolinus. PLoS ONE 2015, 10, e0130095. [Google Scholar] [CrossRef]
- Branconi, R.; Garner, J.G.; Buston, P.M.; Wong, M.Y. A New Non-Invasive Technique for Temporarily Tagging Coral Reef Fishes. Copeia 2019, 107, 85–91. [Google Scholar] [CrossRef]
- Arnold, D.E. Marking Fish with Dyes and Other Chemicals; US Bureau of Sport Fisheries and Wildlife: Washington, DC, USA, 1966; Volume 10.
- Klabukov, I.; Shestakova, V.; Krasilnikova, O.; Smirnova, A.; Abramova, O.; Baranovskii, D.; Atiakshin, D.; Kostin, A.A.; Shegay, P.; Kaprin, A.D. Refinement of animal experiments: Replacing traumatic methods of laboratory animal marking with non-invasive alternatives. Animals 2023, 13, 3452. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, L.; Miyai, C.A.; Sanches, F.H.C.; Arvigo, A.L.; Costa, T.M. Eat First, Fight Later: Competitive Advantage of an Invasive Cichlid over a Native Competitor for Food Resources. Fishes 2025, 10, 340. https://doi.org/10.3390/fishes10070340
Cirillo L, Miyai CA, Sanches FHC, Arvigo AL, Costa TM. Eat First, Fight Later: Competitive Advantage of an Invasive Cichlid over a Native Competitor for Food Resources. Fishes. 2025; 10(7):340. https://doi.org/10.3390/fishes10070340
Chicago/Turabian StyleCirillo, Leonardo, Caio A. Miyai, Fábio H. C. Sanches, Alexandre L. Arvigo, and Tânia M. Costa. 2025. "Eat First, Fight Later: Competitive Advantage of an Invasive Cichlid over a Native Competitor for Food Resources" Fishes 10, no. 7: 340. https://doi.org/10.3390/fishes10070340
APA StyleCirillo, L., Miyai, C. A., Sanches, F. H. C., Arvigo, A. L., & Costa, T. M. (2025). Eat First, Fight Later: Competitive Advantage of an Invasive Cichlid over a Native Competitor for Food Resources. Fishes, 10(7), 340. https://doi.org/10.3390/fishes10070340






