Transcriptome Analysis Reveals Hyperglycemic Hormone and Excitatory Amino Acid Transporter 3 Are Involved in the Thermal Adaptation of Eriocheir sinensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Conditions of E. sinensis
2.2. Sample Collection and Storage
2.3. Reference Genome Assembly
2.4. Transcriptome Sequencing of Samples from Normal and High-Temperature Conditions
2.5. Screening and GO Enrichment Analysis of Thermal-Responsive Genes
2.6. RT-qPCR Validation
2.7. Detection of Hemolymph Glucose at Different Temperatures
3. Results
3.1. Reference Genome Assembly
3.2. Transcriptome Sequencing
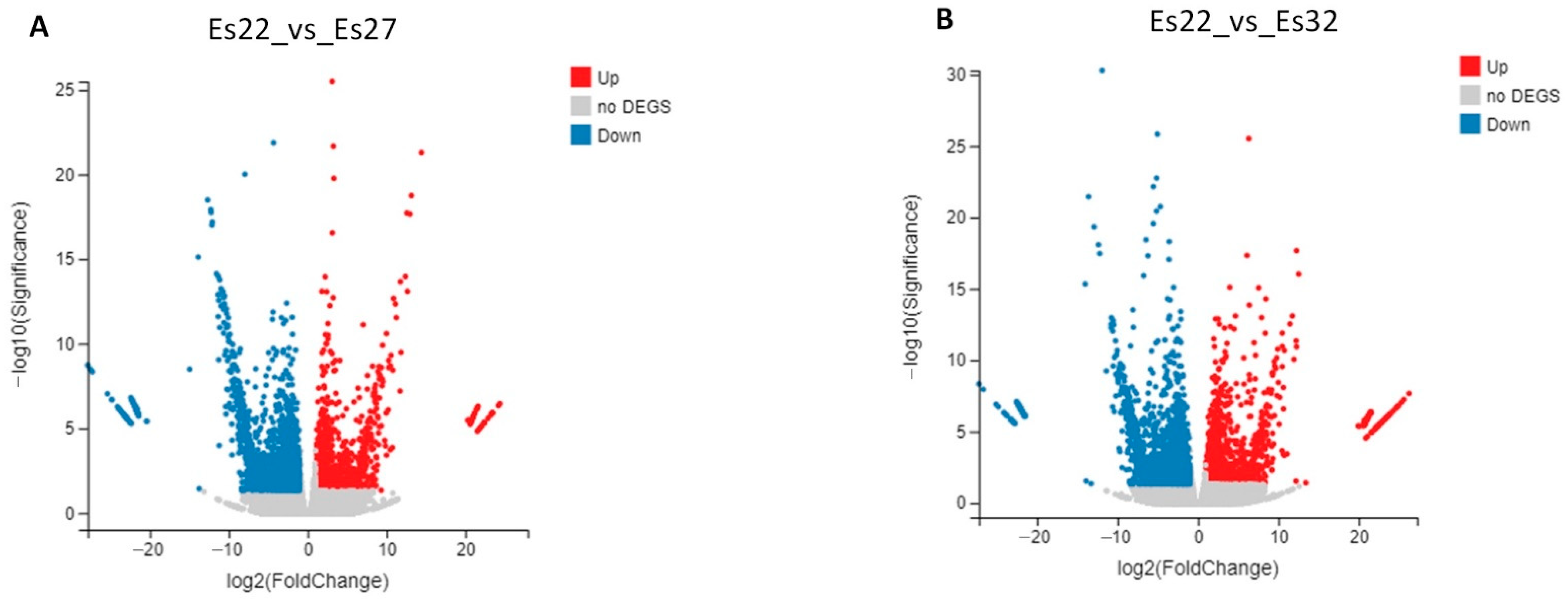
3.3. Analysis of DEGs
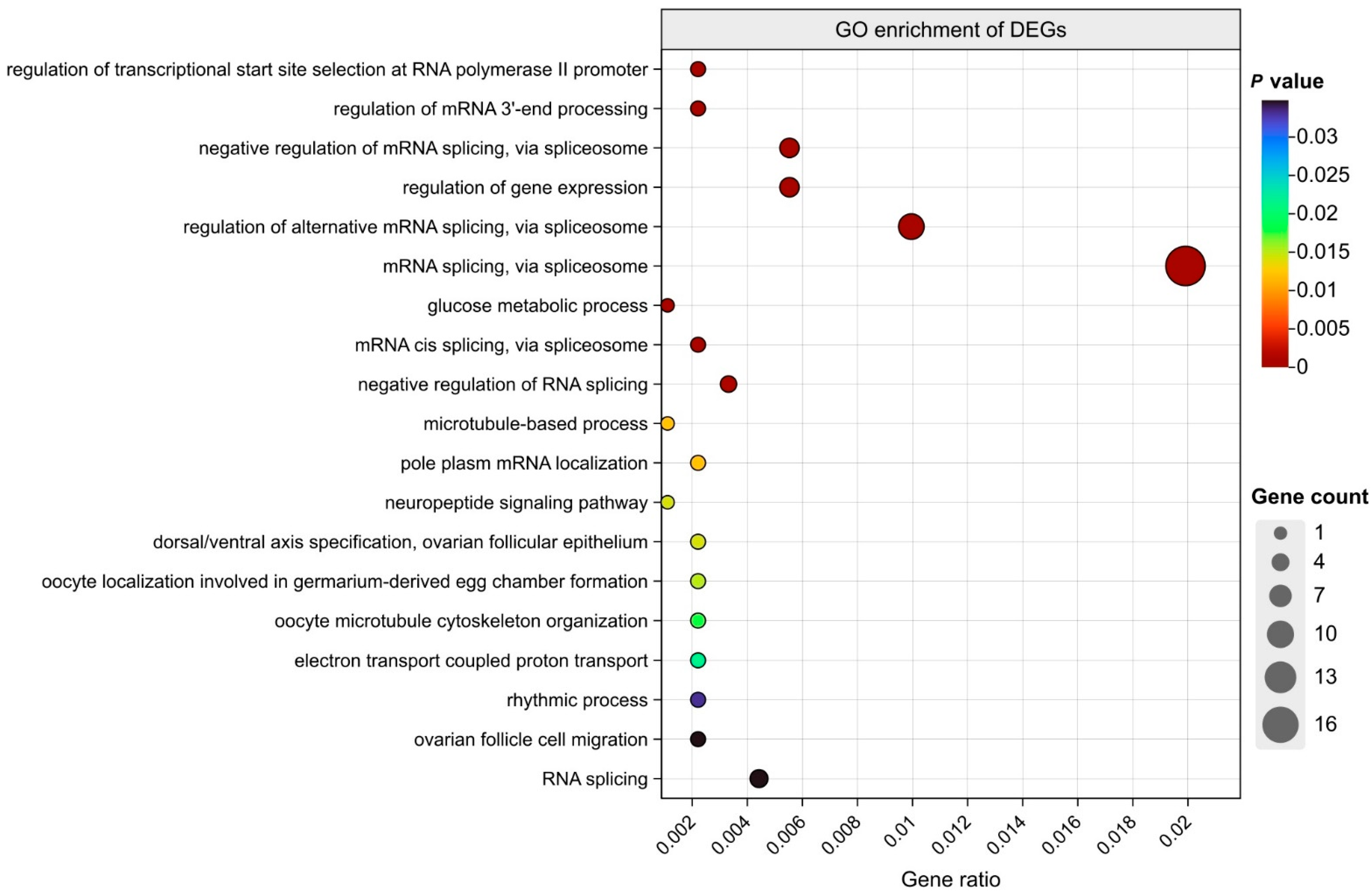
3.4. GO Enrichment Analysis of Common DEGs
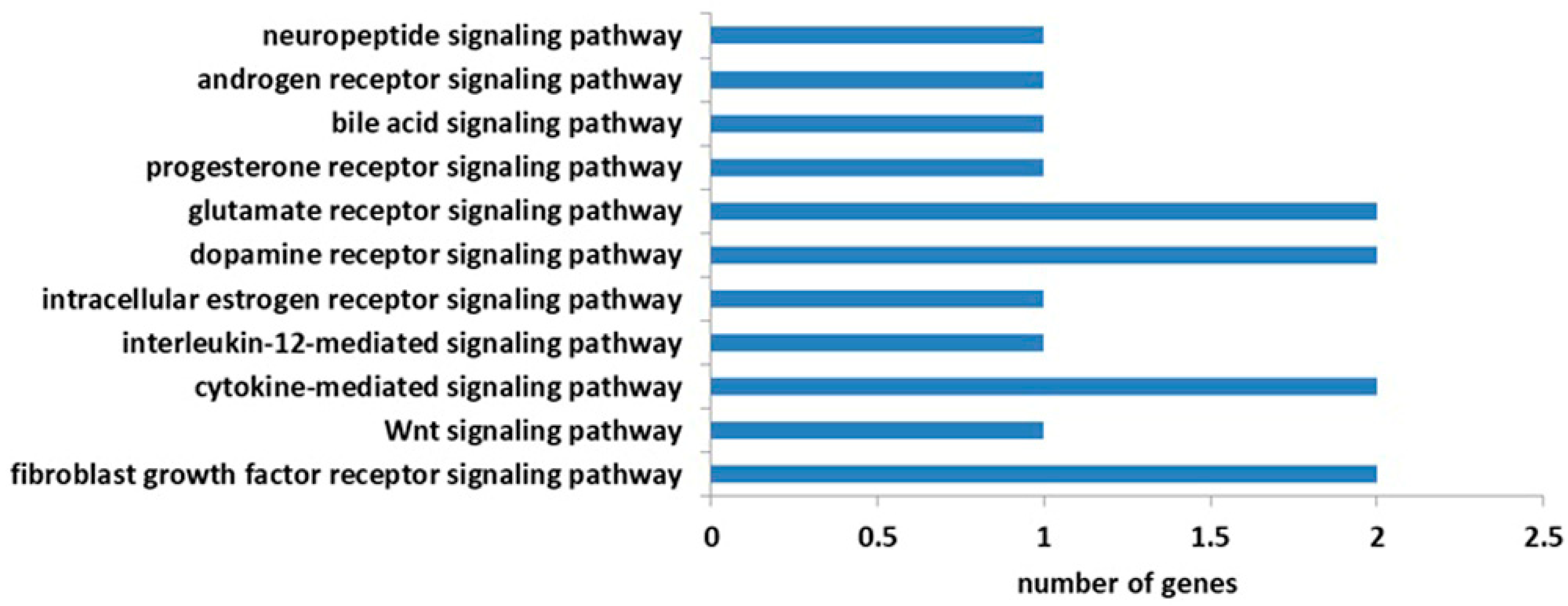
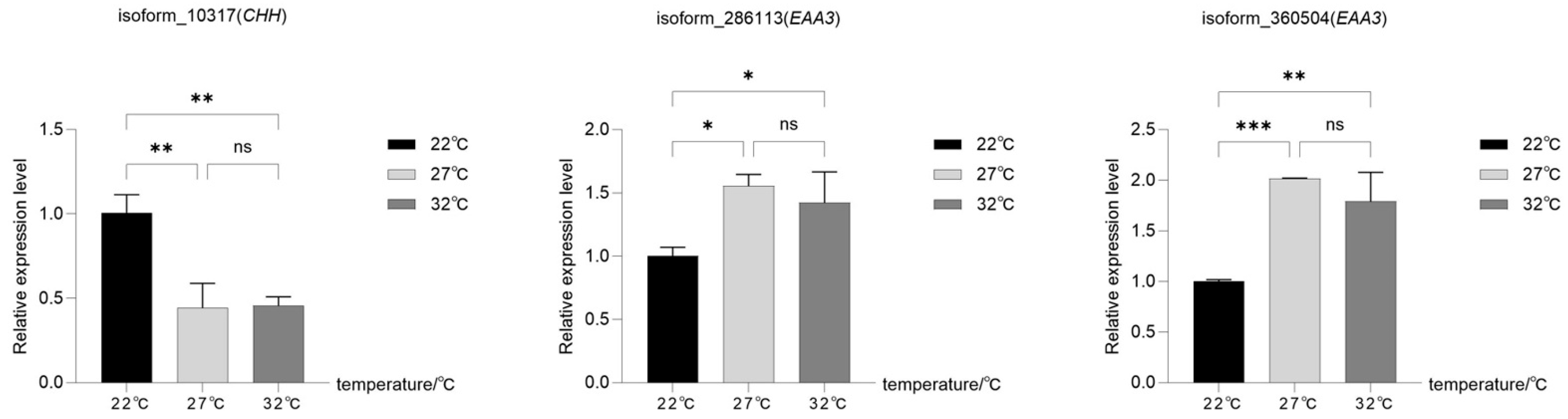
3.5. RT-qPCR Validation of Genes in Neurotransmission Signaling Pathways
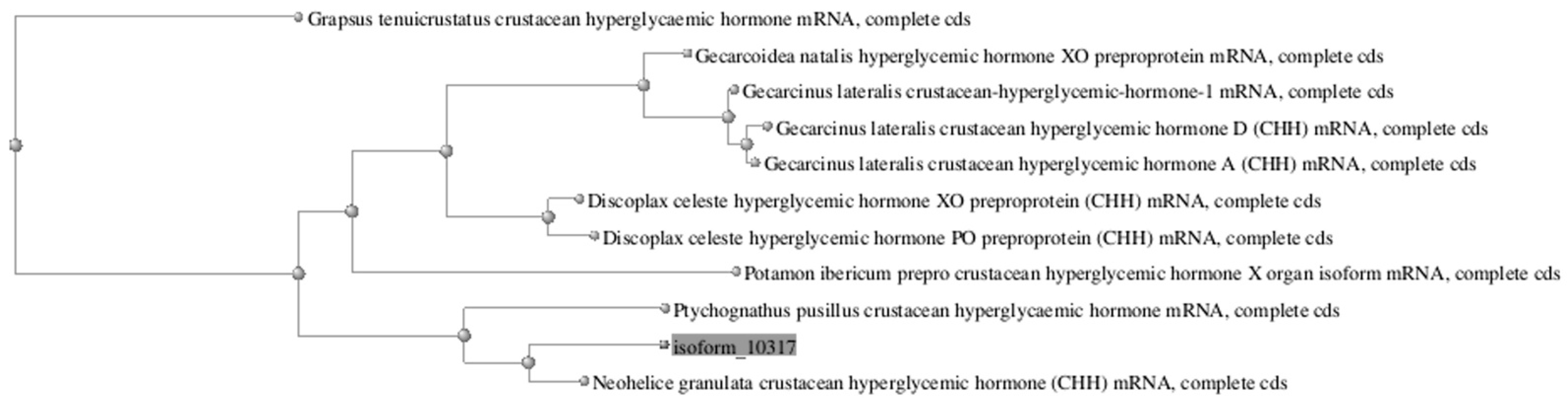
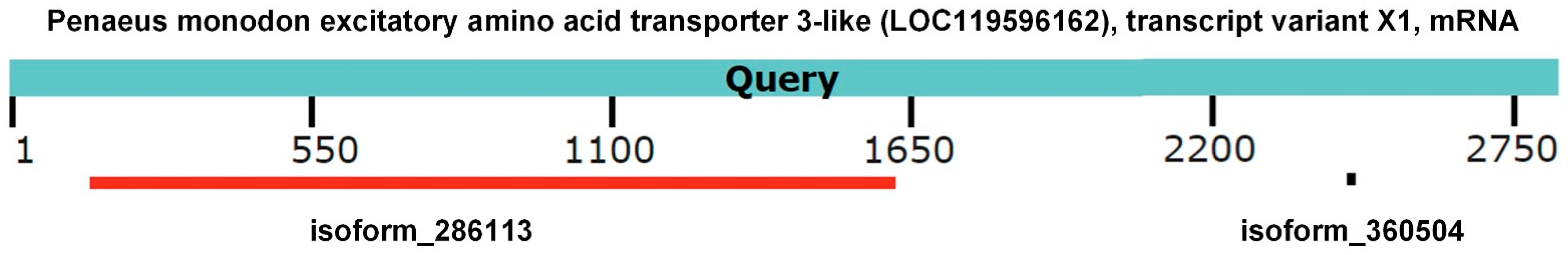
3.6. Sequence Similarity Analysis
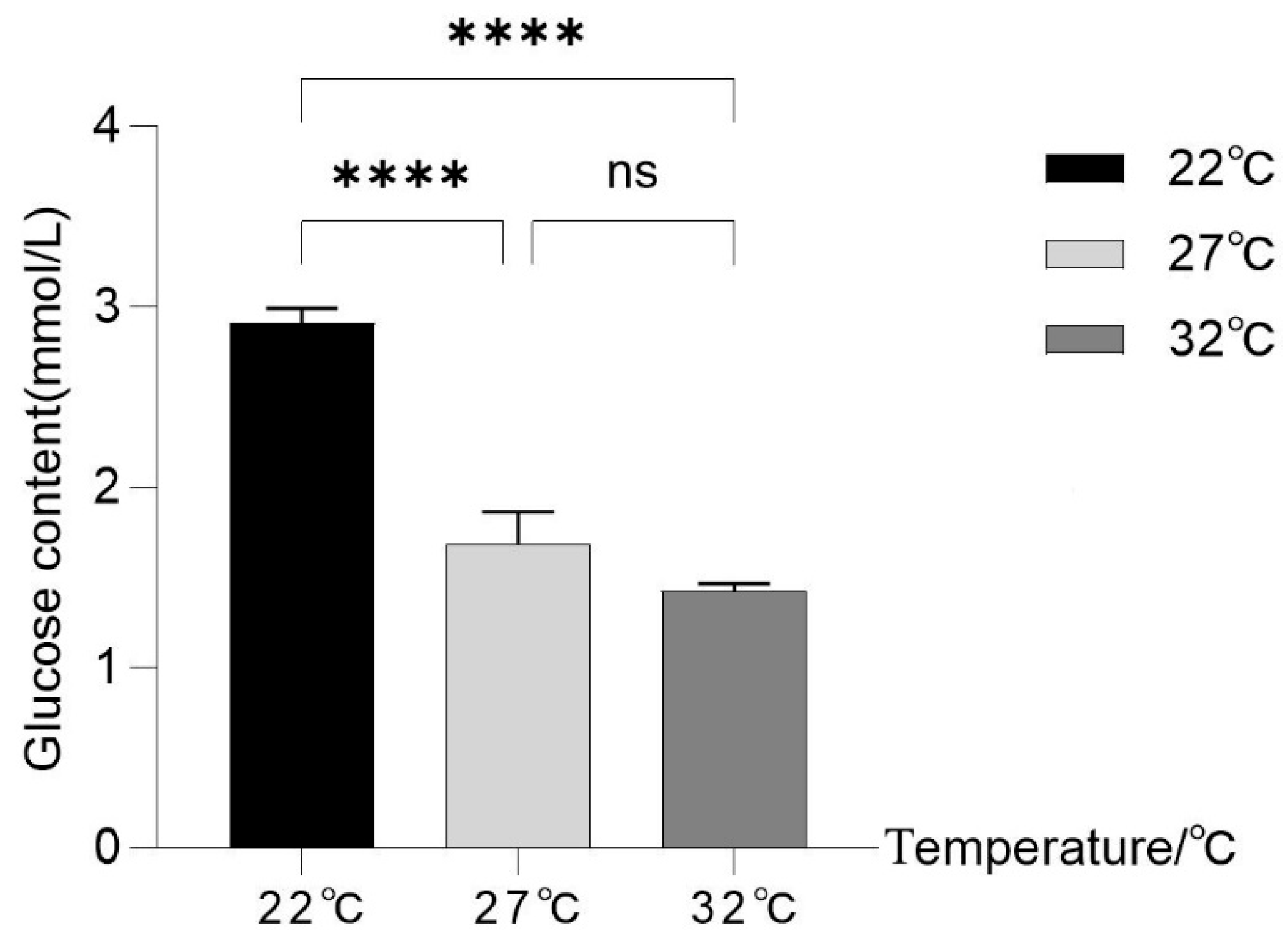
3.7. Influence of Thermal Stress on Hemolymph Glucose
4. Discussion
4.1. Biological Processes Related to Thermal Regulation
4.2. Neurotransmission Signaling Pathways and Genes Related to Temperature Regulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tansey, E.A.; Johnson, C.D. Recent advances in thermoregulation. Adv. Physiol. Educ. 2015, 39, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.L. Fundamental Concepts of Human Thermoregulation and Adaptation to Heat: A Review in the Context of Global Warming. Int. J. Environ. Res. Public Health 2020, 17, 7795. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Kellogg, D.L., Jr. Thermoregulatory and thermal control in the human cutaneous circulation. Front. Biosci. (Schol. Ed.) 2010, 2, 825–853. [Google Scholar] [PubMed]
- Abram, P.K.; Boivin, G.; Moiroux, J.; Brodeur, J. Behavioural effects of temperature on ectothermic animals: Unifying thermal physiology and behavioural plasticity. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1859–1876. [Google Scholar] [CrossRef] [PubMed]
- Volonté, C.; Liguori, F.; Amadio, S. A Closer Look at Histamine in Drosophila. Int. J. Mol. Sci. 2024, 25, 4449. [Google Scholar] [CrossRef] [PubMed]
- Gandara, A.C.P.; Drummond-Barbosa, D. Chronic exposure to warm temperature causes low sperm abundance and quality in Drosophila melanogaster. Sci. Rep. 2023, 13, 12331. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.H.; Dobkowski, K.A.; Seroy, S.K.; Fox, S.; Meenan, N. Feeding preferences and the effect of temperature on feeding rates of the graceful kelp crab, Pugettia gracilis. PeerJ 2023, 11, e15223. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Barón, P.J. Effects of temperature and salinity on the development and survival of the embryos and zoeae I from the southern surf crab Ovalipes trimaculatus (Brachyura: Portunidae). An. Acad. Bras. Cienc. 2021, 93, e20190999. [Google Scholar] [CrossRef] [PubMed]
- Ayres, B.S.; Varela Junior, A.S.; Corcini, C.D.; Lopes, E.M.; Nery, L.E.M.; Maciel, F.E. Effects of high temperature and LPS injections on the hemocytes of the crab Neohelice granulata. J. Invertebr. Pathol. 2024, 205, 108144. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.S.; Zmora, N.; Katayama, H.; Tsutsui, N. Crustacean hyperglycemic hormone (CHH) neuropeptidesfamily: Functions, titer, and binding to target tissues. Gen. Comp. Endocrinol. 2010, 166, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Kotsyuba, E.; Dyachuk, V. Immunocytochemical Localization of Enzymes Involved in Dopamine, Serotonin, and Acetylcholine Synthesis in the Optic Neuropils and Neuroendocrine System of Eyestalks of Paralithodes camtschaticus. Front. Neuroanat. 2022, 16, 844654. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.E.; Stanhope, M.E.; Gandler, H.I.; Lameyer, T.J.; Pascual, M.G.; Shea, D.N.; Yu, A.; Dickinson, P.S.; Hull, J.J. Molecular characterization of putative neuropeptide, amine, diffusible gas and small molecule transmitter biosynthetic enzymes in the eyestalk ganglia of the American lobster, Homarus americanus. Invert. Neurosci. 2018, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.E. Crustacean neuroendocrine systems and their signaling agents. Cell Tissue Res. 2011, 345, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Dircksen, H. Circadian clocks in crustaceans: Identified neuronal and cellular systems. Front. Biosci. 2010, 15, 1040–1074. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, A.C.; Benrabaa, S.A.M.; López-Cerón, D.A.; Chang, E.S.; Mykles, D.L. Effects of temperature on survival, moulting, and expression of neuropeptide and mTOR signalling genes in juvenile Dungeness crab (Metacarcinus magister). J. Exp. Biol. 2018, 221, jeb187492. [Google Scholar]
- Lacerda, J.T.; David, D.D.; Castrucci, A.M.L. The effect of thermal stress on the X-organ/sinus gland proteome of the estuarine blue crab Callinectes sapidus during the intermolt and premolt stages. J. Proteom. 2025, 313, 105382. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wang, Q.D.; Zhang, T.L.; Li, Z.J.; Liu, J.S. Effects of water temperature on growth, feeding and molting of juvenile Chinese mitten crab (Eriocheir sinensis). Aquaculture 2017, 468, 169–174. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, Y.L.; Xu, Z.G.; Liu, B.Q.; Duan, C.C.; Tang, Y.C. Effect of temperature stress on the survival of juvenile Chinese mitten crab (Eriocheir sinensis). Iran. J. Fish. Sci. 2019, 18, 763–774. [Google Scholar]
- Deng, D.; Hu, S.; Lin, Z.; Geng, J.; Qian, Z.; Zhang, K.; Ning, X.; Cheng, Y.; Zhang, C.; Yin, S. High temperature aggravated hypoxia-induced intestine toxicity on juvenile Chinese mitten crab (Eriocheir sinensis). Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 52, 101288. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, Y.; Li, Z.; Luo, L.; Wang, S.; Zhang, R.; Guo, K.; Zhao, Z. Effects of re-enter water on antioxidant, immune, intestinal flora and metabolome of Chinese mitten crab (Eriocheir sinensis) after high temperature air exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2025, 296, 110232. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Li, T.; Yang, M.; Jiang, H.; Ling, J. Integrative Analysis of Hepatopancreas Transcriptome and Proteome in Female Eriocheir sinensis under Thermal Stress. Int. J. Mol. Sci. 2024, 25, 7249. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Pang, A.; Liu, R.; Yang, W.; Wu, H.; Yang, J.; Xuan, J.; Sun, X.; Ding, G.; Zhang, H.; et al. Regulation of gene expression under temperature stress and genome-wide analysis of heat shock protein family in Eriocheir sinensis. Int. J. Biol. Macromol. 2025, 308, 142503. [Google Scholar] [CrossRef] [PubMed]
- Keller, R. Crustacean neuropeptides: Structures, functions and comparative aspects. Experientia 1992, 48, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Simões, L.A.R.; Normann, R.S.; Chung, J.S.; Vinagre, A.S. A brief and updated introduction to the neuroendocrine system of crustaceans. Mol. Cell. Endocrinol. 2024, 590, 112265. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, Z.; She, Q.; Zhao, Y.; Wei, H.; Dong, J.; Xu, W.; Li, X.; Liang, S. Comparative transcriptome analysis provides insights into the molecular basis of circadian cycle regulation in Eriocheir sinensis. Gene 2019, 694, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.Y.; Zhang, C.; Xu, M.J.; Huang, G.Y.; Cheng, Y.X.; Yang, X.Z. The transcriptome sequencing and functional analysis of eyestalk ganglions in Chinese mitten crab (Eriocheir sinensis) treated with different photoperiods. PLoS ONE 2019, 14, e0210414. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Huang, Z.; Xu, Y.; Zhang, B.; Li, Y. Deep sequencing of microRNAs reveals circadian-dependent microRNA expression in the eyestalks of the Chinese mitten crab Eriocheir sinensis. Sci. Rep. 2023, 13, 5253. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Feng, W.; Chen, X.; Tang, Y.; Li, J.; Li, W. Effects of different temperatures on growth and intestinal microbial composition of juvenile Eriocheir sinensis. Front. Physiol. 2023, 14, 1163055. [Google Scholar] [CrossRef] [PubMed]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar] [PubMed]
- Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; Zhang, X.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. GigaScience 2018, 7, gix120. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Mangalam, A.K.; Dwivedi, S.; Naik, S. Primer premier: Program for design of degenerate primers from a protein sequence. Biotechniques 1998, 24, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Kichko, T.I.; Reeh, P.W. TRPV1 controls acid- and heat-induced calcitonin gene-related peptide release and sensitization by bradykinin in the isolated mouse trachea. Eur. J. Neurosci. 2009, 29, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.S.; Zmora, N. Functional studies of crustacean hyperglycemic hormones (CHHs) of the blue crab, Callinectes sapidus—The expression and release of CHH in eyestalk and pericardial organ in response to environmental stress. FEBS J. 2008, 275, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Zhou, Y.; Lee, J.G.; Looger, L.L.; Qian, G.; Ge, C.; Capel, B. Temperature-dependent sex determination is mediated by pSTAT3 repression of Kdm6b. Science 2020, 368, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, X.; Ge, C.; Jin, L.; Ding, Z.; Liu, F.; Zhang, J.; Gao, F.; Du, W. pSTAT3 activation of Foxl2 initiates the female pathway underlying temperature-dependent sex determination. Proc. Natl. Acad. Sci. USA 2024, 121, e2401752121. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Sheng, D.; Guo, J.; Zhou, F.; Wu, S.; Tang, H. Identification of BiP as a temperature sensor mediating temperature-induced germline sex reversal in C. elegans. EMBO J. 2024, 43, 4020–4048. [Google Scholar] [CrossRef] [PubMed]
- Mo, N.; Shao, S.; Cui, Z.; Bao, C. Roles of eyestalk in salinity acclimatization of mud crab (Scylla paramamosain) by transcriptomic analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 52, 101276. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A.; Liu, Y.; Xu, C.; Han, T.; Wang, X.; Li, E. Evidence from transcriptome analysis unravelled the roles of eyestalk in salinity adaptation in Pacific white shrimp (Litopenaeus vannamei). Gen. Comp. Endocrinol. 2022, 329, 114120. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Tomita, J.; Kume, S.; Kume, K. Dopamine modulates metabolic rate and temperature sensitivity in Drosophila melanogaster. PLoS ONE 2012, 7, e31513. [Google Scholar] [CrossRef] [PubMed]
- Kegel, G.; Reichwein, B.; Weese, S.; Gaus, G.; Peter-Katalinić, J.; Keller, R. Amino acid sequence of the crustacean hyperglycemic hormone (CHH) from the shore crab, Carcinus maenas. FEBS Lett. 1989, 255, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pan, L.; Tong, R.; Li, Y.; Si, L.; Chen, Y.; Wu, M.; Wang, Q. Effects of crustacean hyperglycaemic hormone RNA interference on regulation of glucose metabolism in Litopenaeus vannamei after ammonia-nitrogen exposure. Br. J. Nutr. 2022, 127, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Prymaczok, N.C.; Pasqualino, V.M.; Viau, V.E.; Rodriguez, E.M.; Medesani, D.A. Involvement of the crustacean hyperglycemic hormone (CHH) in the physiological compensation of the freshwater crayfish Cherax quadricarinatus to low temperature and high salinity stress. J. Comp. Physiol. B 2016, 186, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Giffard-Mena, I.; Ponce-Rivas, E.; Sigala-Andrade, H.M.; Uranga-Solis, C.; Re, A.D.; Diaz, F.; Camacho-Jimenez, L. Evaluation of the osmoregulatory capacity and three stress biomarkers in white shrimp Penaeus vannamei exposed to different temperature and salinity conditions: Na+/K+ ATPase, Heat Shock Proteins (HSP), and Crustacean Hyperglycemic Hormones (CHHs). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2024, 271, 110942. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, S.; Iqbal, B.M.M.; Vasudevan, S. Induced thermal stress on serotonin levels in the blue swimmer crab, Portunus pelagicus. Biochem. Biophys. Rep. 2016, 5, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Rajendran, S. Thermal stress induced hyperglycemia in the blue swimmer crab, Portunus pelagicus. J. Therm. Biol. 2021, 100, 103076. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Tsai, K.W.; Hsiao, N.W.; Chang, C.Y.; Lin, C.L.; Watson, R.D.; Lee, C.Y. Structural and functional comparisons and production of recombinant crustacean hyperglycemic hormone (CHH) and CHH-like peptides from the mud crab Scylla olivacea. Gen. Comp. Endocrinol. 2010, 167, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.I.; Orlov, I.; Ruggiero, A.M.; Dykes-Hoberg, M.; Lee, A.; Jackson, M.; Rothstein, J.D. Modulation of the neuronal glutamate transporter EAAC1 by the interacting protein GTRAP3-18. Nature 2001, 410, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Bjoras, M.; Gjesdal, O.; Erickson, J.D.; Torp, R.; Levy, L.M.; Ottersen, O.P.; Degree, M.; Storm-Mathisen, J.; Seeberg, E.; Danbolt, N.C. Cloning and expression of a neuronal rat brain glutamate transporter. Brain Res. Mol. Brain Res. 1996, 36, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Underhill, S.M.; Ingram, S.L.; Ahmari, S.E.; Veenstra-VanderWeele, J.; Amara, S.G. Neuronal excitatory amino acid transporter EAAT3: Emerging functions in health and disease. Neurochem. Int. 2019, 123, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bjorn-Yoshimoto, W.E.; Underhill, S.M. The importance of the excitatory amino acid transporter 3 (EAAT3). Neurochem. Int. 2016, 98, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, T.; Jaryal, A.K.; Kumar, V.M.; Mallick, H.N. L-glutamate microinjection in the preoptic area increases brain and body temperature in freely moving rats. NeuroReport 2014, 25, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.; Franklin, R.B.; Costello, L.C. High-affinity L-aspartate transporter in prostate epithelial cells that is regulated by testosterone. Prostate 1993, 22, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Do, P.H.; Tran, P.V.; Bahry, M.A.; Yang, H.; Han, G.; Tsuchiya, A.; Asami, Y.; Furuse, M.; Chowdhury, V.S. Oral administration of a medium containing both D-aspartate-producing live bacteria and D-aspartate reduces rectal temperature in chicks. Br. Poult. Sci. 2017, 58, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Revest, P.A.; Baker, P.F. Glutamate transport in large muscle fibres of Balanus nubilus. J. Neurochem. 1988, 50, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Tabb, J.S.; Ueda, T. Phylogenetic studies on the synaptic vesicle glutamate transport system. J. Neurosci. 1991, 11, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Kane, L.S.; Buttram, J.G.; Urazaev, A.K.; Lieberman, E.M.; Grossfeld, R.M. Uptake and metabolism of glutamate at non-synaptic regions of crayfish central nerve fibers: Implications for axon-glia signaling. Neuroscience 2000, 97, 601–609. [Google Scholar] [CrossRef] [PubMed]

| Gene | Length of Primers (bp) | Sequence of Primers (5′–3′) |
|---|---|---|
| β-actin-qF | 20 | CCCATCTACGAGGGCTACGC |
| β-actin-qR | 23 | CCTTGATGTCTCGCACGATTTCT |
| isoform_10317-qF | 19 | GCCACCCTCTGGATAAACG |
| isoform_10317-qR | 20 | CGGAAGACGAGGTTGCTGTA |
| isoform_286113-qF | 19 | CCCCTCATAGTCTCCTCCC |
| isoform_286113-qR | 25 | GTGCGTCTCGAAGCAGGCCTGGATC |
| isoform_360504-qF | 21 | TCATAGTCTCCTCCCTGGTGT |
| isoform_360504-qR | 17 | CCGTCTTGTGCGTCTCG |
| Sample Name | Raw Reads/M | Clean Reads/M | Q20/% | Q30/% | Clean Reads Ratio/% | Total Mapping/% |
|---|---|---|---|---|---|---|
| Es2201 | 22.88 | 22.04 | 97.66 | 92.64 | 96.33 | 83.94 |
| Es2202 | 22.88 | 22.03 | 97.69 | 92.72 | 96.28 | 83.24 |
| Es2203 | 22.88 | 22.11 | 97.66 | 92.65 | 96.63 | 82.43 |
| Es2701 | 23.2 | 22.04 | 96.52 | 89.89 | 95 | 79.39 |
| Es2702 | 23.04 | 22.04 | 96.59 | 90.14 | 95.66 | 80.21 |
| Es2703 | 23.2 | 22.07 | 96.6 | 90.19 | 95.13 | 80.13 |
| Es3201 | 22.88 | 22.04 | 96.55 | 89.98 | 96.33 | 80.86 |
| Es3202 | 23.36 | 22.09 | 97.97 | 93.58 | 94.56 | 79.78 |
| Es3203 | 22.88 | 22.12 | 97.83 | 93.11 | 96.68 | 81.37 |
| Group | Number of DEGs | Up-Regulated | Down-Regulated |
|---|---|---|---|
| Es22_vs_Es27 | 1243 | 447 | 796 |
| Es22_vs_Es32 | 1486 | 621 | 865 |
| Common | 377 | 149 | 227 |
| Genes | Score | Coverage | E Value | Identity |
|---|---|---|---|---|
| Neohelice granulata crustacean hyperglycemic hormone (CHH) mRNA, complete cds | 861 | 18% | 0 | 93.37% |
| Ptychognathus pusillus crustacean hyperglycaemic hormone mRNA, complete cds | 1103 | 28% | 0 | 86.2% |
| Gecarcoidea natalis hyperglycemic hormone XO preproprotein mRNA, complete cds | 440 | 24% | 1.00 × 10−117 | 73.28% |
| Discoplax celeste hyperglycemic hormone XO preproprotein (CHH) mRNA, complete cds | 425 | 19% | 3.00 × 10−113 | 76.7% |
| Gecarcinus lateralis crustacean-hyperglycemic-hormone-1 mRNA, complete cds | 408 | 16% | 2.00× 10−108 | 77.76% |
| Gecarcinus lateralis crustacean hyperglycemic hormone D (CHH) mRNA, complete cds | 408 | 17% | 2.00× 10−108 | 77.76% |
| Gecarcinus lateralis crustacean hyperglycemic hormone A (CHH) mRNA, complete cds | 404 | 17% | 1.00× 10−106 | 77.57% |
| Potamon ibericum prepro crustacean hyperglycemic hormone X organ isoform mRNA, complete cds | 335 | 15% | 4.00× 10−86 | 76.92% |
| Discoplax celeste hyperglycemic hormone PO preproprotein (CHH) mRNA, complete cds | 419 | 19% | 8.00× 10−64 | 74.22% |
| Grapsus tenuicrustatus crustacean hyperglycaemic hormone mRNA, complete cds | 253 | 15% | 1.00× 10−61 | 72.49% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhou, R.; Zhang, R.; He, Z.; Zhang, M.; Li, R.; Hao, T.; Sun, J. Transcriptome Analysis Reveals Hyperglycemic Hormone and Excitatory Amino Acid Transporter 3 Are Involved in the Thermal Adaptation of Eriocheir sinensis. Fishes 2025, 10, 361. https://doi.org/10.3390/fishes10070361
Li X, Zhou R, Zhang R, He Z, Zhang M, Li R, Hao T, Sun J. Transcriptome Analysis Reveals Hyperglycemic Hormone and Excitatory Amino Acid Transporter 3 Are Involved in the Thermal Adaptation of Eriocheir sinensis. Fishes. 2025; 10(7):361. https://doi.org/10.3390/fishes10070361
Chicago/Turabian StyleLi, Xi, Runlin Zhou, Ruiqi Zhang, Zhen He, Mingzhi Zhang, Ran Li, Tong Hao, and Jinsheng Sun. 2025. "Transcriptome Analysis Reveals Hyperglycemic Hormone and Excitatory Amino Acid Transporter 3 Are Involved in the Thermal Adaptation of Eriocheir sinensis" Fishes 10, no. 7: 361. https://doi.org/10.3390/fishes10070361
APA StyleLi, X., Zhou, R., Zhang, R., He, Z., Zhang, M., Li, R., Hao, T., & Sun, J. (2025). Transcriptome Analysis Reveals Hyperglycemic Hormone and Excitatory Amino Acid Transporter 3 Are Involved in the Thermal Adaptation of Eriocheir sinensis. Fishes, 10(7), 361. https://doi.org/10.3390/fishes10070361






