A Long-Term Overview of Elasmobranch Fisheries in an Oceanic Archipelago: A Case Study of the Madeira Archipelago †
Abstract
1. Introduction
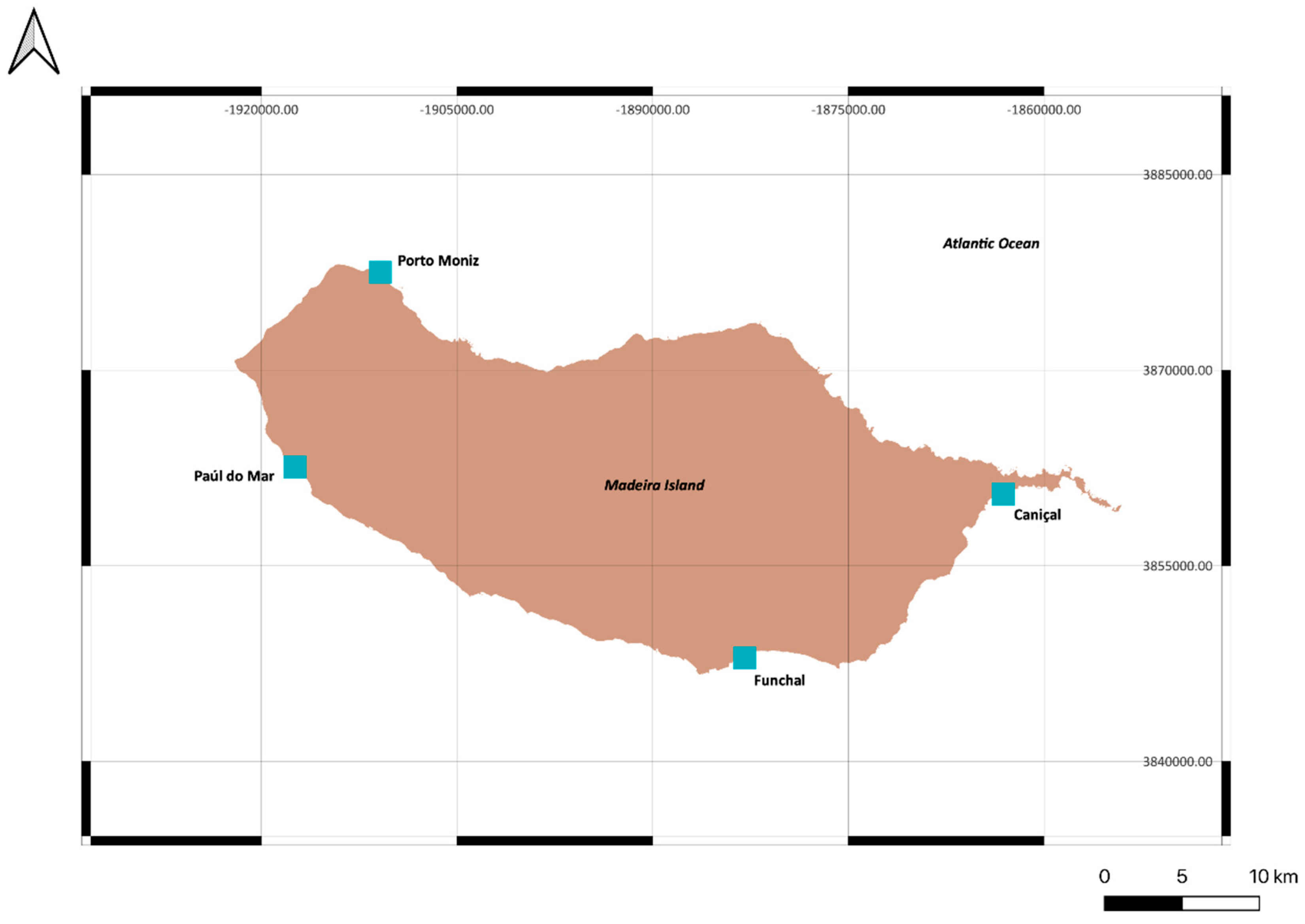
2. Materials and Methods
3. Results
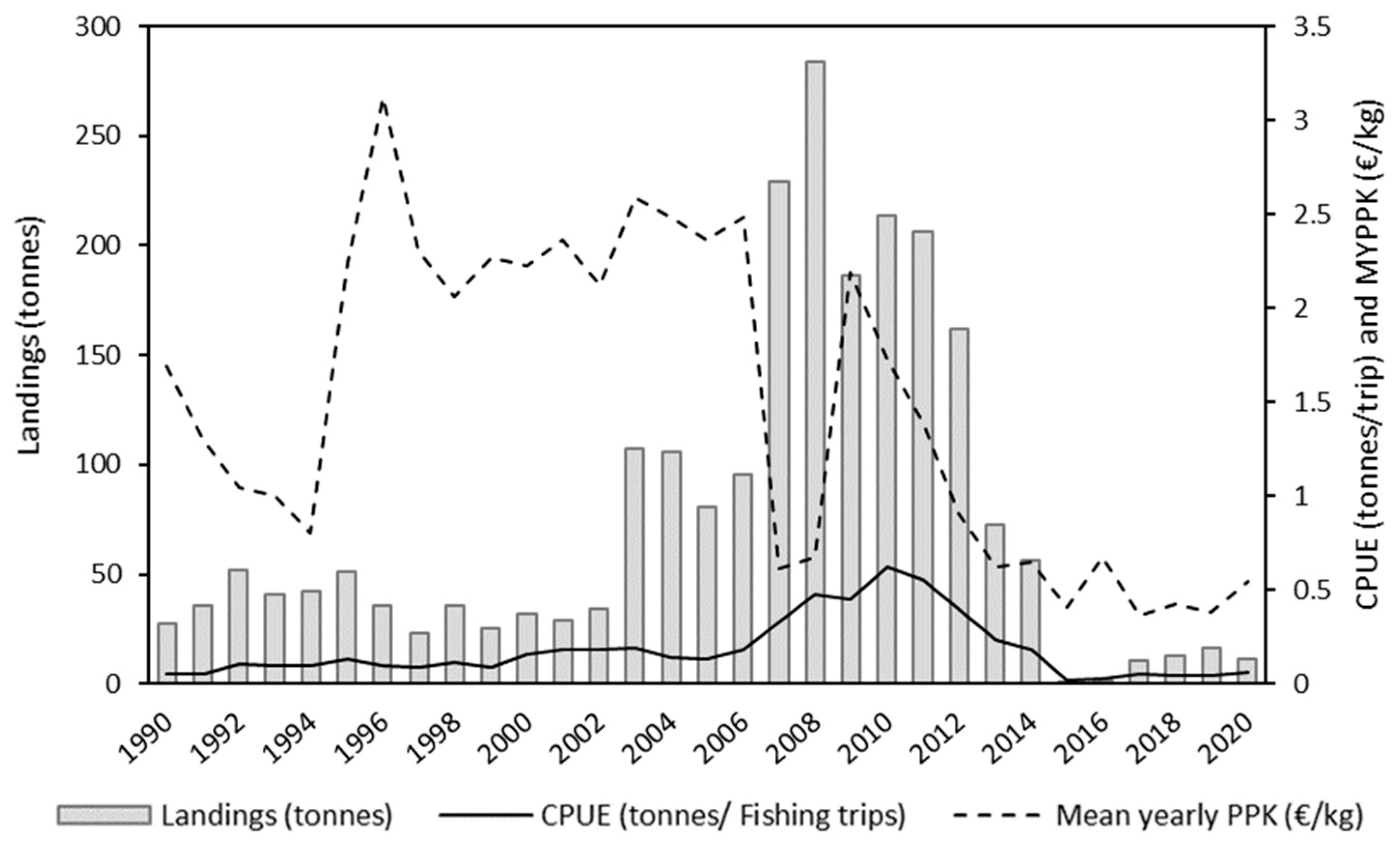
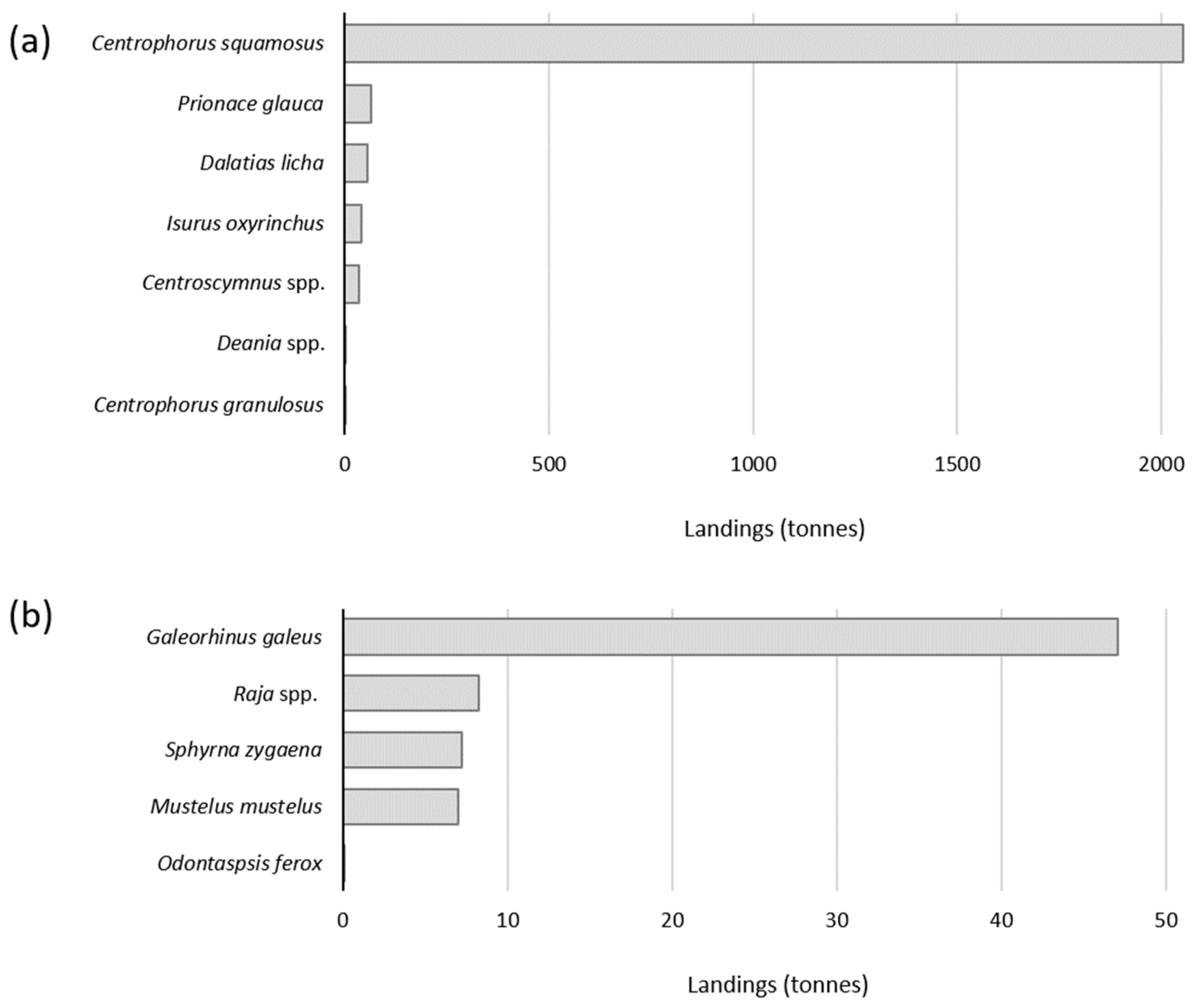
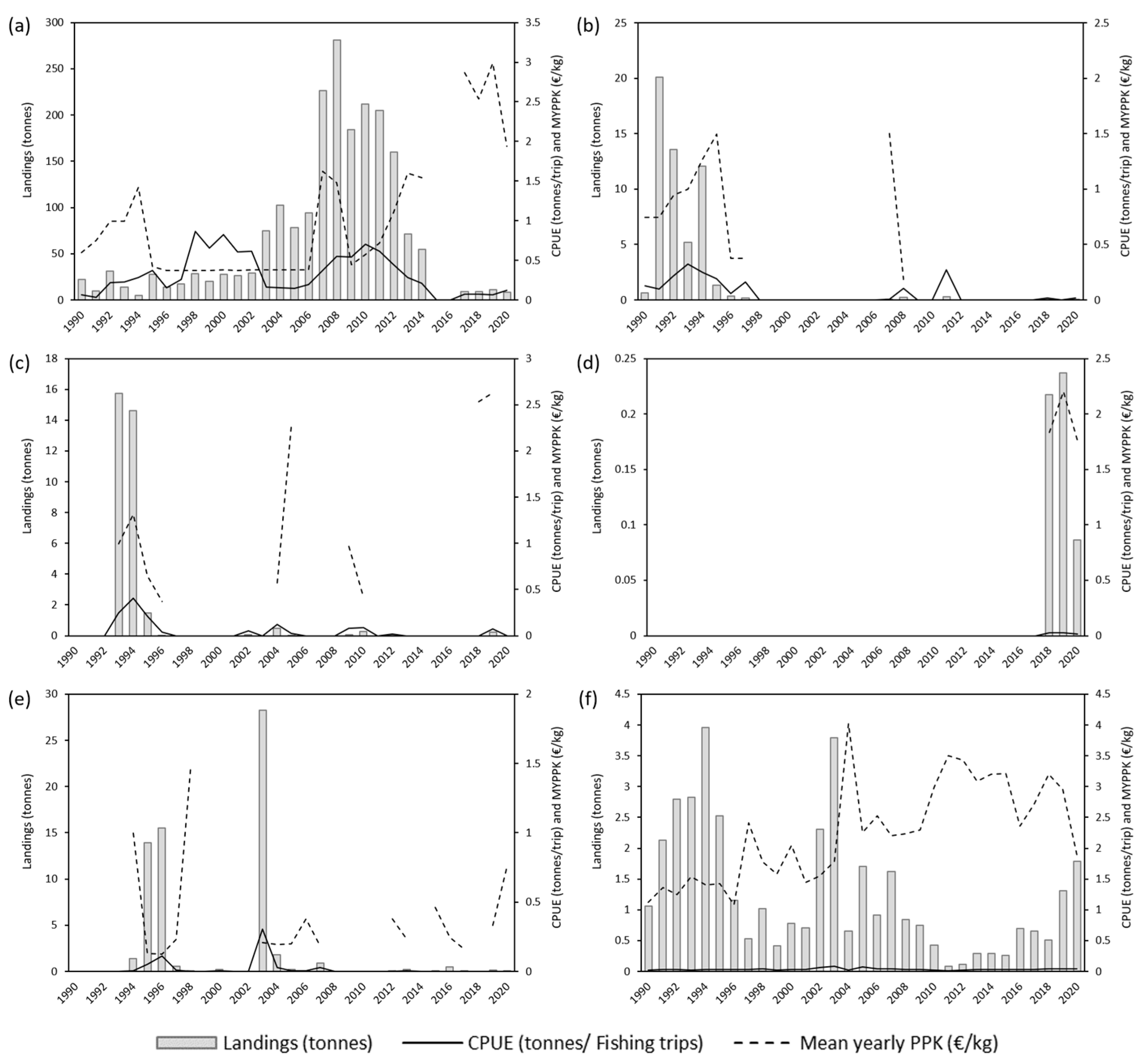
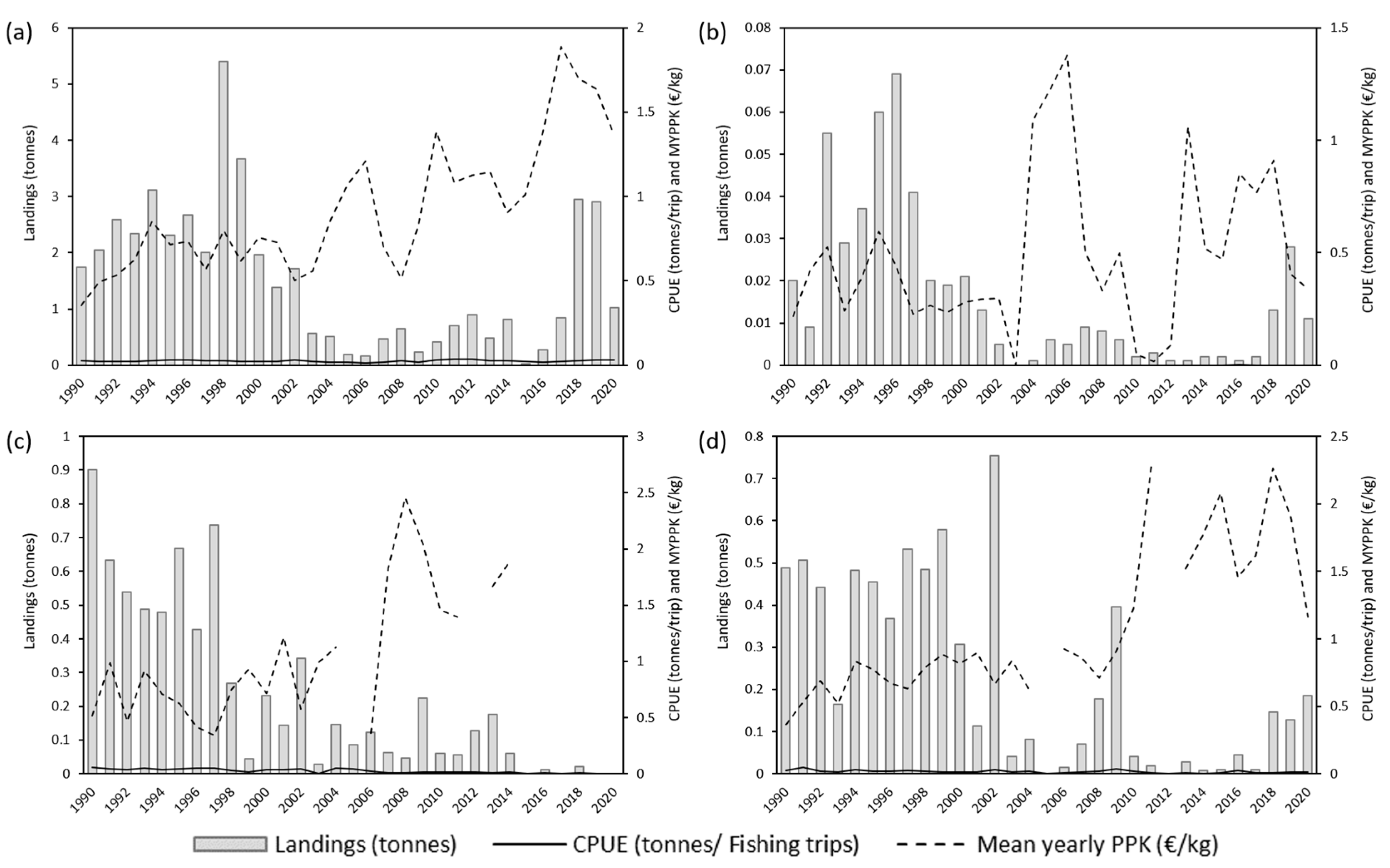
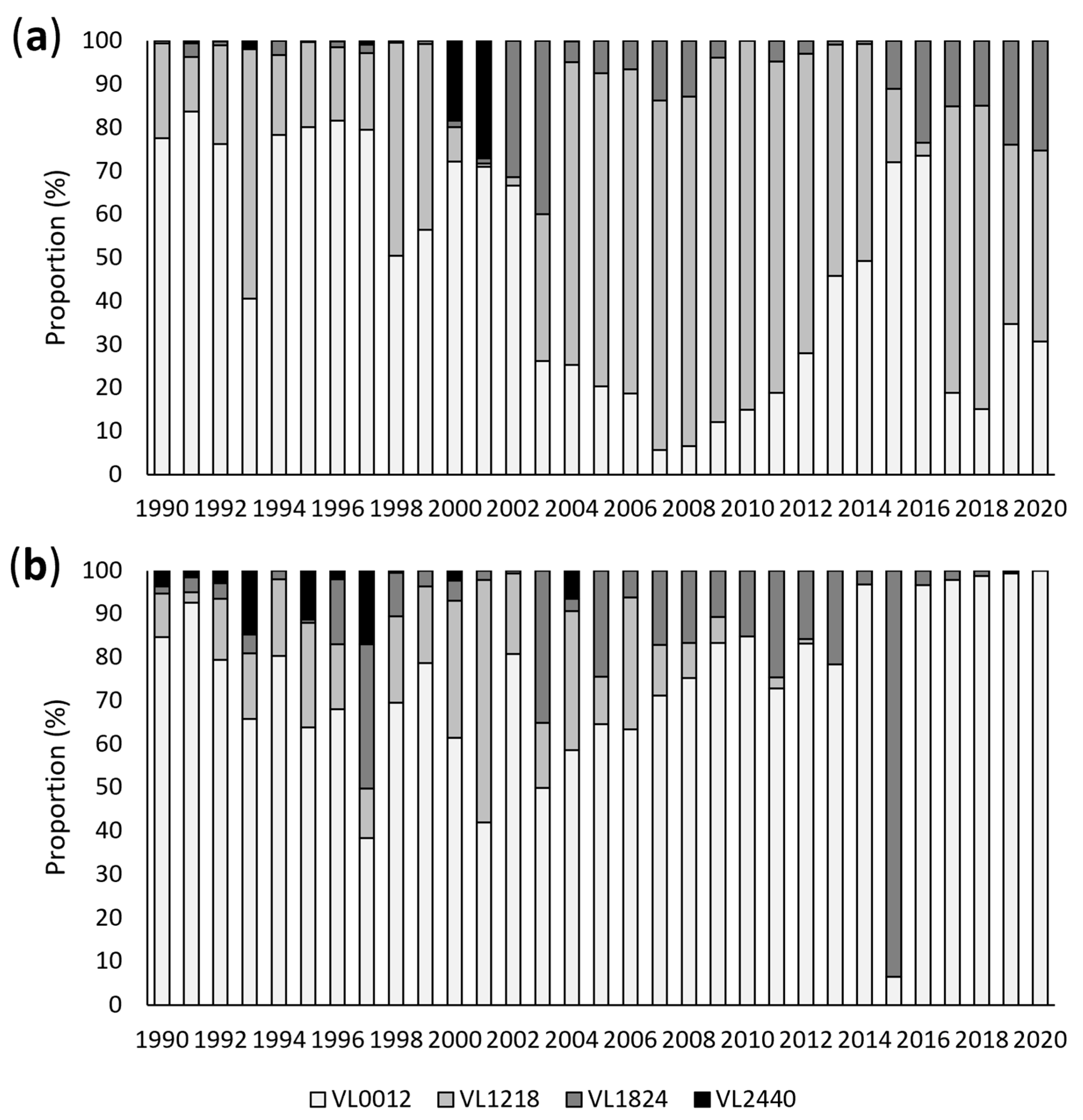
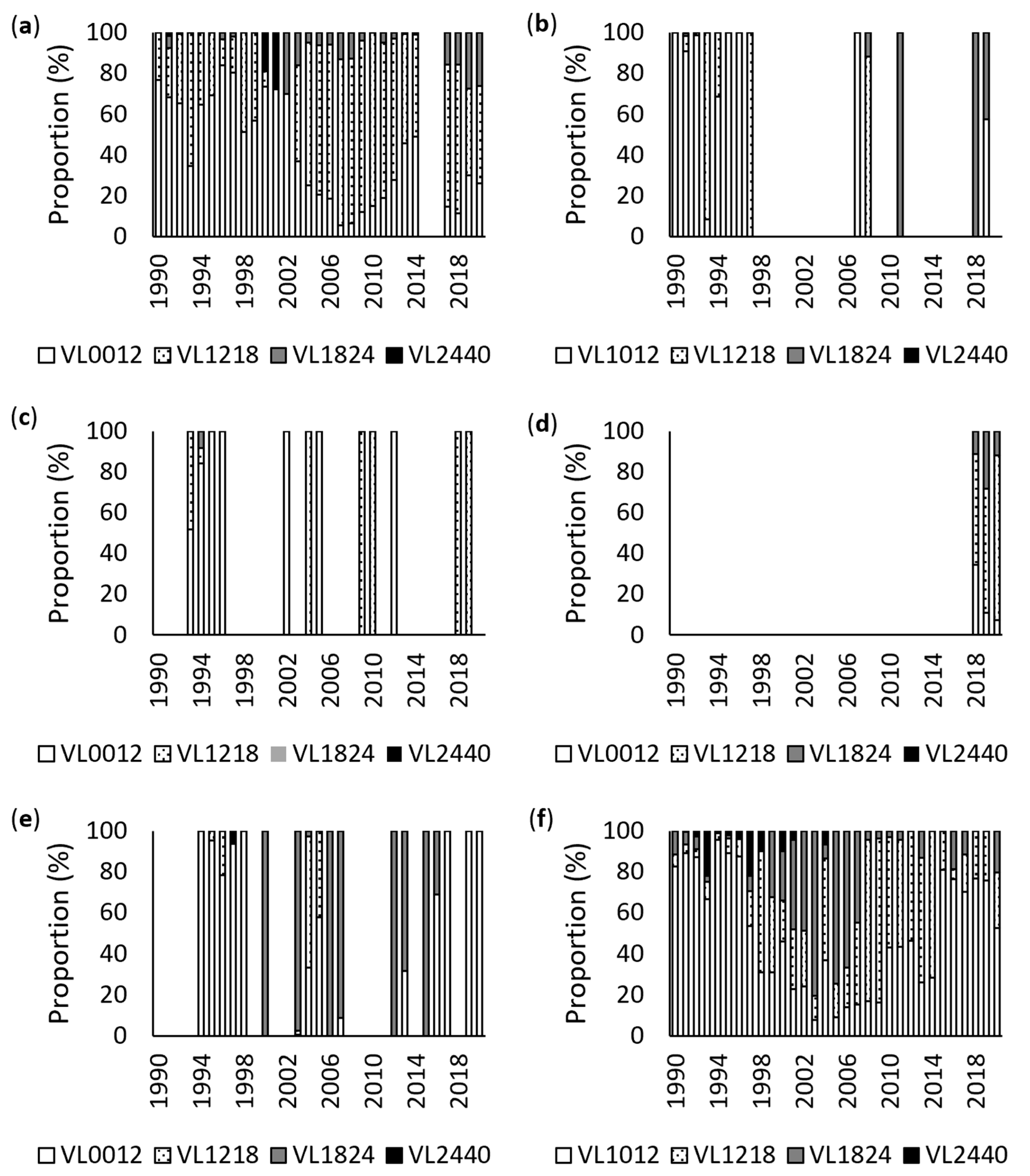
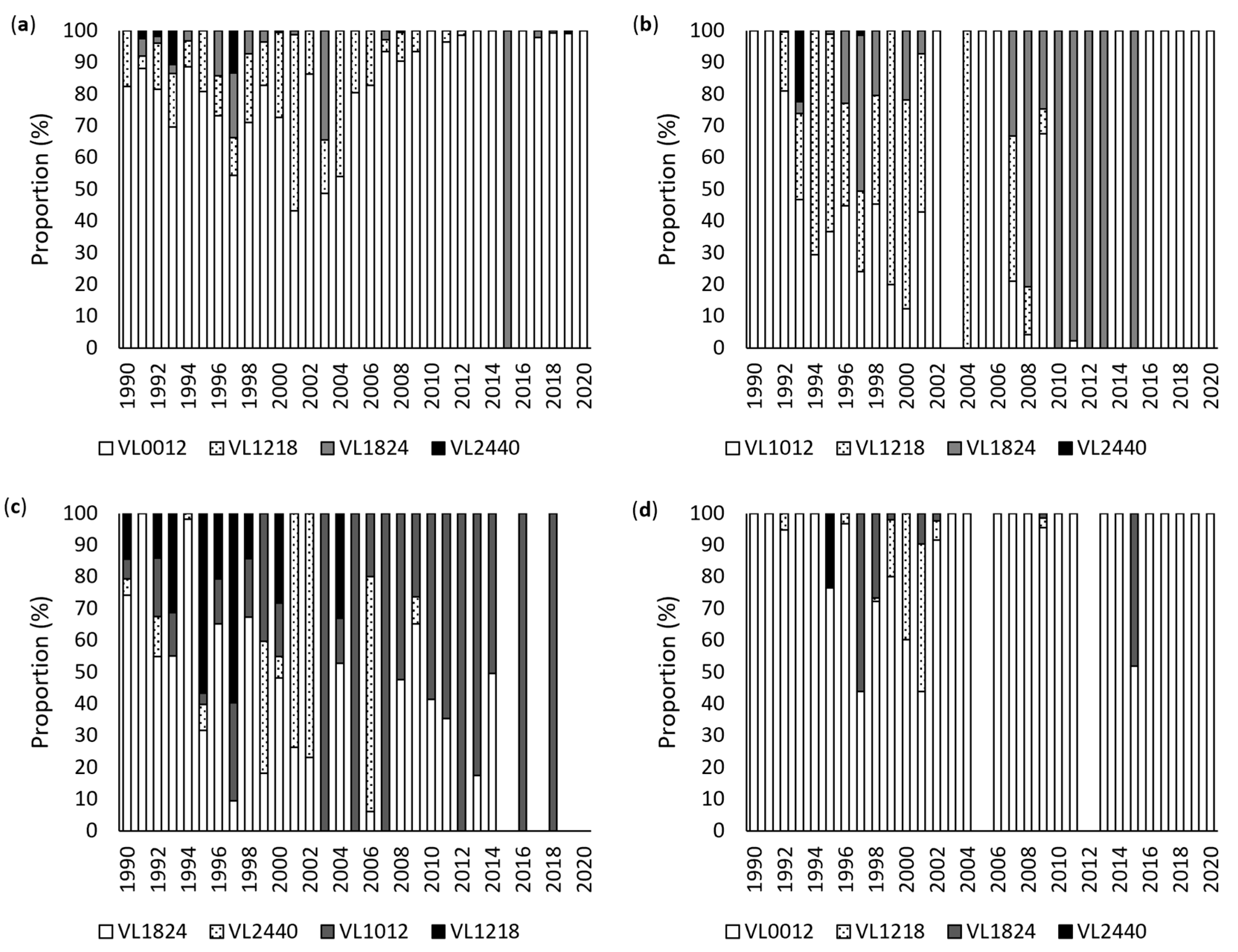
3.1. Annual Analysis
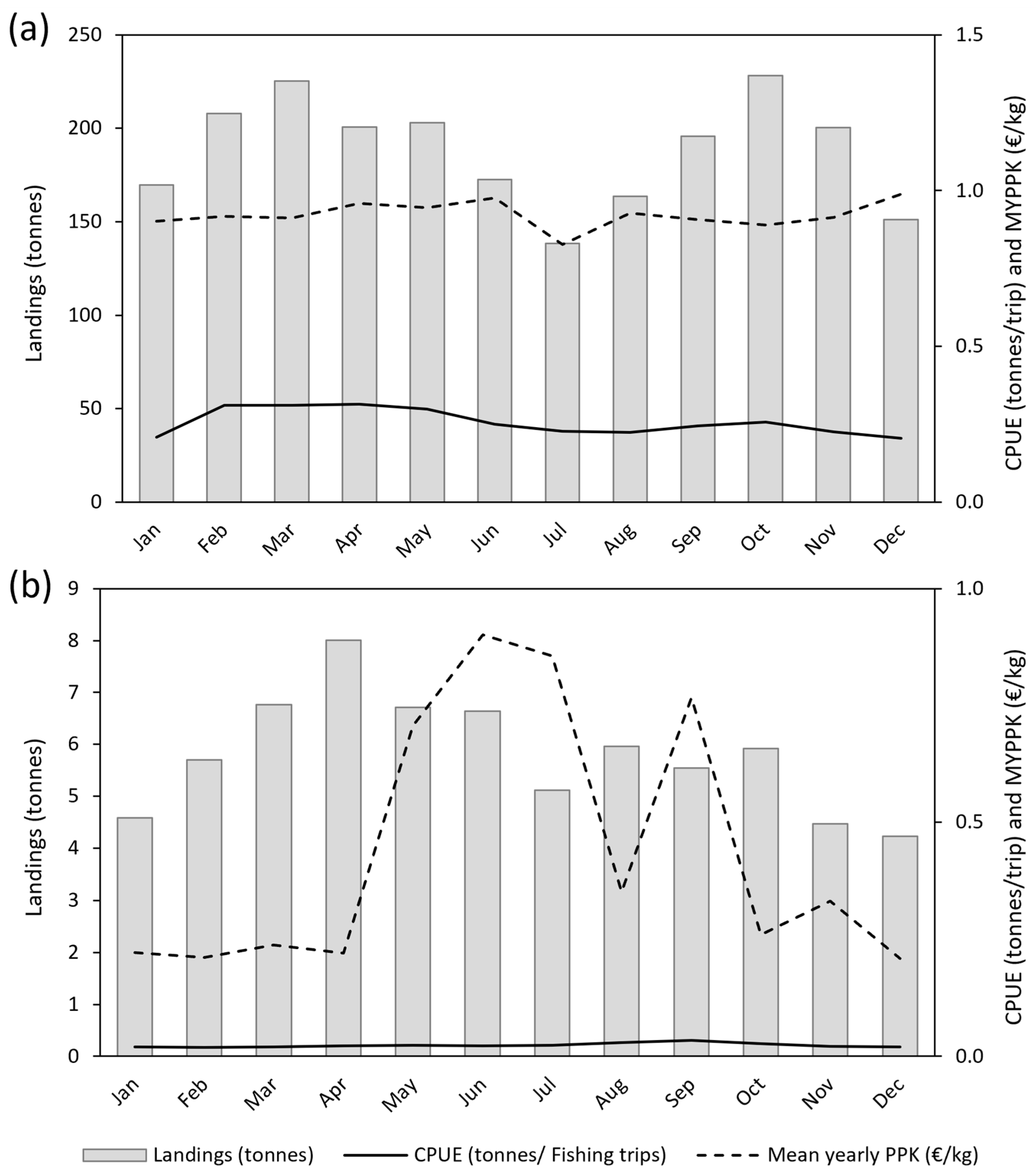
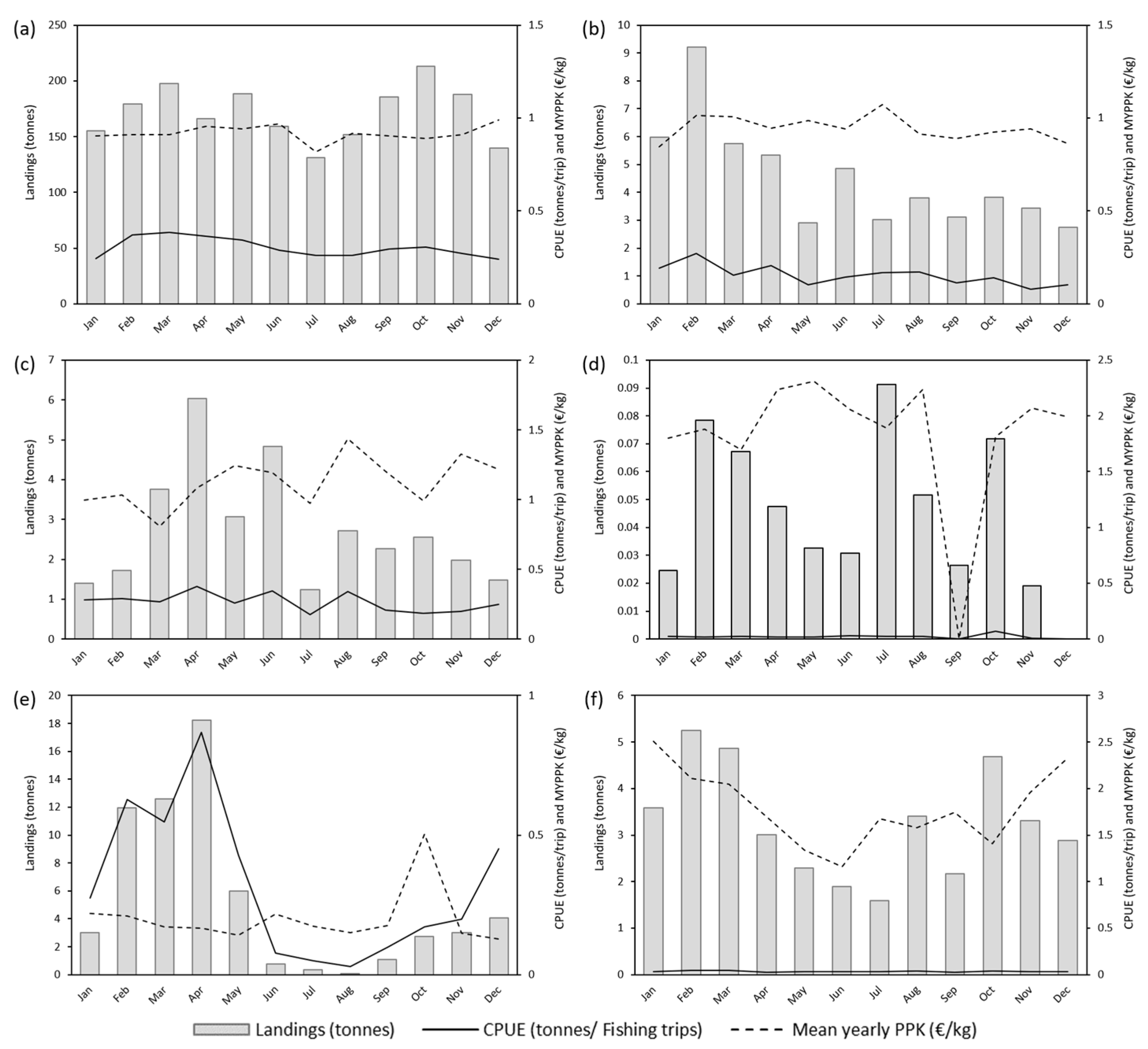
3.2. Monthly Analysis
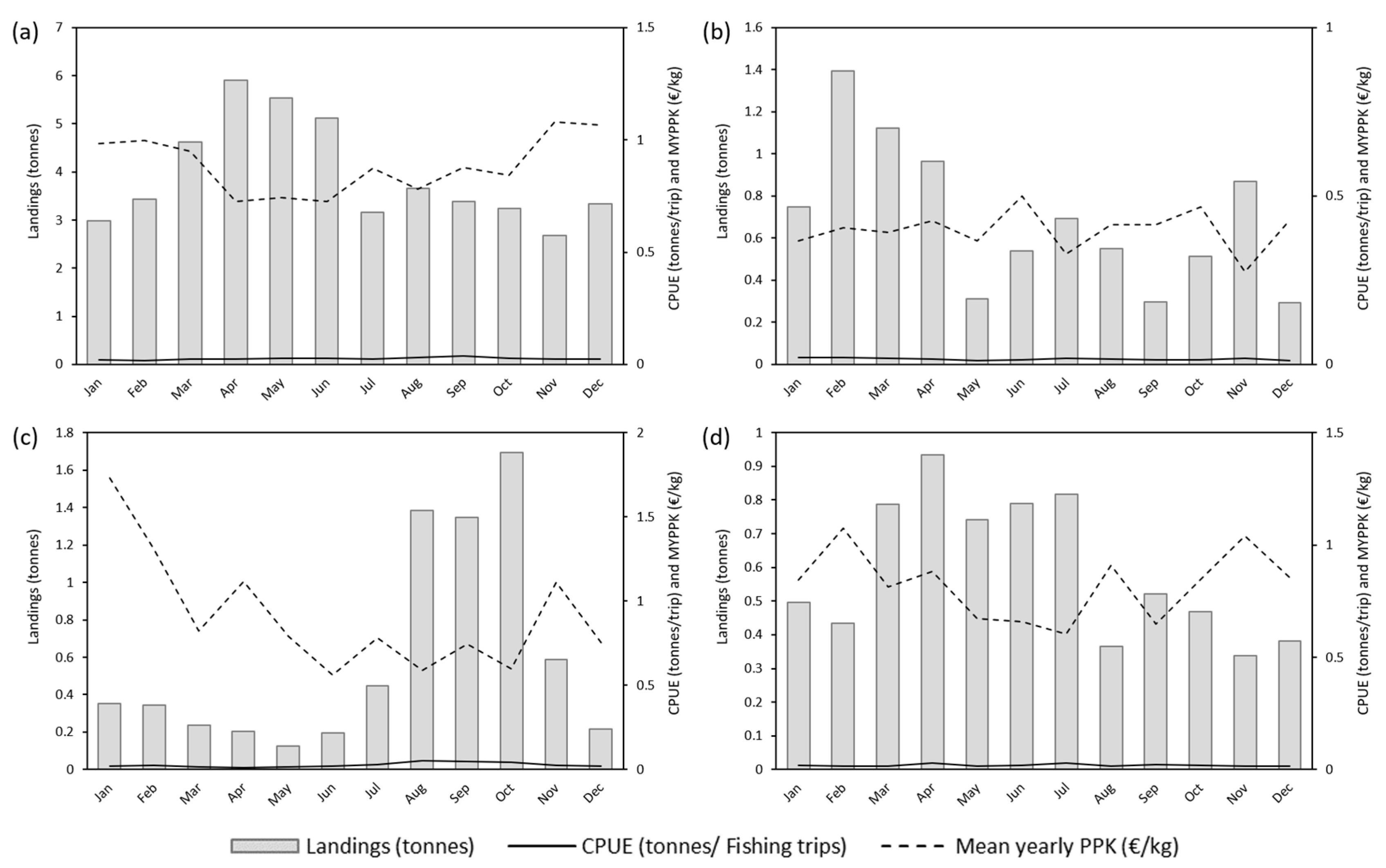
3.3. Vessel Size Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DLL | Mid-water drifting longline |
| BLLs/PLLs/HLs | Bottom longlines, pelagic longlines, and handlines |
| TAC | Total allowable catches |
References
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davisdson, L.N.K.; Fordham, S.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.N.K.; Krawchuk, M.A.; Dulvy, N.K. Why have global shark and ray landings declined: Improved management or overfishing? Fish Fish. 2016, 17, 438–458. [Google Scholar] [CrossRef]
- Bonfil, R. Overview of World Elasmobranch Fisheries; FAO Fisheries Technical Paper, No.341; FAO: Rome, Italy, 1994; ISBN 925-103-566-0. [Google Scholar]
- Gillett, R.D. Marine Fishery Resources of the Pacific Islands; FAO Fisheries and Aquaculture Technical Paper No. 537; FAO: Rome, Italy, 2010; ISBN 978-92-5-106512-9. [Google Scholar]
- Alves, A.; Delgado, J. First Description of a New Local Fishery of Pink Dentex, Dentex (Cheimerius) gibbosus (Rafinesque, 1810) (Pisces: Sparidae) off Madeira Island. In Proceedings of the ICES Annual Science Conference, Copenhagen, Denmark, 1–5 October 2002. Paper No. L:07. [Google Scholar]
- Morato, T. Description of Environmental Issues, Fish Stocks and Fisheries in the EEZs Around the Azores and Madeira; European Commission, Directorate-General for Maritime Affairs and Fisheries: Brussels, Belgium, 2012. [Google Scholar]
- Caldeira, R.M.A.; Groom, S.; Miller, P.; Pilgrim, D.; Nezlin, N.P. Sea-surface signatures of the island mass effect phenomena around Madeira Island, Northeast Atlantic. Remote Sens. Environ. 2002, 80, 336–360. [Google Scholar] [CrossRef]
- Vieira, A. As riquezas novas e os novos mercados de procura e venda. Ilhas Atlânticas Bol. Inst. Hist. Ilha Terceira 1995, LIII, 593–638. [Google Scholar]
- Fowler, S.L.; Cavanagh, R.D. Species Status Reports. In Sharks, Rays and Chimaeras: The Status of the Chondrichthyan Fishes; Fowler, S.L., Cavanagh, R.D., Camhi, M., Burgess, G.H., Caillet, G.M., Fordham, S.V., Simpfendorfer, C.A., Muzick, J.A., Eds.; IUCN/SSC Shark Specialist Group: Gland, Switzerland; IUCN: Cambridge, UK, 2005; pp. 213–361. ISBN 2-8317-0700-5. [Google Scholar]
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Large, P.A.; Hammer, C.; Bergstad, O.A.; Gordon, J.D.M.; Lorance, P. Deep-water fisheries of the Northeast Atlantic: II. Assessment and management approaches. J. Northwest Atl. Fish. Sci. 2003, 31, 151–163. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 1954/2003 of 4 November 2003 on the Management of the Fishing Effort Relating to Certain Community Fishing Areas and Resources and Modifying Regulation (EC) No 2847/93 and Repealing Regulations (EC) No 685/95 and (EC) No 2027/95. Off. J. Eur. Union 2003, L 289, 1–5.
- Delgado, J.; Amorim, A.; Gouveia, L.; Gouveia, N. An Atlantic journey: The distribution and fishing pattern of the Madeira deep sea fishery. Reg. Stud. Mar. Sci. 2018, 23, 107–111. [Google Scholar] [CrossRef]
- ICES. Working Group on the Biology and Assessment of Deep-Sea Fisheries Resources (WGDEEP); No. 40; ICES: Copenhagen, Denmark, 2022; Volume 4. [Google Scholar] [CrossRef]
- Council Regulation (EU) 2021/91 of 28 January 2021 Fixing, for the Years 2021 and 2022, the Fishing Opportunities for Union Fishing Vessels for Certain Deep-Sea Fish Stocks. Off. J. Eur. Union 2021, L 31, 20–30.
- Freitas, M.; Vieira, S.; Costa, L.; Delgado, J.; Biscoito, M.; González, J.A. First records of Chimaera opalescens (Holocephali: Chimaeriformes: Chimaeridae) from Madeira and north-west African coast. Acta Ichthyol. Piscat. 2017, 47, 81–84. [Google Scholar] [CrossRef]
- Severino, R.B.; Afonso-Dias, I.; Delgado, J.; Afonso-Dias, M. Aspects of the biology of the leaf-scale gulper shark Centrophorus squamosus (Bonnaterre, 1788) off Madeira archipelago. Arquipélago Life Mar. Sci. 2009, 26, 57–61. Available online: http://hdl.handle.net/10400.3/450 (accessed on 3 February 2024).
- Biscoito, M.; Ribeiro, C.; Freitas, M. Annotated checklist of the fishes of the archipelago of Madeira (NE Atlantic): I-Chondrichthyes. Zootaxa 2018, 4429, 459–494. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Ralph, G.M.; Comeros-Raynal, M.T.; Kemp, J.; García Criado, M.; Allen, D.J.; Dulvy, N.K.; Walls, R.H.L.; Russell, B.; Pollard, D.; et al. European Red List of Marine Fishes; Publications Office of the European Union: Luxembourg, 2015. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice-Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Clarke, M.W. Sharks, skates and rays in the northeast Atlantic: Population status, advice and management. J. Appl. Ichthyol. 2009, 25, 3–8. [Google Scholar] [CrossRef]
- Denney, N.H.; Jennings, S.; Reynolds, J.D. Life-history correlates of maximum population growth rates in marine fishes. Proc. R. Soc. B Biol. Sci. 2002, 269, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Reis, S.; González, J.A.; Isidro, E.; Biscoito, M.; Freitas, M.; Tuset, V.M. Reproduction and growth of Aphanopus carbo and A. intermedius (Teleostei: Trichiuridae) in the northeastern Atlantic. J. Appl. Ichthyol. 2013, 29, 1008–1014. [Google Scholar] [CrossRef]
- Yildiz, T.; Ulman, A.; Karakulak, F.S.; Uzer, U.; Demirel, N. Bio-economic indicators of fisheries: Impact of variations in landings and fish size on market prices in Istanbul Fish Market. PeerJ 2023, 11, e15141. [Google Scholar] [CrossRef] [PubMed]
- ICES. Report of the Working Group on Elasmobranch Fishes (WGEF); ICES: Copenhagen, Denmark, 2024; Volume 6, Issue 75. [Google Scholar] [CrossRef]
- Roxo, A.; Mendes, S.; Correia, J. Portuguese Commercial Fisheries of Swordfish, Xiphias gladius. Rev. Fish. Sci. Aquacult. 2017, 25, 150–157. [Google Scholar] [CrossRef]
- Aranha, S.G.; Teodósio, A.; Marsili, T.; Pires da Rocha, P.; Modesto, T.; Guerreiro, P.M.; Tambutte, A.; Alves, A.; Relvas, P.; Dias, E. Under pressure: Deep-sea elasmobranchs experience high mortality and stress in a crustacean trawling fishery. Front. Fish. Sci. 2025, 3, 1473376. [Google Scholar] [CrossRef]
- Torres, P.; Cunha, R.T.; Rodrigues, A.S. The elasmobranch fisheries of the Azores. Mar. Policy 2016, 73, 108–118. [Google Scholar] [CrossRef]
- Capapé, C.; Ben Souissi, J.; Mejri, H.; Guelorget, O.; Hemida, F. The reproductive biology of the school shark, Galeorhinus galeus Linnaeus 1758 (Chondrichthyes: Triakidae), from the Maghreb shore (southern Mediterranean). Acta Adriat. 2005, 46, 109–124. Available online: https://hrcak.srce.hr/84 (accessed on 25 March 2024).
- Thorburn, J.; Neat, F.; Burrett, I.; Henry, L.A.; Bailey, D.M.; Jones, C.S.; Noble, L.R. Ontogenetic variation in movements and depth use, and evidence of partial migration in a benthopelagic elasmobranch. Front. Ecol. Evol. 2019, 7, 353. [Google Scholar] [CrossRef]
- Vasconcelos, J.; Sousa, R.; Henriques, P.; Amorim, A.; Delgado, J.; Riera, R. Two sympatric, not externally discernible, and heavily exploited deepwater species with coastal migration during spawning season: Implications for sustainable stocks management of Aphanopus carbo and Aphanopus intermedius around Madeira. Can. J. Fish. Aquat. Sci. 2020, 77, 124–131. [Google Scholar] [CrossRef]
- Campos, A.; Leitão, P.; Sousa, L.; Gaspar, P.; Henriques, V. Spatial patterns of fishing activity inside the Gorringe bank MPA based on VMS, AIS and e-logbooks data. Mar. Policy 2023, 147, 105356. [Google Scholar] [CrossRef]
- O’Keefe, M.; Bengil, E.G.T.; Palmer, J.L.; Beton, D.; Çağlar, Ç.; Godley, B.J.; Özkan, M.; Snape, R.T.E.; Broderick, A.C. Diversity and distribution of elasmobranchs in the coastal waters of Cyprus: Using bycatch data to inform management and conservation. Front. Mar. Sci. 2023, 10, 1181437. [Google Scholar] [CrossRef]
- Liu, K.M.; Huang, L.H.; Su, K.Y. Assessment of the impact on 20 pelagic fish species by the Taiwanese small-scale longline fishery in the Western North Pacific using ecological risk assessment. Animals 2022, 12, 2124. [Google Scholar] [CrossRef] [PubMed]
- Fauconnet, L.; Ribeiro, A.; Catarino, D.; Giacomello, E.; Das, D.; Morato, T.; Rosa, A.; Afonso, P. Guia dos Tubarões de Profundidade dos Açores; Publicações Científicas Okeanos: Ponta Delgada, Portugal, 2020; ISBN 978-989-99834-2-7. [Google Scholar]
- Cameron, L.W.; Roche, W.K.; Beckett, K.; Payne, N.L. A review of elasmobranch catch-and-release science: Synthesis of current knowledge, implications for best practice and future research directions. Conserv. Physiol. 2023, 11, coad100. [Google Scholar] [CrossRef] [PubMed]
- Bowlby, H.D.; Benoît, H.P.; Joyce, W.; Sulikowski, J.; Coelho, R.; Domingo, A.; Cortés, E.; Hazin, F.; Macias, D.; Biais, G.; et al. Beyond post-release mortality: Inferences on recovery periods and natural mortality from electronic tagging data for discarded lamnid sharks. Front. Mar. Sci. 2021, 8, 619190. [Google Scholar] [CrossRef]
- Lima, F.D.; Parra, H.; Alves, R.B.; Santos, M.A.; Bjorndal, K.A.; Bolten, A.B.; Vandeperre, F. Correction: Effects of gear modifications in a North Atlantic pelagic longline fishery: A multiyear study. PLoS ONE 2024, 19, e0315684. [Google Scholar] [CrossRef] [PubMed]
- Suuronen, P.; Gilman, E. Monitoring and managing fisheries discards: New technologies and approaches. Mar. Policy 2020, 116, 103554. [Google Scholar] [CrossRef]
- Stahl, J.P.; Tucker, J.B.; Hawn, L.A.; Bradford, A.L. The Role of Electronic Monitoring in Assessing Post-Release Mortality of Protected Species in Pelagic Longline Fisheries; U.S. Department of Commerce: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Chan, A.N.; Long, K.; Alger, B. Examining the Potential of Electronic Monitoring to Augment Protected Species Bycatch Data Collection; U.S. Department of Commerce: Washington, DC, USA, 2024. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, M.; Pinho-Duarte, F.; Gaspar, M.; Ideia, P.; Delgado, J.; Cerqueira, S.C.; Sousa, R. A Long-Term Overview of Elasmobranch Fisheries in an Oceanic Archipelago: A Case Study of the Madeira Archipelago. Fishes 2025, 10, 358. https://doi.org/10.3390/fishes10070358
Freitas M, Pinho-Duarte F, Gaspar M, Ideia P, Delgado J, Cerqueira SC, Sousa R. A Long-Term Overview of Elasmobranch Fisheries in an Oceanic Archipelago: A Case Study of the Madeira Archipelago. Fishes. 2025; 10(7):358. https://doi.org/10.3390/fishes10070358
Chicago/Turabian StyleFreitas, Mafalda, Filipa Pinho-Duarte, Madalena Gaspar, Pedro Ideia, João Delgado, Sara C. Cerqueira, and Ricardo Sousa. 2025. "A Long-Term Overview of Elasmobranch Fisheries in an Oceanic Archipelago: A Case Study of the Madeira Archipelago" Fishes 10, no. 7: 358. https://doi.org/10.3390/fishes10070358
APA StyleFreitas, M., Pinho-Duarte, F., Gaspar, M., Ideia, P., Delgado, J., Cerqueira, S. C., & Sousa, R. (2025). A Long-Term Overview of Elasmobranch Fisheries in an Oceanic Archipelago: A Case Study of the Madeira Archipelago. Fishes, 10(7), 358. https://doi.org/10.3390/fishes10070358





