The Temporal Dynamics of the Impact of Overfishing on the Resilience of the Sarotherodon melanotheron (Rüppel, 1858) Fish Species’ Population in the West African Lake Toho
Abstract
1. Introduction
2. Materials and Methods
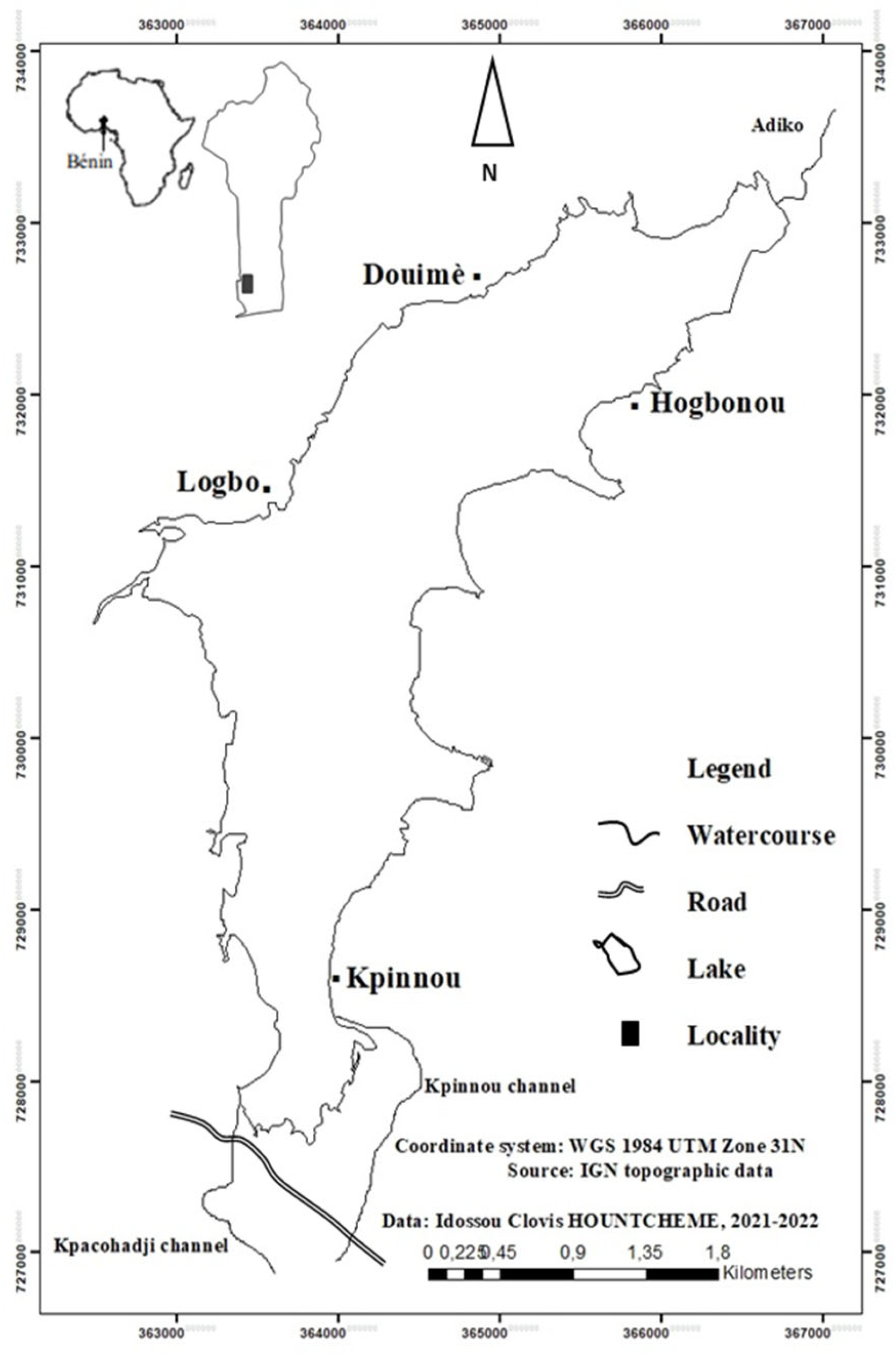
2.1. Study Area
2.2. Fish Sampling and Data Analysis
2.3. Data Organization
2.3.1. Size at First Sexual Maturity (Lm50) and Sexually Mature Fish
2.3.2. Sexually Mature Fish in the Catches
2.3.3. Estimation of Growth Parameters
2.3.4. Mortality and Exploitation Rates
2.3.5. Size at First Capture (Lc50 and the Optimal Length (Lopt))
2.3.6. Relative Yield per Recruit (Y’/R) and Relative Biomass per Recruit (B’/R)
2.3.7. Yield per Recruit (Y/R) and the Maximum Sustainable Yield (MSY)
2.3.8. Justification for the Use Micha’s Scale in 2002–2003 and Brown-Peterson’s Scale in 2011
2.3.9. Water Temperature
3. Results
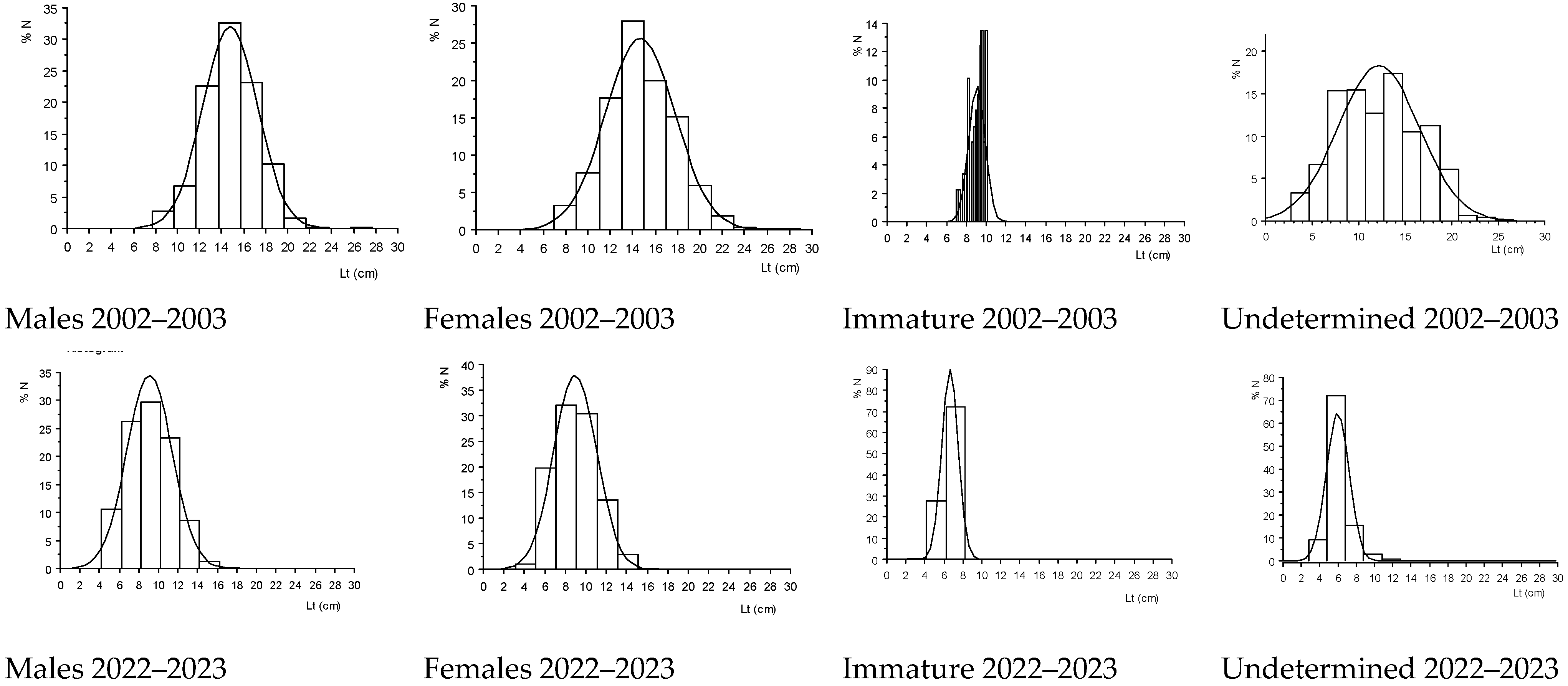
3.1. Structural Characteristics of the S. melanotheron Population
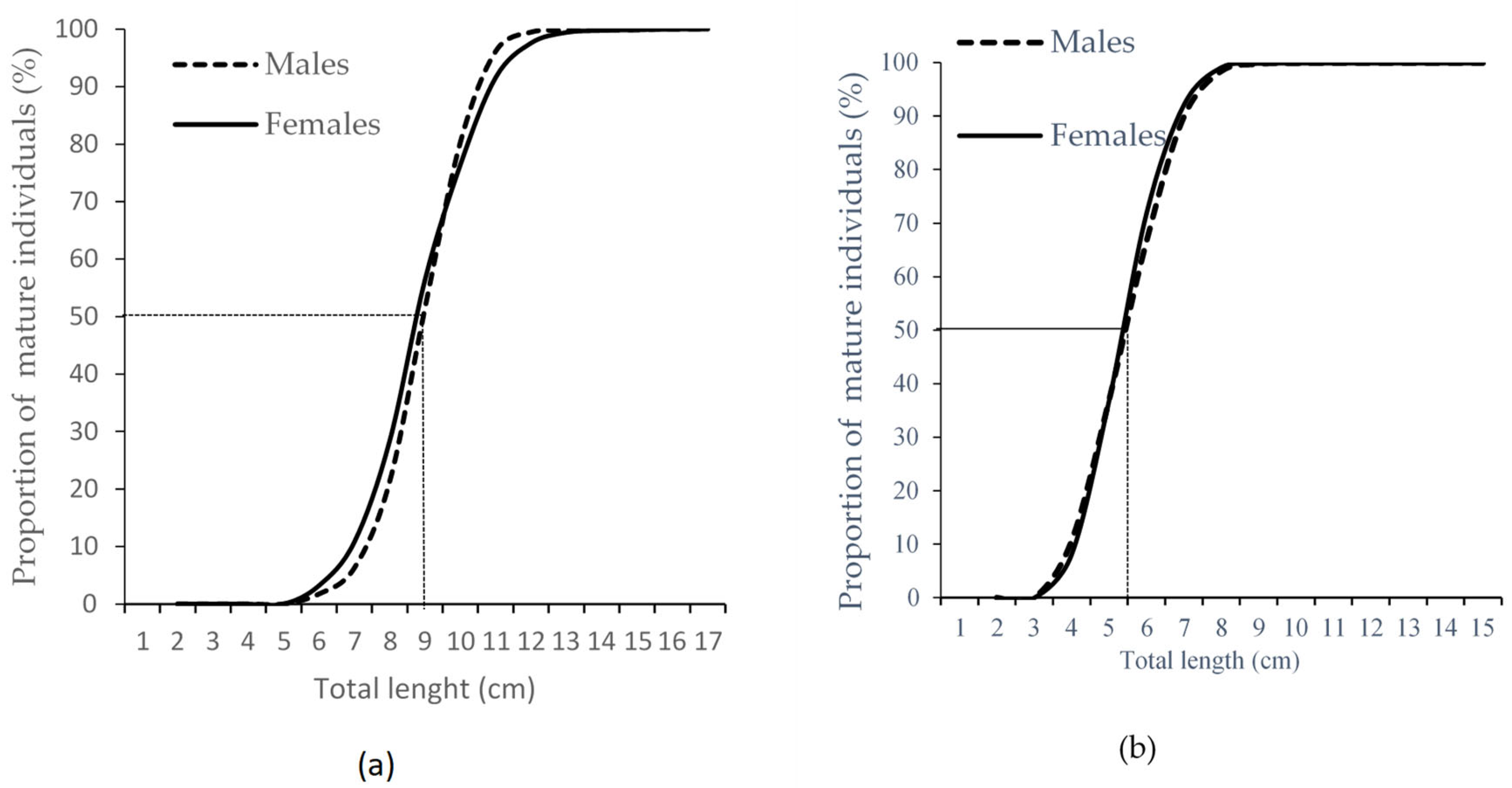
3.1.1. Size at First Sexual Maturity (Lc (Lm50))
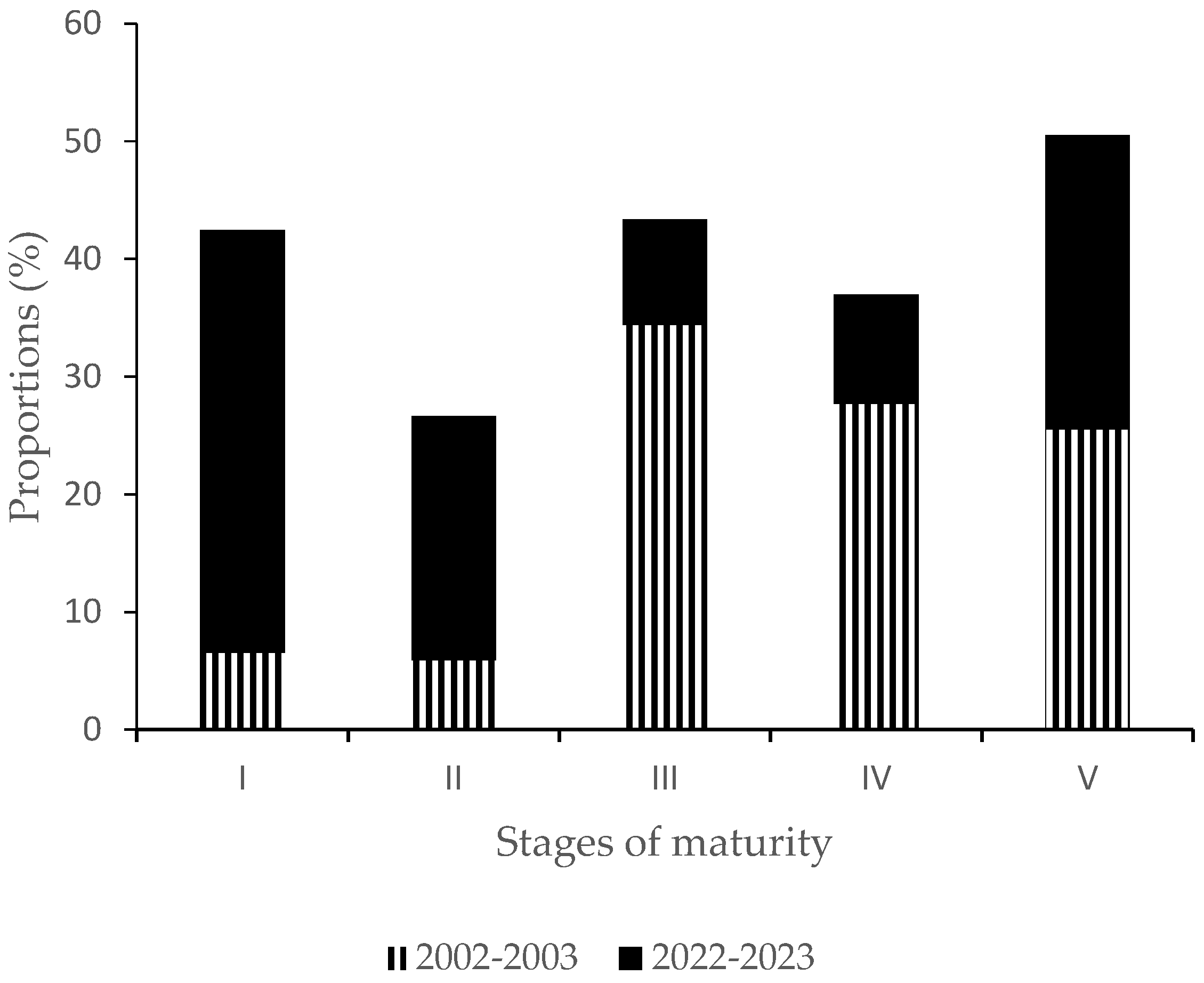
3.1.2. Percentage of Sexually Mature Fish in Catches
3.1.3. Estimation of Growth Parameters
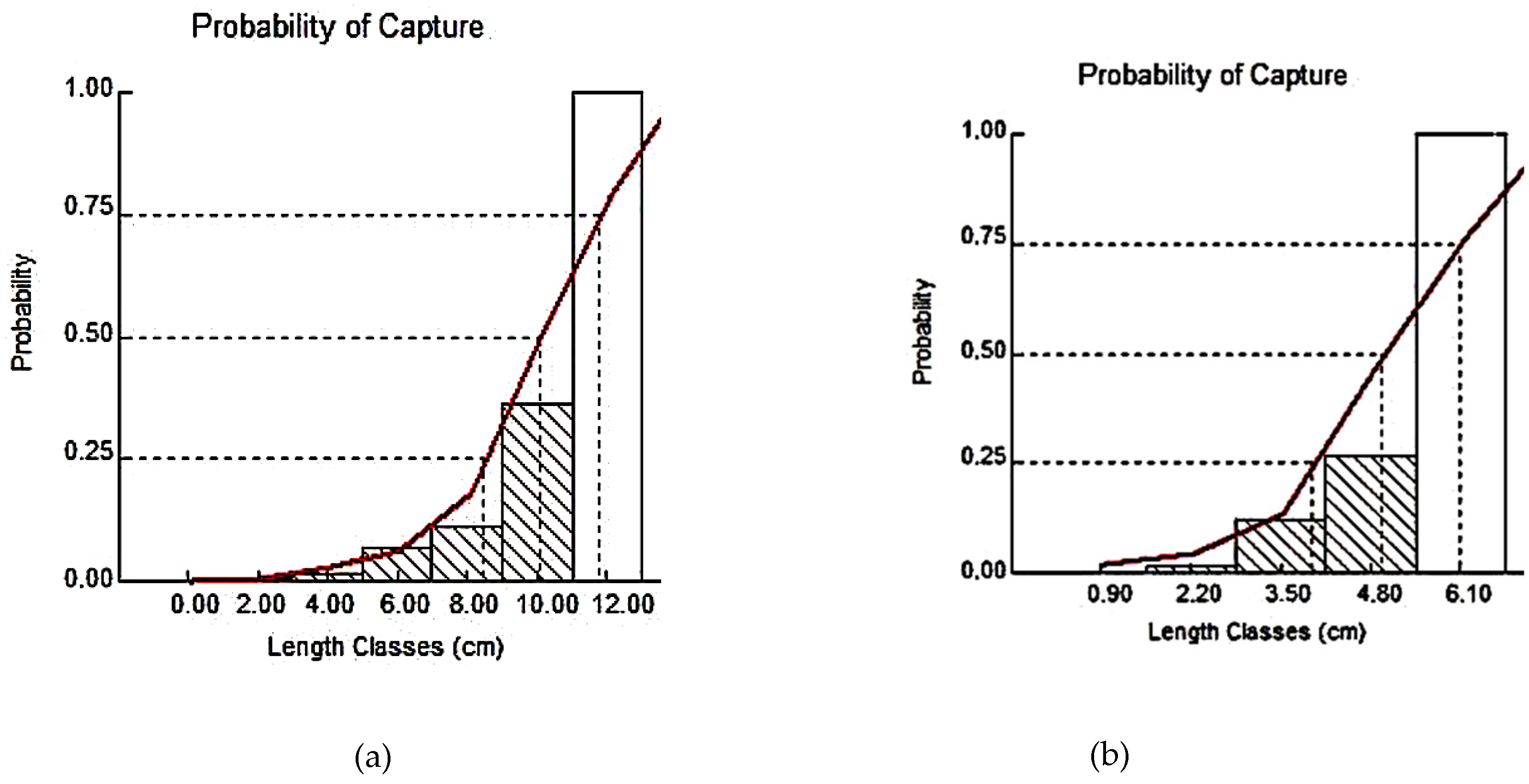
3.1.4. First-Capture Sizes (L50C) and Optimal Lengths (Lopt)
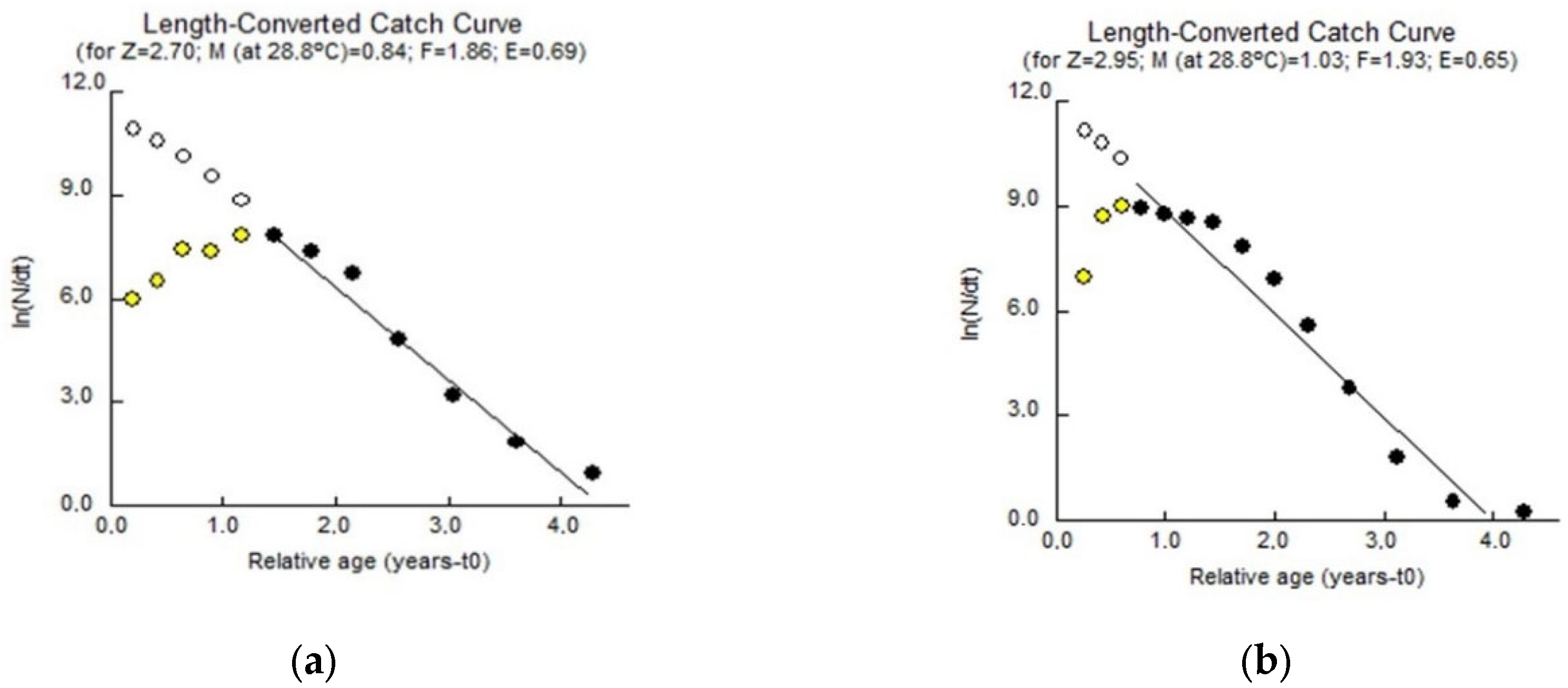
3.1.5. Mortality and Exploitation Rates
3.1.6. Relative Yield Per Recruit (Y’/R) and Relative Biomass Per Recruit (B’/R)
3.1.7. Maximum Sustainable Yield (MSY)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curtean-Bănăduc, A.; Olosutean, H.; Bănăduc, D. The evaluation of ecosystem resources and services—A short historical review. Acta Oec. Carp. 2023, XVI, 81–88. [Google Scholar]
- Li, H.; Yu, X.; Wu, B.; Yu, L.; Wang, D.; Wang, K.; Wang, S.; Chen, D.; Li, Y.; Duan, X.; et al. Temporal and Spatial Distribution Characteristics of Fish Resources in a Typical River-Lake Confluence Ecosystem During the Initial Period of Fishing Ban. Fishes 2024, 9, 492. [Google Scholar] [CrossRef]
- Bănăduc, D.; Voicu, R.; Voicu, L.; Baki, A.B.M.; Serrano, I.; Barb, C.; Curtean-Bănăduc, A. Coştei hydrographic diversion node, a historical environment quality and biological resources accessibility game changer (Middle Danube Watershed); anthropogenic induced problems and sustainable solutions—An ichthyologic perspective. Transylv. Rev. Syst. Ecol. Res. 2021, 23, 87–114. [Google Scholar]
- Da Silveira, E.L.; Semmar, N.; Ballester, E.L.C.; Vaz-dos-Santos, A.M. Integrative Analysis to manage Aquatic Resources Based on Fish Feeding Patterns in Neotropical Rivers. Fishes 2023, 8, 157. [Google Scholar] [CrossRef]
- Bănăduc, D.; Simić, V.; Cianfaglione, K.; Barinova, S.; Afanasyev, S.; Öktener, A.; McCall, G.; Simić, S.; Curtean-Bănăduc, A. Freshwater as a sustainable resource and generator of secondary resources in the 21st century: Stressors, threats, risks, management and protection strategies, and conservation approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Olosutean, H.; Bănăduc, D. Influence of environmental variables on the structure and diversity of ephemeropteran communities: A case study of the Timiș River, Romania. Acta Zool. Bulg. 2016, 68, 215–224. [Google Scholar]
- Bănăduc, D.; Barinova, S.; Cianfaglione, K.; Curtean-Bănăduc, A. Editorial: Multiple freshwater stressors-Key drivers for the future of freshwater environments. Front. Environ. Sci. 2023, 11, 1143706. [Google Scholar] [CrossRef]
- Bănăduc, D.; Afanasyev, S.; Akeroyd, J.R.; Năstase, A.; Năvodaru, I.; Tofan, L.; Curtean-Bănăduc, A. The Achilles Heel of Danube River-Danube Delta-Black Sea Region Fish Diversity under a Black Sea Impact Scenario Due to Sea Level Rise—A Prospective Review. Fishes 2023, 8, 355. [Google Scholar] [CrossRef]
- Oluwakayode-Oluyi, O.O.; Emmanuel, B.E.; Samuel, O.B. Children’s involvement in artisanal fishing in selected fishing communities of Badagry, Lagos (South-West Nigeria). Transylv. Rev. Syst. Ecol. Res. 2025, 27, 59–66. [Google Scholar] [CrossRef]
- Ajiboye, O.R.; Lawal-Are, A.O.; Obiakara-Amaechi, A.I. Trace metal contamination in two fish species from Epe Lagoon (South-West Nigeria): Health risk assessment. Transylv. Rev. Syst. Ecol. Res. 2024, 26, 71–80. [Google Scholar] [CrossRef]
- Sabai, D. The role of integrated coastal management approach in the protection of coastal and marine resources in the eastern coast of Tanzania. Transylv. Rev. Syst. Ecol. Res. 2023, 25, 77–86. [Google Scholar] [CrossRef]
- Olopade, O.A.; Dienye, H.E.; Nwosu, E.I. Some aspects of the growth features and condition factor of Arius gigas (Boulenger, 1911) from Obuama Creek (River State, Nigeria). Transylv. Rev. Syst. Ecol. Res. 2021, 23, 63–74. [Google Scholar] [CrossRef]
- Chu, C.; Barker, J.; Gutowsky, L.; de Kerckhove, D.A. Conceptual Management Framework for Multiple Stressor Interactions in Freshwater Lakes and Rivers; Ontario Ministry of Natural Resources and Forestry: Peterborough, ON, Canada, 2018. [Google Scholar]
- Allan, J.D.; Abell, R.; Hogan, Z.E.B.; Revenga, C.; Taylor, B.W.; Welcomme, R.L.; Winemiller, K. Overfishing of Inland Waters. Bioscience 2005, 55, 1041–1051. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018; Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; ISBN 9789251305621. [Google Scholar]
- Owusu, V. Effects of the closed fishing season on small-scale fisheries and coastal livelihoods in Ghana. Fish. Aquat. Sci. 2024, 27, 753–768. [Google Scholar] [CrossRef]
- Organisation de coopération et de développement économiques (OCDE); Organisation des Nations Unies pour l’alimentation et l’agriculture (FAO). Perspectives Agricoles de l’OCDE et de la FAO 2023–2032; OCDE/FAO: Paris, France, 2023; 330p. [Google Scholar]
- Wohlfarth, G.W.; Hulata, G.G. Applied genetics of tilapias. In ICLARM Stud. Rev. 6, 2nd ed.; ICLARM: Makati, Philippines, 1983; 26p. [Google Scholar]
- Teugels, G.G.; Thys van den Audenaerde, D.F.E. Cichlidae. In The Fresh and Brackish Water Fishes of West Africa, Volume 2; Paugy, D., Lévêque, C., Teugels, G.G., Eds.; Coll. Faune et Flore Tropicale 40; Institut de Recherche de Développement: Paris, France; Musée royale de l’Afrique Centrale: Tervuren, Belgium, 2003; pp. 521–600. [Google Scholar]
- Lederoun, D.; Emmanuel, V.; Chikou, A.; Vreven, E.; Snoeks, J.; Moreau, J.; Vandewalle, P.; Lalèyè, P. Population parameters and exploitation rate of Sarotherodon melanotheron melanotheron rüppell, 1852 (Cichlidae) in Lake Toho, Benin. J. Biodivers. Environ. Sci. 2015, 6, 259–271. [Google Scholar]
- Codjo, V.; Zannou, A.; Biaou, G. Baisse des ressources halieutiques du lac Toho au Sud du Bénin: Perceptions des pêcheurs et efficacité des pratiques de gestion et stratégies d’adaptation. Tropicultura 2018, 36, 713–721. [Google Scholar]
- Meijboom, F.L.B.; Bovenkerk, B. Fish resilience as an ethical issue. J. Fish Biol. 2024, 106, 6–11. [Google Scholar] [CrossRef]
- Sanon, V.-P.; Toé, P.; Caballer Revenga, J.; El Bilali, H.; Hundscheid, L.J.; Kulakowska, M.; Magnuszewski, P.; Meulenbroek, P.; Paillaugue, J.; Sendzimir, J.; et al. Multiple-Line Identification of Socio-Ecological Stressors Affecting Aquatic Ecosystems in Semi-Arid Countries: Implications for Sustainable Management of Fisheries in Sub-Saharan Africa. Water 2020, 12, 1518. [Google Scholar] [CrossRef]
- Ayoubi, H.E.; Failler, P. Industrie des Pêches et de L’aquaculture au Bénin; Rapport N°5 de la Revue de L’industrie des Pêches et de L’aquaculture Dans la Zone de la COMHAFAT; 2013; 141p. Available online: https://www.researchgate.net/publication/277776052_Industrie_des_peches_et_de_l'aquaculture_au_Benin (accessed on 20 May 2025).
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.M.; Torres, F.C., Jr. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Page, L.M.; Burr, B.M. A Field Guide to Freshwater Fishes of North America North of Mexico; Houghton Mifflin Company: Boston, MA, USA, 1991; 432p. [Google Scholar]
- Gobert, B.; Reynal, L. Les méthodes d’évaluation des ressources halieutiques. In La Pêche aux Antilles; IRD Edition: Marseille, France, 2002. [Google Scholar] [CrossRef]
- Trewavas, E. Tilapiine Fishes of the Genera Sarotherodon, Oreochromis and Danakilia; British Museum of Natural History: London, UK, 1983; 583p. [Google Scholar]
- Froese, R. Keep it simple three indicators to deal with overfishing. Fish Fish. 2004, 5, 86–91. [Google Scholar] [CrossRef]
- Trewavas, E.; Teugels, G.G. Sarotherodon. In Check-List of the Freshwater Fishes of Africa (CLOFFA); Daget, J., Gosse, J.-P., Teugels, G.G., Thys van den Audenaerd, D.F.E., Eds.; ISNB: Brussels, Belgium; MRAC: Brussels, Belgium; ORSTOM: Paris, France, 1991; Volume 4, pp. 425–437. [Google Scholar]
- Gabriel, W.L.; Mace, P.M. Examen des points de référence biologiques dans le contexte de l’approche de précaution. In Actes du 5ᵉ Atelier National d’évaluation des Stocks du NMFS: Fournir des Conseils Scientifiques Pour la Mise en œuvre de L’approche de Précaution Dans le Cadre de la loi Magnuson-Stevens sur la Conservation et la Gestion des pêcheries; Restrepo, V.R., Ed.; NOAA Technical Memorandum NMFS-F/SPO-40; U.S. Department of Commerce, National Marine Fisheries Service: Silver Spring, Maryland, USA, 1999; pp. 34–45. [Google Scholar]
- Ahouansou Montcho, S. Etude de l’écologie et de la Production Halieutique du lac Toho au Bénin; Diplôme d’Etude Supérieures Spécialisées en Aménagement et Gestion des Ressources Naturelles; Université d’Abomey-Calavi: Cotonou, Benin, 2003; 113p. [Google Scholar]
- FAO. Points de référence en aménagement des pêcheries. In Document Technique sur les Pêcheries; N° 347; FAO: Rome, Italy, 1997; 111p. [Google Scholar]
- Ahouansou Montcho, S.; Akele, D.G.; Hountcheme, I.A.C.; Montchowui, E.; Nyonkuru, C.; Laleye, P. Impact de la pêche sur la population de Oreochromis niloticus introduite dans le lac Toho (Bénin). Ann. Sci. Agron. 2023, 26, 89–102. [Google Scholar]
- Paugy, D.; Lévêque, C.; Teugels, G.G. Faune des Poissons D’eaux Douces et Saumâtres de l’Afrique de l’Ouest; IRD: Paris, France, 2003. [Google Scholar]
- Micha, J.C. Etude des Populations Piscicoles de l’Ouganda et Tentation de Sélection et D’adaptation de Quelques Espèces à L’étang de Pisciculture; Centre Technique Forestier Tropicale: Nogent-sur-Marne, France, 1973; 110p. [Google Scholar]
- Laleye, P. Ecologie Comparée de Deux Espèces de Chrysichthys, Poissons Siluriformes (Claroteidae) du Complexe lac Nokoué- Lagune de Porto/Novo au Bénin. Ph.D. Thesis, Faculté des Sciences Agronomiques, Université de Liège, Liège, Belgium, 1995; 199p. [Google Scholar]
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A standardized terminology for describing reproductive development in fishes. Mar. Coast. Fish. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Ahouansou Montcho, S.; Laleye, P.A. Some aspects of biology of Oreochromis niloticus L. (Perciformes: Cichlidae) recently introduced in Lake Toho (Benin, West Africa). Int. J. Biol. Chem. Sci. 2008, 2, 114–122. [Google Scholar] [CrossRef]
- Ahouansou Montcho, S. Diversité et Exploitation des Poissons de la Rivière Pendjari (Bénin, Afrique de l’Ouest). Ph.D. Thesis, Université d’Abomey-Calavi, Cotonou, Benin, 2011; 234p. [Google Scholar]
- Ahouansou-Montcho, S.; Agadjihouèdé, H.; Montchowui, E.; Lalèyè, P.A.; Moreau, J. Population parameters of Oreochromis niloticus (Cichlidae) recently introduced in lake Toho (Benin, West Africa). Int. J. Aqut. Stud. 2015, 2, 141–145. [Google Scholar]
- Sturges, H. The choice of a class interval. J. Am. Stat. Assoc. 1926, 21, 65–66. [Google Scholar] [CrossRef]
- Amenzoui, K.; Ferhan-Tachinante, F.; Yahyaoui, A.; Mesfioui, A.H.; Kifani, S. Etude de quelques aspects de la reproduction de la région de Saedina pilchardus (Walbaum, 1792) de la région de Laâyoune (Maroc). Bull. Inst. Sci. 2005, 27, 43–50. [Google Scholar]
- N’Guessan, Y.; N’Guessan, C.D.; Amande, J.M.; Kouame, J.-P.A.; Abekan, E.; Assan, F.N.; N’Da, K. Sex-ratio, stades de maturité, taille de première maturité et facteur de condition de Canthidermis maculata capturé dans l’océan Atlantique Est. Int. J. Biol. Chem. Sci. 2018, 11, 2876. [Google Scholar] [CrossRef]
- Dagnelie, P. Théorie et Méthodes Statistiques, 2nd ed.; Presses Agronomiques de Gembloux: Gembloux, Belgium, 1973; Volume 1. [Google Scholar]
- Albaret, J.J.; Legendre, M. Biologie et écologie des Mugilidae en lagune Ebrié (Côte d’Ivoire) intérêt potentiel pour l’aquaculture lagunaire. Rev. Hydrobiol. Trop. 1985, 18, 281–303. [Google Scholar]
- Gayanilo, F.C.; Sparre, P.; Pauly, D. FAO-ICLARM. Stock Assessment Tools II. User’s Guide; FAO Computerized Information Series (Fisheries); FAO: Rome, Italy, 2005; 168p. [Google Scholar]
- Pauly, D. Une Sélection des Méthodes Simples Pour L’estimation des Stocks de Poissons Tropicaux; Circulaire sur les Pêches N° 729; FAO: Rome, Italy, 1982; 60p. [Google Scholar]
- Pauly, D.; Munro, J.L. Once more: Growth comparisons fish and invertebrates. Fishbyte 1984, 2, 21. [Google Scholar]
- Pauly, D. Theory and Management of Tropical Multispecies Stocks: A Review with Emphasis on the Southeast Asian Demersal Fisheries; ICLARM Studies and Review N° 1; ICLARM: Manila, Philippines, 1979; pp. 1–35. [Google Scholar]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Gulland, J.A. The Fish Resources of the Ocean West Polyfleet; Document Technique de la FAO N0 97; Survey Fishing News (Books) Ltd.: West Polyfleet, UK, 1971; 428p. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. Manual of Methods for Fish Stock Assessment: Part II. Tables of Yield Function; FAO Fisheries Biology; Technical Paper; FAO: Rome, Italy, 1966; pp. 1–67. [Google Scholar]
- Gilbert, A.; Andréfouët, S.; Gascuel, D. Dynamique des Populations, Modélisation Halieutique, Approche de Précaution et Stratégie de Co-Gestion Adaptative des Pêcheries de Bénitiers de Trois îles de Polynésie Française. Rapport Final, Convention 6.0080. 2007. 94p. Available online: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/pleins_textes_6/Fau_trop/36564.pdf (accessed on 20 May 2025).
- Thompson, W.F.; Bell, F.H. Biological statistics of the Pacific halibut fishery. In Effect of Changes in Intensity Upon Total Yield and Yield per Unit of Gear; Report No. 8; International Fisheries Commission: Seattle, WA, USA, 1934; 49p. [Google Scholar]
- Viaho, C.C.; Lederoun, D.; Baglo, I.S.; Ahouansou Montcho, S.; Adandédjan, D.; Agblonon Houelome, T.; Gbedey, N.M.; Laleye, P. Paramètres de population et taux d’exploitation de Sarotherodon melanotheron melanotheron Rüppell (1852, Cichlidae) et Ethmalosa fimbriata (Bowdich, 1825, Clupeidae) dans le lac Ahémé et ses chenaux avant le dragage (Bénin, Afrique de l’Ouest). Int. J. Biol. Chem. Sci. 2021, 15, 1991–2007. [Google Scholar] [CrossRef]
- Niyonkuru, C.; Lalèyè, P.A.; Moreau, J. Impact of acadja fisheries on the population dynamics of Sarotherodon melanotheron and Hemichromis fasciatus in a Lake Nokoué (Benin, West Africa). Knowl. Manag. Aquatic. Ecosyst. 2010, 397, 1. [Google Scholar] [CrossRef][Green Version]
- Lederoun, D.; Amoussou, G.; Baglo, I.S.; Adjibogoun, H.; Vodougnon, H.; Moreau, J.; Lalèyè, P. Growth, mortality and yield of Sarotherodon melanotheron melanotheron (Rüppell, 1852) in the Lake Nokoué and Porto-Novo Lagoon complex Benin, West Africa. Aquat. Living Resour. 2020, 33, 18. [Google Scholar] [CrossRef]
- Anagonou, S.P.G. Diversité et Exploitation des Poissons des lacs Datchi et Wozo au Sud-Bénin. Master’s Thesis, Université d’Abomey-Calavi, Abomey-Calavi, Bénin, 2016; 73p. [Google Scholar]
- Villanueva, M.C.S. Biodiversité et Relations Trophiques dans Quelques Milieux Estuariens et Lagunaires de l’Afrique de l’Ouest: Adaptations aux Pressions Environnementales. Ph.D. Thesis, Institut National Polytechnique de Toulouse, Toulouse, France, 2004; 272p. [Google Scholar]
- Cissé, M.; Kamelan, T.M.; Siaka, B.; Kouamelan, E.P. Population parameters of major fish species in Ayamé 1 man-made lake seven years after the return of non-native fishermen coming back. Rev. De L’environnement et de la Biodiversité-Programme D’appui Strat. à La Rech. Sci. Abidj. Côte D’ivoire 2021, 6, 23–26. [Google Scholar]
- Koranteng, K.A.; Ofori-Danson, P.K.; Entsua-Mensah, M. Fish and fisheries of Muni lagoon in Ghana, West Africa. Biodivers. Conserv. 2000, 9, 487–499. [Google Scholar] [CrossRef]
- Arizi, E.K.; Aggrey-Fynn, J.; Obodai, E.A. Growth, Mortality and Exploitation Rates of Sarotherodon melanotheron in the Dominlin Lagoon of Ghana. Momona Ethiopian. J. Sci. 2015, 7, 258–274. [Google Scholar]
- Jenning, S.; Kaisier, M.; Reynold, J. Marine Fisheries Ecology; Blackwell Science: Oxford, UK, 2000; 391p. [Google Scholar]
- Legendre, M.; Slembrouck, J.; Kerdchuen, N.; Oteme, Z. Evaluation D’une Méthode Extensive D’alevinage des Clariidae en Cages Implantées en Étangs; N04; Document ORSTOM: Montpellier, France, 1991; 35p. [Google Scholar]
- Law, R. Fishing, selection, and phenotypic evolution. ICES J. Mar. Sci. 2000, 57, 659–668. [Google Scholar] [CrossRef]
- Hutchings, J.A.; Baum, J.K. Measuring marine fish biodiversity: Temporal changes in abundance, life history and demography. Philos. Trans. R. Soc. B. 2005, 360, 315–338. [Google Scholar] [CrossRef]
- Christensen, V.; Guénette, S.; Heymans, J.J.; Walters, C.J.; Watson, R.; Zeller, D.; Pauly, D. Hundred-Year decline of North Atlantic predatory fishes. Fish Fish. 2003, 4, 1–24. [Google Scholar] [CrossRef]
- Barry, J.P.; Tegner, M.J. Inferring demographic processes from size-frequency distributions: Simple models indicate specific patterns of growth and mortality. Fish. Bull. 1990, 88, 13–19. [Google Scholar]
- Akou Loba, V.; Gbitry Bolou, A. La Problématique du Développement dans l’Ouest de la Côte d’Ivoire; Archives nationales de Côte d’Ivoire; Eléments de Diagnostics et de Réflexion; Abidjan, Côte d’Ivoire, 2018; 37p. [Google Scholar]
- Koigny, K.J.H.; Diarrassouba, A.; Yelkouni, M.; Assie, D.R.H. Analyse des facteurs de dégradation des ressources halieutiques du lac Buyo dans la Réserve de biosphère Taï en Cote d’Ivoire. Vertigo 2024, 24. [Google Scholar] [CrossRef]
- Kantoussan, J. Impacts de la Pression de Pêche sur L’organisation des Peuplements de Poissons: Application aux Retenues Artificielles de Sélingué et de Manantali, Mali, Afrique de l’Ouest. Ph.D. Thesis, Agrocampus Rennes, Rennes, France, 2007; 195p. [Google Scholar]
- Pulin, R.S.V.; Lazard, J.; Legendre, M.; Amon Kothias, J.-B.; Pauly, D. Le Troisième Symposium International sur le Tilapia en Aquaculture; ICLARM: Penang, Malaysia, 1966; 642p. [Google Scholar]
- Anderson, K.H.; Farnsworth, K.D.; Thygesen, U.H.; Beyer, J.E. The evolutionary pressure from fishing on size at maturation of Baltic cod. Ecol. Model. 2007, 204, 246–252. [Google Scholar] [CrossRef]
- Miethe, T.; Dytham, C.; Dieckmann, U.; Pitchford, J.W. Marine reserves and the evolutionary effects of fishing on size at maturation. ICES J. Mar. Sci. 2010, 67, 412–425. [Google Scholar] [CrossRef]
- Pauly, D.; Chua, T.E. The overfishing of marine ressources: Socioeconomic background in Southeast Asia. Ambio 1988, 17, 200–206. [Google Scholar]
- Hutchings, J.A.; Reynolds, J.D. Marine fish population collapses: Consequences for recovery and extinction risk. BioScience 2004, 54, 297–309. [Google Scholar] [CrossRef]
- Brown, J.H.; Sibly, R.M. Life-history evolution under a production constraint. Proc. Natl. Acad. Sci. USA 2006, 103, 17595–17599. [Google Scholar] [CrossRef]
- Hutchings, J.A.; Myers, R.A. What can be learned from the collaps of a renewable resource? Atlantic cod, Gadus morhua, of Newfounland and labrador. Can. J. Fish. Aquat. Sci. 1994, 51, 2126–2146. [Google Scholar] [CrossRef]
- Hountcheme, I.A.C.; Ahouansou Montcho, S.; Lederoun, D.; Kpossou, S.; Agadjihouede, H. La pêcherie du lac Toho, acteurs, engins et commercialisation du poisson. Ann. Sci. Agric. 2024, 26, 47–58. [Google Scholar]
- Gouré Bi, T.F.; Yeo, G.M.; Amian, A.F.R.; Niamien, K.J.; Blé, M.C. Age et croissance du tilapia estuarin Sarotherodon melanotheron dans le lac de barrage d’Ayamé 1 (Côte d’Ivoire, Afrique de l’Ouest). J. Appl. Biosci. 2023, 191, 20219–20230. [Google Scholar] [CrossRef]
- Adéoti, B.O.E.; Yabi, I.; Medeou, F.; Ogouwale, E. Caractérisation de la pêcherie continentale dans les communes d’Adjohoun et de Dangbo au sud-est Bénin. Afr. Sci. 2018, 14, 170–184. [Google Scholar]
- Hutchings, J.A. Life histories of fish. Fish Biol. 2002, 1, 149–174. [Google Scholar] [CrossRef]



| Sex/Maturity | Number (N) | Total Length (cm) (min.–max.) | Average Length (cm) | Total Weight (g) (min.–max.) | Average Weight (g) |
|---|---|---|---|---|---|
| April 2002 to March 2003 | 3511 | 2.7–27.5 | 0.4–411.1 | 58.12 ± 33.44 | |
| Females | 870 | 7.0–27.5 | 14.67 ± 2.44 | 6.0–411.1 | 68.88 ± 32.74 |
| Males | 753 | 7.7–27.0 | 14,78 ± 1.95 | 8.0–360.2 | 63.57 ± 22.42 |
| Immature | 230 | 7.5–9.2 | 12.15 ± 1.59 | 6.5–16.0 | 37.43 ± 13.55 |
| Undetermined | 1658 | 2.7–27.5 | 12.15 ± 3.65 | 0.4–333.8 | 47.60 ± 38.22 |
| April 2022 to March 2023 | 9158 | 2.2–19.4 | 7.95 ± 2.26 | 0.2–133.7 | 12.88 ± 9.11 |
| Females | 3008 | 3.1–16.4 | 8.96 ± 1.71 | 1.2–79.9 | 16.17 ± 8.42 |
| Males | 3708 | 2.2–19.4 | 9.09 ± 1,91 | 1.3–133.7 | 16.86 ± 9.27 |
| Immature | 1823 | 2.3–7.7 | 7.46 ± 1.12 | 0.2–7.1 | 9.14 ± 4.13 |
| Undetermined | 619 | 2.8–11.7 | 5.96 ± 0.90 | 0.9–24.6 | 4.56 ± 2.19 |
| Stage | 2002–2003 | 2022–2023 | Chi-Squared Test |
|---|---|---|---|
| I | 121 | 3068 | p < 0.0001 |
| II | 109 | 1774 | |
| III | 637 | 769 | |
| IV | 513 | 793 | |
| V | 473 | 2135 | |
| Total | 1853 | 8539 |
| Period | Par | Apr. | May | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2002– 2003 | Nt | 342 | 581 | 89 | 135 | 387 | 199 | 452 | 277 | 475 | 89 | 19 | 466 | 3511 |
| NMS | 2 | 6 | 0 | 2 | 11 | 10 | 52 | 53 | 10 | 13 | 0 | 0 | 159 | |
| (%) MS | 0.58 | 1.03 | 0 | 1.48 | 2.84 | 5.03 | 11.50 | 19.13 | 2.10 | 1.61 | 0 | 0 | 4.53 | |
| 2022– 2023 | Nt | 853 | 257 | 991 | 446 | 582 | 1113 | 909 | 905 | 855 | 884 | 568 | 768 | 9131 |
| NMR | 16 | 20 | 23 | 14 | 9 | 16 | 12 | 4 | 8 | 6 | 0 | 14 | 142 | |
| (%) Ms | 1.88 | 7.78 | 2.32 | 3.14 | 1.55 | 1.44 | 1.32 | 0.44 | 0.94 | 0.68 | 0 | 1.82 | 1.56 |
| Growth Parameter | 2002–2003 | 2022–2023 | Observed Variation |
|---|---|---|---|
| Asymptotic length (L∞, cm) | 32.2 | 23.8 | Decrease |
| Growth coefficient (K, year−1) | 0.32 | 0.38 | Increase |
| Theoretical age at zero length (t0, years) | −0.50 | −0.44 | Slight increase |
| Estimated maximum longevity (tmax, years) | 9.37 | 7.89 | Decrease |
| Growth performance (Φ’) | 2.53 | 2.33 | Decrease |
| Parameter | 2002–2003 | 2022–2023 |
|---|---|---|
| Z | 2.70 | 2.95 |
| M | 0.84 | 1.03 |
| F | 1.86 | 1.92 |
| K | 0.32 | 0.38 |
| M/K | 2.63 | 2.71 |
| F/K | 5.81 | 5.05 |
| Lc50/L∞ | 0.31 | 0.20 |
| Lm50/L∞ (males) | 0.84 | 1.10 |
| Lm50/L∞ (females) | 0.25 | 0.23 |
| Country | Name | Years | L∞ (cm) | K (Year−1) | t0 (Year) | tmax (Year) | Φ’ | M | Z (Year−1) | F (Year−1) | E (Year−1) | L50C (cm) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benin | Lake Toho | 2022–2023 | 23.80 | 0.38 | −0.44 | 7.89 | 2.33 | 1.03 | 2.95 | 1.92 | 0.65 | 4.97 | Present study, 2022–2023 |
| Benin | Lake Toho | 2012–2013 | 21.53 | 0.58 | −0.31 | 5.17 | 2.43 | 1.37 | 1.95 | 0.58 | 0.30 | 6.70 | Lederoun et al., 2015 [20] |
| Benin | Lake Toho | 2002–2003 | 32.20 | 0.32 | −0.50 | 9.37 | 2.53 | 0.84 | 2.70 | 1.86 | 0.69 | 10.06 | Present study, 2002–2003 |
| Benin | Lake Ahémé | 2016–2017 | 18.90 | 0.71 | −0.26 | 4.23 | 2.40 | 1.62 | 2.56 | 0.94 | 0.37 | 7.89 | Viaho et al., 2021 [56] |
| Benin | Lake Nokoué (in Acadja) | 2003–2004 | 26.80 | 0.52 | 0.02 | 5.80 | 2.57 | 1.20 | 1.53 | 0.33 | 0.21 | - | Niyonkuru, 2010 [57] |
| Benin | Lake Nokoué (in Acadja) | 2003–2004 | 24.10 | 0.55 | 0.02 | 5.50 | 2.50 | 1.28 | 1.66 | 0.38 | 0.23 | - | Niyonkuru, 2010 [57] |
| Benin | Lake Nokoué and Porto-Novo Lagoon | 2015 | 24.68 | 0.86 | −0.20 | 3.49 | 2.42 | 1.71 | 2.46 | 0.75 | 0.31 | 9.20 | Lederoun et al. (2020) [58] |
| Benin | Lake Wozo | 2016 | 23.63 | 0.82 | - | - | 2.68 | 1.67 | 3.77 | 2.10 | 0.55 | - | Anagonou, 2016 [59] |
| Côte d’Ivoire | Lagoon Ebrié | 1991–1992 | 34 | 0.42 | - | - | - | 0.98 | - | 0.22 | - | - | Villanueva, 2004 [60] |
| Côte d’Ivoire | Ayamé 1 Dam | 2017–2018 | 33.26 | 0.56 | −0.28 | 5.35 | 2.79 | 1.23 | 1.88 | 0.65 | 0.35 | - | Cissé et al., 2021 [61] |
| Ghana | Lagoon Muni | 1994 | 15.50 | 0.70 | −0.10 | 4.200 | 2.03 | 1.84 | 5.38 | 3.55 | 0.48 | 10.13 | Koranteng et al., 2000 [62] |
| Ghana | Lagoon Dominli | 2011–2012 | 20.48 | 0.97 | −0.81 | 3.10 | 2.60 | 2.02 | 3.86 | 1.84 | 0.48 | 10.1 | Arizi et al., 2015 [63] |
| Gambia | Estuaries | 1991–1992 | 37 | 0.39 | - | - | - | 0.89 | - | 0.85 | - | - | Villanueva, 2004 [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hountcheme, C.A.I.; Montcho, S.A.; Agadjihouede, H.; Bănăduc, D. The Temporal Dynamics of the Impact of Overfishing on the Resilience of the Sarotherodon melanotheron (Rüppel, 1858) Fish Species’ Population in the West African Lake Toho. Fishes 2025, 10, 357. https://doi.org/10.3390/fishes10070357
Hountcheme CAI, Montcho SA, Agadjihouede H, Bănăduc D. The Temporal Dynamics of the Impact of Overfishing on the Resilience of the Sarotherodon melanotheron (Rüppel, 1858) Fish Species’ Population in the West African Lake Toho. Fishes. 2025; 10(7):357. https://doi.org/10.3390/fishes10070357
Chicago/Turabian StyleHountcheme, Clovis Ayodédji Idossou, Simon Ahouansou Montcho, Hyppolite Agadjihouede, and Doru Bănăduc. 2025. "The Temporal Dynamics of the Impact of Overfishing on the Resilience of the Sarotherodon melanotheron (Rüppel, 1858) Fish Species’ Population in the West African Lake Toho" Fishes 10, no. 7: 357. https://doi.org/10.3390/fishes10070357
APA StyleHountcheme, C. A. I., Montcho, S. A., Agadjihouede, H., & Bănăduc, D. (2025). The Temporal Dynamics of the Impact of Overfishing on the Resilience of the Sarotherodon melanotheron (Rüppel, 1858) Fish Species’ Population in the West African Lake Toho. Fishes, 10(7), 357. https://doi.org/10.3390/fishes10070357








