Effect of Flaxseed Gum on the Gelling and Structural Properties of Fish Gelatin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Modification of Fish Gelatin Samples
2.3. Determination of the Gel Strength
2.4. Determination of the Melting Temperature
2.5. Determination of the Rheological Properties
2.6. Determination of Particle Size, Zeta Potential, and Turbidity
2.7. Determination of Fluorescence Spectroscopy
2.8. FTIR
2.9. ESEM
2.10. Data Analysis
3. Results
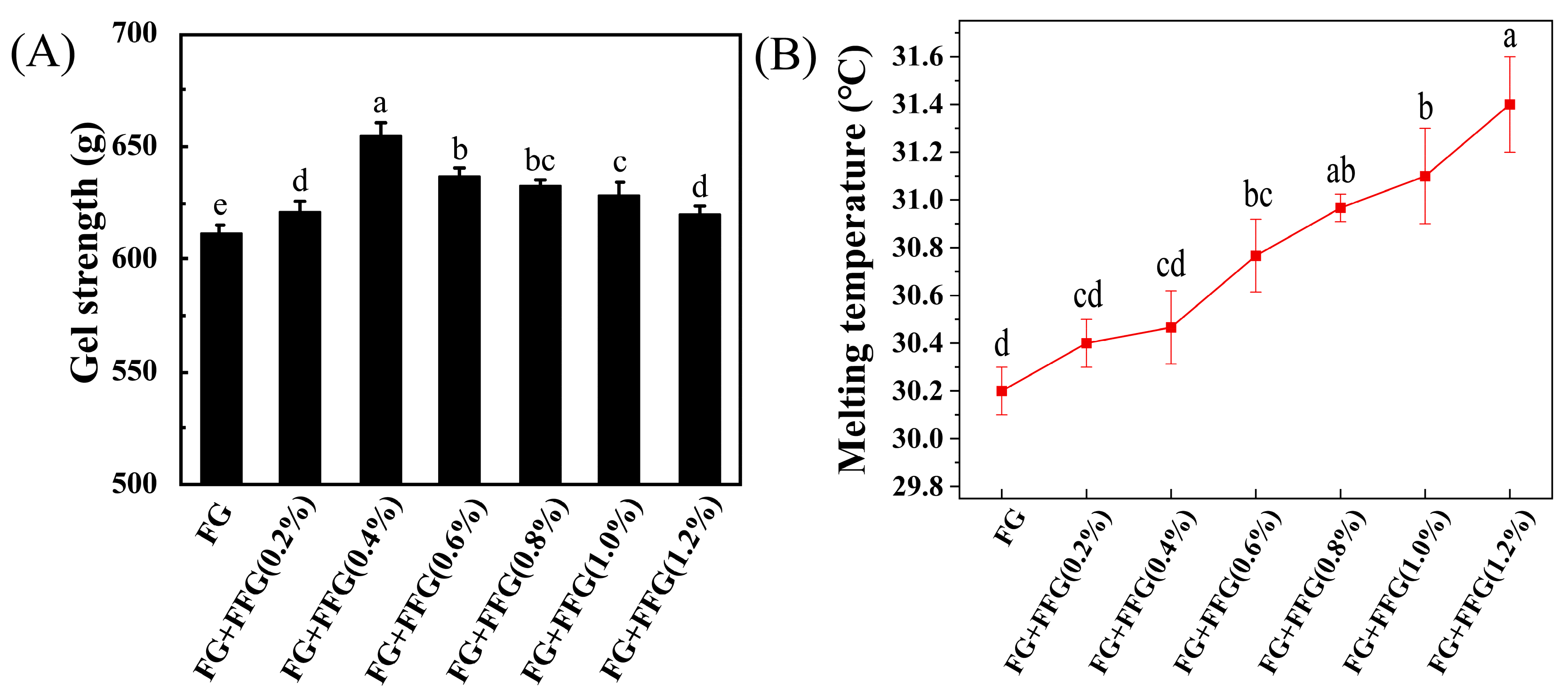
3.1. Effect of FFG Concentration on the Gel Strength of FG
3.2. Effect of FFG Concentration on the Melting Temperature of FG
3.3. Effect of FFG Concentration on the Rheological Properties of FG
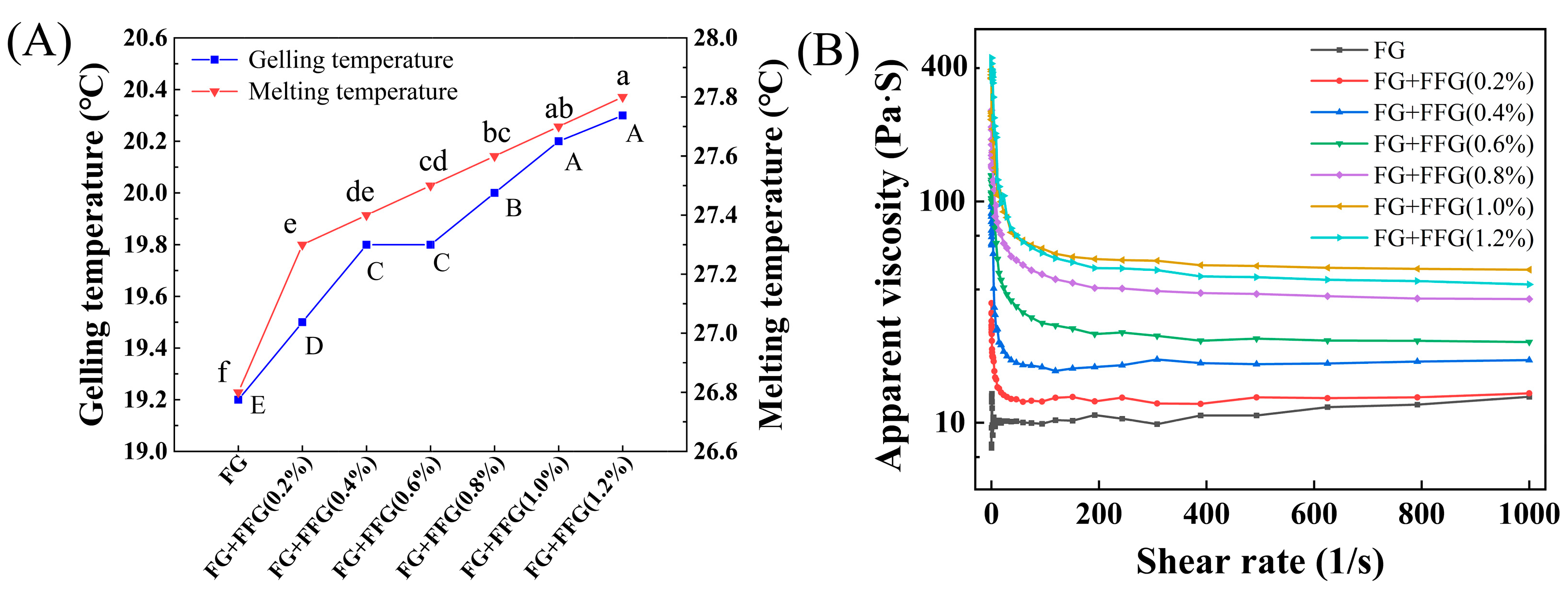
3.3.1. Effect of FFG Concentration on the Gelling–Melting Temperature of FG
3.3.2. Effect of FFG Concentration on the Viscosity of FG
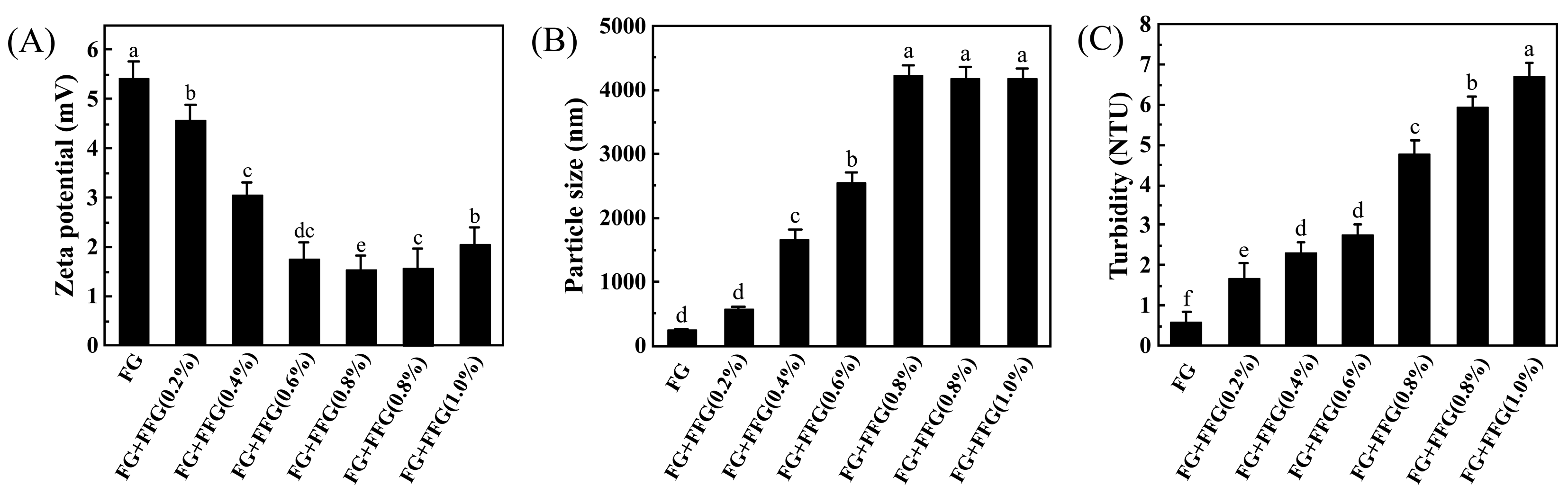
3.4. Effect of FFG Concentration on the Zeta Potential of FG
3.5. Effect of FFG Concentration on the Particle Size of FG
3.6. Effect of FFG Concentration on the Turbidity of FG
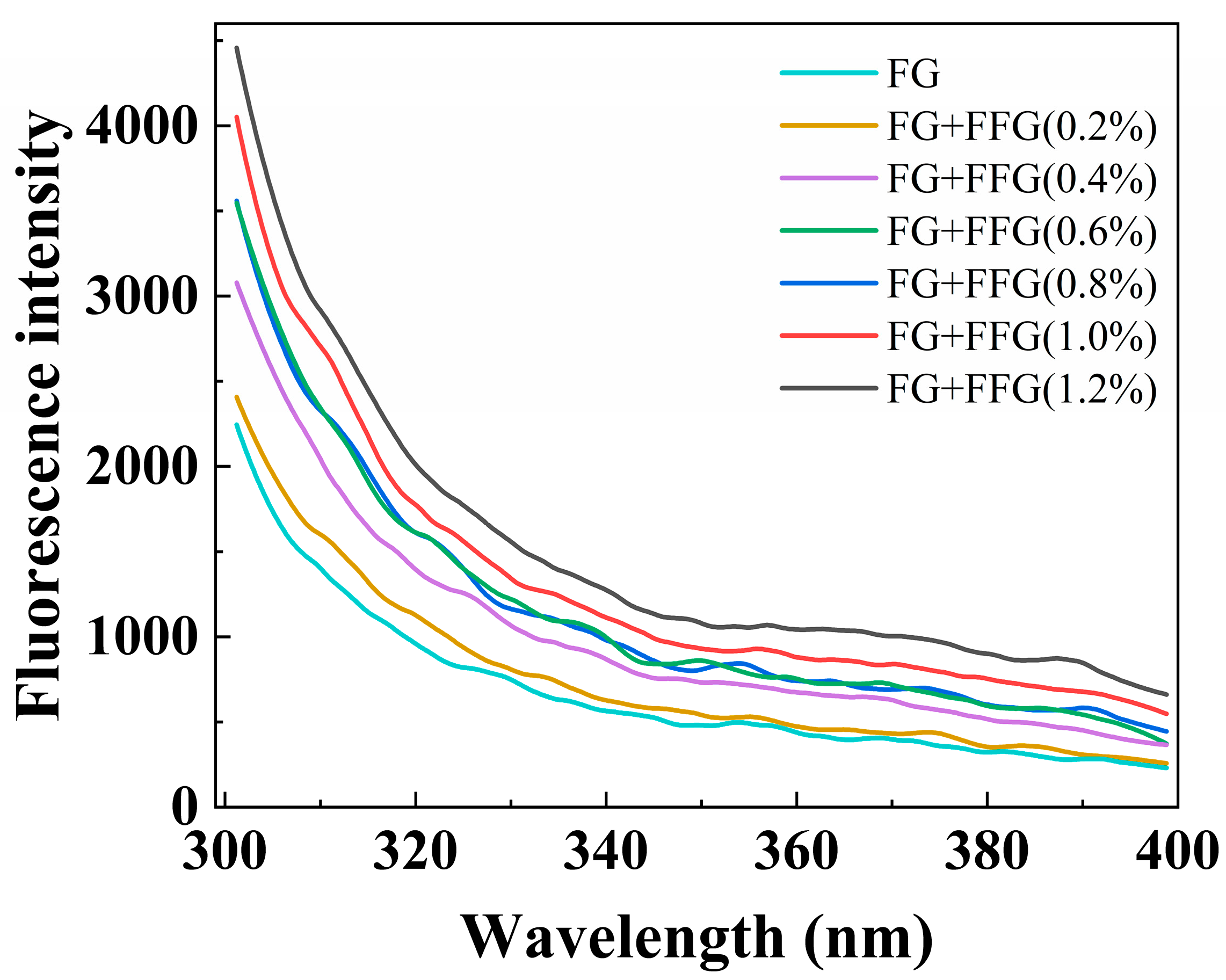
3.7. Analysis of Fluorescence Spectroscopy
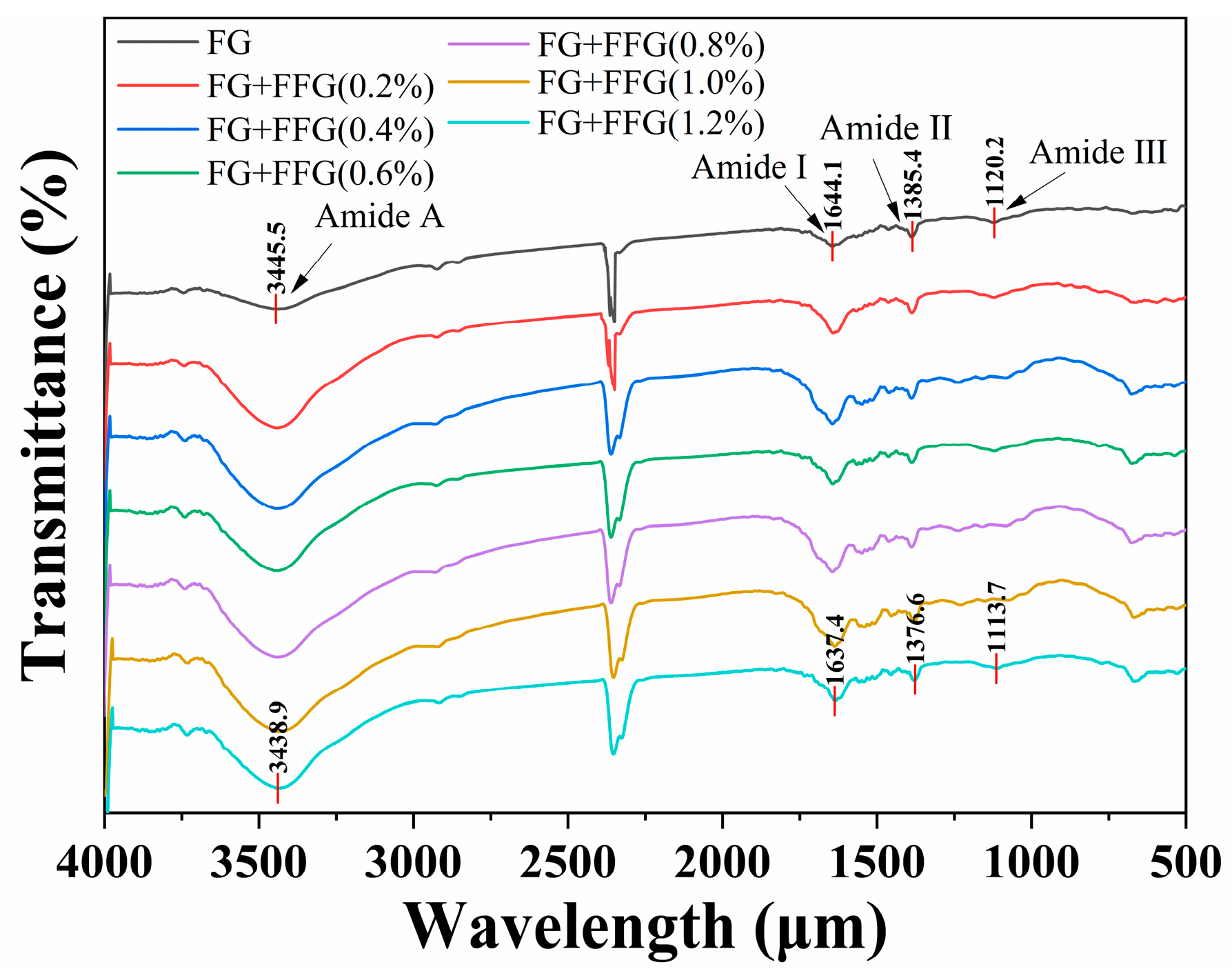
3.8. Analysis of FTIR
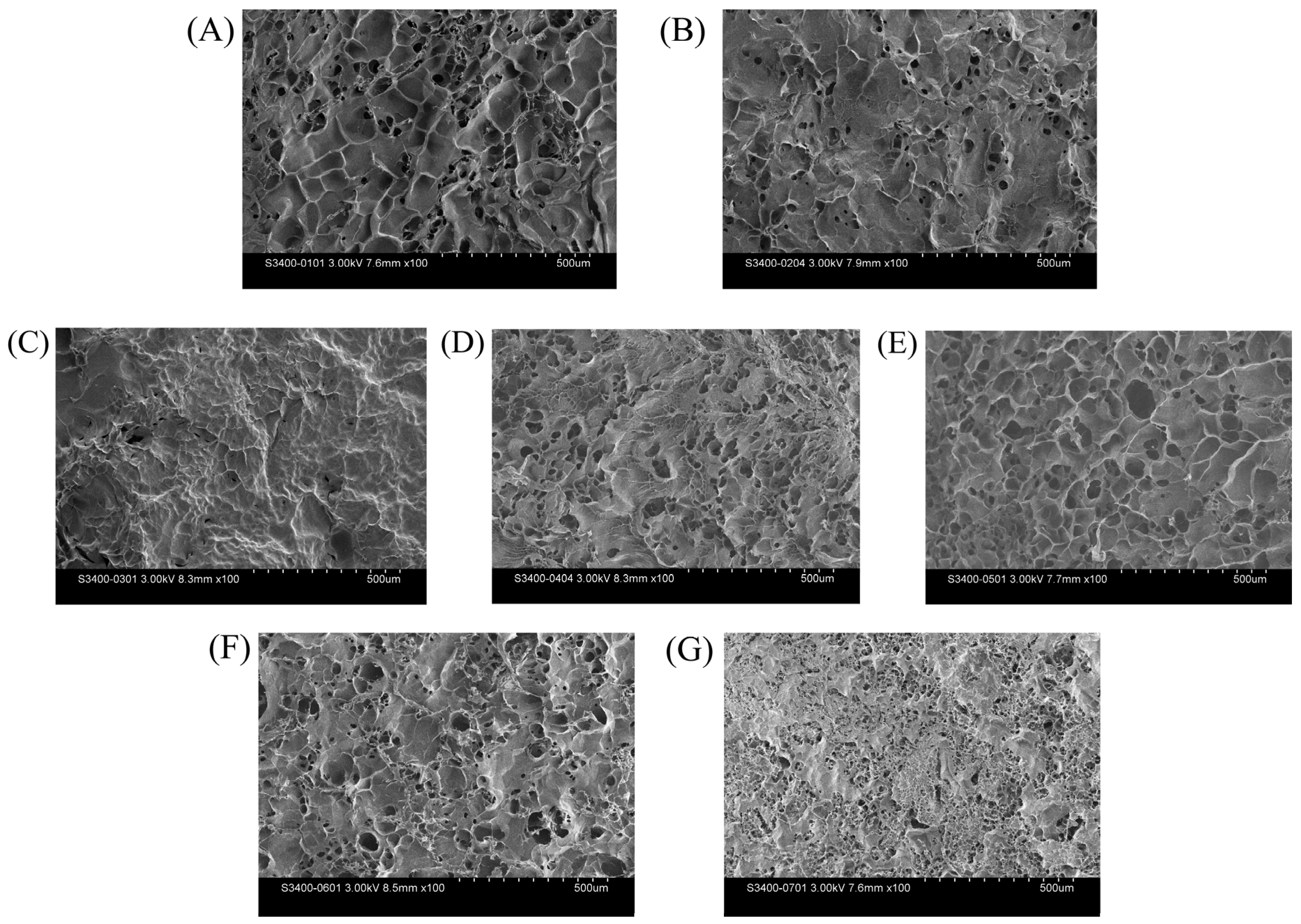
3.9. The Influence of FFG on the Microstructure of FG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Z.Z.; Sha, X.M.; Huang, T.; Yuan, C.H.; Chen, W.M.; Li, X.X.; Tu, Z.C. Gelling properties and structure modification of tilapia skin gelatin by the addition of γ-polyglutamic acid at different pH levels. Int. J. Food Sci. Technol. 2021, 56, 5812–5823. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Shangguan, X.; Sha, X.; Wang, H.; Zhang, L.; Bansal, N. Fish gelatin modifications: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 260–269. [Google Scholar] [CrossRef]
- Alfaro, A.d.T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish gelatin: Characteristics, functional properties, applications and future potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Sha, X.M.; Tu, Z.C.; Wang, H.; Huang, T.; Duan, D.L.; He, N.; Li, D.J.; Xiao, H. Gelatin quantification by oxygen-18 labeling and liquid chromatography-high-resolution mass spectrometry. J. Agric. Food Chem. 2014, 62, 11840–11853. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Thi, P.L.; Lee, Y.; Tran, D.L.; Thai Thanh Hoang, T.; Park, K.D. Horseradish peroxidase-catalyzed hydrogelation of fish gelatin with tunable mechanical properties and biocompatibility. J. Biomater. Appl. 2020, 34, 1216–1226. [Google Scholar] [CrossRef]
- Kaewruang, P.; Benjakul, S.; Prodpran, T. Effect of phosphorylation on gel properties of gelatin from the skin of unicorn leatherjacket. Food Hydrocoll. 2014, 35, 694–699. [Google Scholar] [CrossRef]
- Yang, D.Y.; Gao, S.; Yang, H.S. Effects of sucrose addition on the rheology and structure of iota-carrageenan. Food Hydrocoll. 2020, 68, 99–105. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Wang, H.; Liu, W.; Zhang, L.; Zhang, Y.; ShangGuan, X.C. Comparison of rheological behaviors and nanostructure of bighead carp scales gelatin modified by different modification methods. J. Food Sci. Technol. 2017, 54, 1256–1265. [Google Scholar] [CrossRef]
- Tu, Z.C.; Huang, T.; Wang, H.; Sha, X.M.; Shi, Y.; Huang, X.Q.; Man, Z.Z.; Li, D.J. Physico-chemical properties of gelatin from bighead carp (Hypophthalmichthys nobilis) scales by ultrasound-assisted extraction. J. Food Sci. Technol. 2015, 52, 2166–2174. [Google Scholar] [CrossRef]
- Yu, Z.L.; Zeng, W.C.; Zhang, W.H.; Liao, X.P.; Shi, B. Effect of ultrasonic pretreatment on kinetics of gelatin hydrolysis by collagenase and its mechanism. Ultrason. Sonochem. 2016, 29, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Sow, L.C.; Chong, J.M.N.; Liao, Q.X.; Yang, H. Effects of κ-carrageenan on the structure and rheological properties of fish gelatin. J. Food Eng. 2018, 239, 92–103. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Dowling, K.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Effect of extraction temperature on composition, structure and functional properties of flaxseed gum. Food Chem. 2017, 215, 333–340. [Google Scholar] [CrossRef]
- Cardoso Carraro, J.C.; de Souza Dantas, M.I.; Rocha Espeschit, A.C.; Duarte Martino, H.S.; Rocha Ribeiro, S.M. Flaxseed and human health: Reviewing benefits and adverse effects. Food Rev. Int. 2012, 28, 203–230. [Google Scholar] [CrossRef]
- Chen, C.; Huang, X.; Wang, L.J.; Li, D.; Adhikari, B. Effect of flaxseed gum on the rheological properties of peanut protein isolate dispersions and gels. LWT-Food Sci. Technol. 2016, 74, 528–533. [Google Scholar] [CrossRef]
- Moakes, R.J.A.; Sullo, A.; Norton, I.T. Preparation and characterisation of whey protein fluid gels: The effects of shear and thermal history. Food Hydrocoll. 2015, 45, 227–235. [Google Scholar] [CrossRef]
- Tabarestani, H.S.; Maghsoudlou, Y.; Motamedzadegan, A.; Mahoonak, A.R.S. Optimization of physico-chemical properties of gelatin extracted from fish skin of rainbow trout (Onchorhynchus mykiss). Bioresour. Technol. 2010, 101, 6207–6214. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Shangguan, X.; Wang, H.; Sha, X.M.; Bansal, N. Rheological behavior, emulsifying properties and structural characterization of phosphorylated fish gelatin. Food Chem. 2018, 246, 428–436. [Google Scholar] [CrossRef]
- Wu, B.C.; McClements, D.J. Microgels formed by electrostatic complexation of gelatin and OSA starch: Potential fat or starch mimetics. Food Hydrocoll. 2015, 47, 87–93. [Google Scholar] [CrossRef]
- Joshi, N.; Rawat, K.; Bohidar, H.B. pH and ionic strength induced complex coacervation of pectin and gelatin A. Food Hydrocoll. 2018, 74, 132–138. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Liu, C.; Luo, L.; Chen, J.; Luo, S.; McClements, D.J.; Wu, L. Effect of limited enzymatic hydrolysis on structure and emulsifying properties of rice glutelin. Food Hydrocoll. 2016, 61, 251–260. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Sha, X.M.; Ye, Y.H.; Xiao, W.R.; Tu, Z.C. Effects of γ-polyglutamic acid on the gelling properties and non-covalent interactions of fish gelatin. J. Texture Stud. 2020, 51, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Cona, C.; Bailey, K.; Barker, E. Characterization methods to determine interpenetrating polymer network (IPN) in hydrogels. Polymers 2024, 16, 2050. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, H.; Fang, Y.; Lu, J.; Yang, W.; Qiao, Z.; Lou, Q.; Xu, D.; Zhang, J. Comparison of gelling properties and flow behaviors of microbial transglutaminase (MTGase) and pectin modified fish gelatin. J. Texture Stud. 2019, 50, 400–409. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Sarabia, A.I.; Solas, M.T.; Montero, P. Effect of microbial transglutaminase on the functional properties of megrim (Lepidorhombus boscii) skin gelatin. J. Sci. Food Agric. 2001, 81, 665–673. [Google Scholar] [CrossRef]
- Kolodzlejska, I.; Kaczorowski, K.; Piotrowska, B.; Sadowska, M. Modification of the properties of gelatin from skins of Baltic cod (Gadus morhua) with transglutaminase. Food Chem. 2004, 86, 203–209. [Google Scholar] [CrossRef]
- Cheng, C.; Tu, Z.C.; Wang, H. pH-induced complex coacervation of fish gelatin and carboxylated chitosan: Phase behavior and structural properties. Food Res. Int. 2023, 167, 34–42. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P.; Kishimura, H. Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocoll. 2012, 29, 389–397. [Google Scholar] [CrossRef]
- Hassan, N.; Ahmad, T.; Zain, N.M.; Awang, S.R. A fuzzy graph based chemometrics method for gelatin authentication. Mathematics 2020, 8, 1969. [Google Scholar] [CrossRef]
- Geng, H.; Sun, W.; Zhan, S.; Jia, R.; Lou, Q.; Huang, T. Glycosylation with different saccharides on the gelling, rheological and structural properties of fish gelatin. Food Hydrocoll. 2024, 150, 22–32. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, B.; Qiao, D.; Yan, X.; Zhao, S.; Jia, C.; Niu, M.; Xu, Y. Addition of κ-carrageenan increases the strength and chewiness of gelatin-based composite gel. Food Hydrocoll. 2022, 128, 54–59. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Wang, H.; Shangguan, X.; Zhang, L.; Zhang, N.-H.; Bansal, N. Pectin and enzyme complex modified fish scales gelatin: Rheological behavior, gel properties and nanostructure. Carbohyd. Polym. 2017, 156, 294–302. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.S.G.; Pinto, L.A.A. Physical cross-linkers: Alternatives to improve the mechanical properties of fish gelatin. Food Eng. Rev. 2012, 4, 165–170. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bode, F.; Grillo, I.; Dreiss, C.A. Exploring the kinetics of gelation and final architecture of enzymatically cross-linked chitosan/gelatin gels. Biomacromolecules 2015, 16, 1401–1409. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Sha, X.M.; Huang, T.; Zhang, L.; Wang, G.Y.; Tu, Z.C. Microbial transglutaminase (MTGase) modified fish gelatin-γ-polyglutamic acid (γ-PGA): Rheological behavior, gelling properties, and structure. Food Chem. 2021, 348, 123–134. [Google Scholar] [CrossRef]
- Hong, T.; Iwashita, K.; Shiraki, K. Viscosity control of protein solution by small solutes: A review. Curr. Protein Pept. Sci. 2018, 19, 746–758. [Google Scholar] [CrossRef]
- Su, K.; Sun, W.; Li, Z.; Huang, T.; Lou, Q.; Zhan, S. Complex modification orders alleviate the gelling weakening behavior of high microbial transglutaminase (MTGase)-catalyzed fish gelatin: Gelling and structural analysis. Foods 2023, 12, 3027. [Google Scholar] [CrossRef]
- Ramanathan, G.; Hassan, M.; Rochev, Y. Optimising the viscoelastic properties of hyaluronic acid hydrogels through colloidal particle interactions: A response surface methodology approach. Colloids Surf. A 2024, 703, 34–41. [Google Scholar] [CrossRef]
- Feng, Q.; Jia, F.J.; Peng, Z.G.; Zheng, Y. Development of temperature-responsive suspension stabilizer and its application in cementing slurry system. Colloids Surf. A 2003, 658, 13–21. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Cai, J.; Zhang, X.; Duhoranimana, E.; Su, J. Gelatin and pectin complex coacervates as carriers for cinnamaldehyde: Effect of pectin esterification degree on coacervate formation, and enhanced thermal stability. Food Hydrocoll. 2019, 87, 712–722. [Google Scholar] [CrossRef]
- Guo, C.; Gemeinhart, R.A. Understanding the adsorption mechanism of chitosan onto poly(lactide-co-glycolide) particles. Eur. J. Pharm. Biopharm. 2008, 70, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Yang, Q.; Du, Y.N.; Chen, H.Q. Chitosan can improve the storage stability of ovalbumin fibrils at pH higher than isoelectric point. Food Hydrocoll. 2023, 136, 65–71. [Google Scholar] [CrossRef]
- Yang, Y.; Anvari, M.; Pan, C.H.; Chung, D. Characterisation of interactions between fish gelatin and gum arabic in aqueous solutions. Food Chem. 2012, 135, 555–561. [Google Scholar] [CrossRef]
- Bae, W.; Yoon, T.Y.; Jeong, C. Direct evaluation of self-quenching behavior of fluorophores at high concentrations using an evanescent field. PLoS ONE 2021, 16, 21–28. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Guo, X.; Deng, X.; Kang, S.; Zhu, X.; Guo, X. Effect of phosphorylation on the structure and emulsification properties of different fish scale gelatins. Foods 2022, 11, 804. [Google Scholar] [CrossRef]
- Lacy, H.A.; Jencová, V.; Hauzerová, S.; Stanishevsky, A. High-throughput fabrication, structural characterization, and cellular interaction of compositionally diverse fish gelatin/polycaprolactone (PCL) nanofibrous materials. J. Appl. Polym. Sci. 2023, 140, 15–18. [Google Scholar] [CrossRef]
- Kaewruang, P.; Benjakul, S.; Prodpran, T. Characteristics and gelling property of phosphorylated gelatin from the skin of unicorn leatherjacket. Food Chem. 2014, 146, 591–596. [Google Scholar] [CrossRef]
- Fang, Q.; Ma, N.; Ding, K.Y.; Zhan, S.N.; Lou, Q.M.; Huang, T. Interaction between negatively charged fish gelatin and cyclodextrin in aqueous solution: Characteristics and formation mechanism. Gels 2021, 7, 260. [Google Scholar] [CrossRef]
- Pan, J.; Li, Q.; Jia, H.; Xia, L.; Jin, W.; Shang, M.; Xu, C.; Dong, X. Physiochemical and functional properties of tiger puffer (Takifugu rubripes) skin gelatin as affected by extraction conditions. Int. J. Biol. Macromol. 2018, 109, 1045–1053. [Google Scholar] [CrossRef]
- Tang, W.; Pan, Q.; He, J.; Liu, J. Plant-based meat: The influence on texture by protein-polysaccharide interactions and processing techniques. Food Res. Int. 2025, 202, 76–82. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-T.; Kuang, Y.-T.; Peng, C.-Y.; Hu, X.-W.; Yuan, P.; Sha, X.-M.; Hu, Z.-Z. Effect of Flaxseed Gum on the Gelling and Structural Properties of Fish Gelatin. Fishes 2025, 10, 346. https://doi.org/10.3390/fishes10070346
Wu T-T, Kuang Y-T, Peng C-Y, Hu X-W, Yuan P, Sha X-M, Hu Z-Z. Effect of Flaxseed Gum on the Gelling and Structural Properties of Fish Gelatin. Fishes. 2025; 10(7):346. https://doi.org/10.3390/fishes10070346
Chicago/Turabian StyleWu, Ting-Ting, Ya-Ting Kuang, Chun-Yan Peng, Xin-Wu Hu, Ping Yuan, Xiao-Mei Sha, and Zi-Zi Hu. 2025. "Effect of Flaxseed Gum on the Gelling and Structural Properties of Fish Gelatin" Fishes 10, no. 7: 346. https://doi.org/10.3390/fishes10070346
APA StyleWu, T.-T., Kuang, Y.-T., Peng, C.-Y., Hu, X.-W., Yuan, P., Sha, X.-M., & Hu, Z.-Z. (2025). Effect of Flaxseed Gum on the Gelling and Structural Properties of Fish Gelatin. Fishes, 10(7), 346. https://doi.org/10.3390/fishes10070346