Abstract
The white snook (Centropomus viridis) is an emerging aquaculture species with high market acceptance, exhibiting catadromous and protandric hermaphroditic characteristics in adulthood. This study aimed to preliminarily characterize certain hematological and biochemical parameters, as well as blood cell morphology, for identifying possible variations between sexes maintained under aquaculture recirculating system (RAS) conditions. The white snook broodstock was anesthetized with clove oil, and biometric values, as well as sex classification, were measured. Then, blood samples were collected from 14 females (7132 ± 1610 g) and 20 males (2200 ± 0.963 g) via caudal vessel puncture to analyze selected hematological parameters, blood biochemistry, and cellular morphology. Fulton’s condition factor (K) showed no differences between sexes, indicating a healthy fish status. Females showed significantly higher serum cholesterol, glucose, and triglyceride levels than males. Also, hematocrit (HCT) and mean corpuscular volume (MCV) were elevated in females. No sex-related differences were observed in red or white cell counts or in blood cell dimensions. Morphological characterization identified erythrocytes, thrombocytes, and three types of leukocytes: lymphocytes (small and large lymphocytes), neutrophils, and monocytes, with no eosinophils or basophils detected in either sex. These findings provide fundamental reference values for the hematological and biochemical profiles of C. viridis broodstock in captivity and highlight sex-specific differences relevant for reproductive and health monitoring. However, it should be considered that the sample size used to establish reference ranges for the species is small, so it is recommended to implement a monitoring plan for this and other broodstocks of this emerging species.
Keywords:
fish health; blood chemistry; fish blood cells; hematological physiology; blood cell morphology; catadromous fishes Key Contribution:
Due to lack of knowledge on blood physiology in C. viridis, this study provides novel hematology understanding of male and female C. viridis broodstock under captive conditions, which will help provide a basis to implement monitoring systems that allow for the early detection of physiological changes and potential disease, and to promote successful reproduction in breeding programs.
1. Introduction
Maintaining healthy broodstock in captivity is fundamental to achieving the success of any breeding program [1]. A healthy broodstock not only ensures greater efficiency in gamete production but also guarantees the quality of the progeny, their viability, and their survival [2]. Under captive conditions, environmental, nutritional, and management factors can be controlled, and assuring the welfare of broodstock becomes even more crucial [3], as any alteration in their health status can compromise gonadal maturation, spawning, or fertilization [3].
Thus, before exposing fishes to hormonal therapies, handling, gamete extraction, or bioassay, it is essential to verify their optimal health conditions [1]. For this purpose, blood tests represent a valuable tool [4] to detect health issues, establish baseline physiological parameters [1,2,5], and identify early signs of stress, physiological imbalances, or diseases. This is an indispensable strategy to ensuring reproductive success and sustainable aquaculture [6].
Hematological parameters are essential markers of fish health because they reveal physiological reactions to illnesses [7] and environmental stress [8,9,10]; an essential tool for fish health evaluation in RAS systems [11]. The main hematological parameters include red blood cell (RBC) count, hematocrit (HCT), hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and white blood cell (WBC) count, which includes lymphocytes, monocytes, granulocytes (neutrophils, eosinophils, and basophils), and thrombocytes [6,7,8,9]. In addition, the examination of blood smears allows for the detection of infections, monitoring the presence of parasites, and the identification of cellular abnormalities [1,12,13,14,15]. regarding blood chemistry, parameters such as total protein, cholesterol, glucose, and triglycerides provide key information on the nutritional and metabolic status of fishes [16], reflecting the body’s protein balance, cardiovascular health, and metabolic efficiency [6,15].
The white snook (Centropomus viridis) is a catadromous species [17] and, during the reproductive stage, it presents protandric hermaphroditism [18]. Technology for the mass production of juveniles has already been developed [19], and bioeconomic analyses have shown profitability in cage culture [20]. The white snook possesses a high commercial value, which reinforces the species as a potential species for aquaculture [21].
Several studies have evaluated hematological and biochemical parameters in fishes, including pacific fat sleeper (Dormitator latifrons) [13], silver tiger fish (Datnioides polota) [4], Atlantic horse mackerel (Trachurus trachurus) [22], and brook trout (Salvelinus fontinalis) [23], where differences between sexes, stages, metabolism, and hormone levels are characterized, providing essential information for effective management in aquaculture.
It is worth noting that only studies on blood biochemistry in juveniles of this species exist [24]. Therefore, it is essential to study the hematology of adult white snook to optimize their culture conditions and implement tools to evaluate their health, detect stress, determine their nutritional status, and diagnose diseases, guaranteeing their welfare and adequate management in production systems [4]. Hence, this research aims to characterize certain cellular hematology, cell morphology, and blood biochemistry parameters, identifying possible variations between sexes in C. viridis broodstock maintained under aquaculture recirculating system.
2. Materials and Methods
2.1. Aquaculture System and Fish Husbandry
The white snook broodstock used in the present study belonged to the Marine Fish Pilot hatchery of the Centro de Investigación en Alimentación y Desarrollo (CIAD), in Mazatlán, Sinaloa, México. The females had been in the CIAD’s facilities for 8 to 10 years since their capture, while the males were young and had been in captivity for 8 months since their capture. The broodstock of 22 females and 26 males was randomly distributed in four recirculating aquaculture systems (RASs) with a salinity of 33 ppt and temperature pf 22 °C. Each RAS included two 14-ton main tanks, a 750 L reservoir, a 1300 L solids settler, a 400 L mechanical sand filter, a 1000 L biological filter (model Kaldnes k1, Aqua pond, Leighton Buzzard, UK), and a protein skimmer. The pH was measured with a Testa Scientific Uni pH meter (Trans Instruments, Singapore). The concentrations of total ammonium (TAN), nitrite, and nitrate were evaluated using a commercial Saltwater Master Test Kit (model 410M). The dissolved oxygen concentration was measured with a YSI Pro20. Salinity was measured with a refractometer (Trans Instruments). Water temperature was monitored with a 30 cm mercury glass thermometer (Brannan, Cleator Moor, UK). The photoperiod was natural, with 11 h of light and 13 h of darkness. A summary of the physicochemical variables is shown in Table 1.

Table 1.
Physicochemical variables of aquaculture recirculating system (RAS) of C. viridis. Results are presented as Mean ± SD.
Sex was distinguished by genital pore observation; females present three pores (urethra, genital, and anus), and males have two pores (urogenital and anus). Additionally, the organisms have an identification chip linked to information obtained during a gamete biopsy for sex identification, a procedure performed one month before any reproductive management and, in this case, before blood sampling management. The temperature was regulated to maintain broodstock in the pre-spawning stage during the blood sample.
The broodstock is always maintained in the RAS, and management is only performed as required. To ensure a parasite-free system and the optimal health status of the broodstock, constant monitoring for parasites is carried out. Two 20 cm long ropes are placed inside the tanks, 30 cm away from the tank wall and in favor of the inflow. These ropes are changed every 7 days to detect the possible life stages of pathogenic ectoparasites.
Snook broodstock were fed every third day to apparent satiety, offering white shrimp (Litopenaeus vannamei) cephalothorax, skipjack (Katsuwonus pelamis) muscle, and a semi-moist feed. The semi-moist feed was prepared in situ, consisting of 29% skipjack muscle, 29% white shrimp cephalothorax, 14% squid (Doryteuthis opalescens), and 26% fishmeal supplemented with vigantol (Vitamin A: 50.000 UI, Vitamin D3: 75.000 UI, Vitamin E: 50 mg), catosal (Butafosfan: 100 mg, Vitamin B12: 0.05 mg), and ascorbic acid.
2.2. Proximal Analysis
The total protein, total lipids, ash, and moisture of the feed were determined using the techniques described by the AOAC [25]. The dry weight and ash content of the diets were determined by drying ground samples at 60 °C for 24 h, followed by carbonization in a muffle furnace at 550 °C for six hours. The total nitrogen was analyzed by the micro-Kjeldahl method (UDK 129, Velp, Italy) to calculate crude protein by conversion (%N × 6.25). The crude lipids were calculated using the Soxhlet method, using petroleum ether as a carrier solvent. Differences from the sum of protein, lipids, and ash percentages are reported as nitrogen-free extract (NFE). All procedures were performed in triplicate.
2.3. Blood Sample Collection and Biometrics
The main tanks were drained to a volume of 8400 L for blood collection, and the fish were sedated with clove oil (7.66 μL/L) to obtain analgesic anesthesia [26]. This state showed two different anesthetic states. First, light sedation was observed, evidenced by partial loss of balance and reduced mobility, followed by a second stage of deep sedation, characterized by total loss of balance with a duration of 20 to 40 min. Subsequently, inside the main tank, each breeder was transferred to a 189 L icebox with a clove oil concentration of 21.16 μL/L to induce a state of surgical anesthesia. This state was obtained after between five to ten minutes and was characterized by a total loss of stimuli, although they maintained active opercular ventilation. At this point, they were carefully removed from the water using a plastic canvas stretcher (which avoids removal of the protective mucus from the skin and prevents ocular lesions). Then, blood sampling was performed, during which blood could only be collected from 14 females and 20 males. Female blood extraction was carried out with a 16G (1.60 × 51 mm) disposable intravenous (I.V.) catheter (Equipos medicos, Viszcarra; Punzocat PTFE, catalog number 17058; San Luis Porosí, México), while a 3 mL (21G, 32 mm) disposable syringe was used for males. Further, 1 mm of blood was extracted by punction in the caudal vessel [27], where 0.5 mL was placed in 1.5 mL Eppendorf tubes for serum extraction for blood biochemistry analysis, and the other 0.5 mL was placed in tubes with 55 µL of heparin sodium salt from porcine intestinal mucosa (Sigma, catolog number H3393-250K, Darmstadt, Alemania) and manually mixed for 30 s to avoid coagulation, for later use in hematological parameter evaluation. During the time out of water, fish were weighed and had their length measured, and the time of management did not exceed one and a half minutes. Recovery was carried out in the main tanks in an assisted manner, until the fish completely regained their balance, thereby avoiding injuries due to loss of swimming control.
With the biometric data (wet weight and total length), Fulton’s condition factor (K) was determined for each fish.
where ‘‘W” is body weight and ‘‘L” defines total length.
K = (W/L3) × 100
A summary of the male and female biometric data is shown in Table 2.
2.4. Hematological Analysis
From blood without heparin, a capillary tube was filled two-thirds full with blood, centrifuged for 10 min in a microhematocrit centrifuge (SOLBAT, MOD. C600-4P; Puebla, México), and then the packed cell volume was measured using a hematocrit reader and reported as a percentage [28].
Hemoglobin was determined using the HGB Assay kit (catalog number MAK 115, Sigma-Aldrich, Darmstadt, Alemania). The total red and white blood cell count was measured using the Natt-Herricks method [29]. A blood sample was collected with a pipette (Research® plus Eppendorf, catalog number EP3123000918, Darmstadt, Alemania) and diluted in ratio 1:200 with Natt-Herrick’s staining solution (Laboratory supplies, GOLDEN, catalog number 63455-500; Mazatlán, sinaloa). A Neubauer hemocytometer (Bright-Line™, catalogo number Z359629, Darmstadt, Alemania) with a 20 μL chamber capacity was fully loaded with the cell suspension, and then RBCs and WBCs were manually counted. For the RBC count, only the corner squares and the center squares inside the big center square were used, and the four big squares located at the corners of the chamber were used for the WBC count, with the support of an optical microscope (Keyence, Olympus BX53 SKU: bx53, San Miguel México).
Cellular differentiation of leukocytes was observed through blood smears treated with a fast hemacolor stain (Hycel de México, catalog number 548; Zapopan, Jalisco). Photographs of cells found in the smears were taken with the support of a camera microscope (Olympus BX53; Infinity 1–2 camera, color, CMOS, 1/2” 2 MP, USB 2.0). The images were processed, and cell and nuclei lengths were measured using Sigma Scan Pro 5.0 software. Sixty digital pictures of an average of 250 ± 100 cells were captured from each blood smear. WBCs (lymphocytes, granulocytes, monocytes) and thrombocytes were counted as a percentage of the total WBCs, and their size and morphology were reported as reference intervals from the smallest to the largest cell found [27]. Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) indices were calculated using standard formulas [4], using HCT, RBC, and HGB data.
2.5. Blood Biochemistry Analysis
Triglyceride, cholesterol, and glucose levels in the blood serum were determined using Valtek commercial kits (Melax Group; Zapopan, Jalisco, México). The triglyceride assay was performed using the glycerol-3-phosphate-oxidase method (GPO-PAP; SKU: 180-170) at 505 nanometers. Cholesterol was analyzed using the cholesterol esterase method (CHOD-PAP; SKU:060-170) at 505 nm. The glucose oxidase method (GOD-PAD; SKU: 110–150) for glucose concentration was performed at 505 nanometers. Total protein was determined using the BioSystems kit (SKU: 11500; Carrer de la Costa Brava, Barcelona, España). All blood parameters analyzed in each sample were measured in triplicate, and the results were read using a quartz probe in a spectrophotometer.
2.6. Statistical Analysis
The results are expressed as mean ± standard deviation (SD). For statistical analysis, data were evaluated for normality (Kolmogorov–Smirnov test) and homoscedasticity (Levene’s test). An independent standard Student’s t-test was used to compare differences between sexes in biological indices, hematology, blood biochemistry, and blood cell size parameters. A one-way ANOVA was performed to analyze differences between the proximate profiles of the feeds. When differences were detected, a posteriori Tukey test was applied. All the statistical procedures were performed using SigmaStat 12.0 (Systat Software Inc., San Jose, CA, USA). In all cases, a significant level of p < 0.05 was considered.
3. Results
3.1. Fish Biometrics
A summary of the biometric indices of C. viridis males and females is shown in Table 2. Males had a significantly lower average weight and total length than females (p < 0.05); however, Fulton’s condition factor (K) showed no difference between sexes (p > 0.05), and K values were found to be in the optimal range.

Table 2.
Biometric variables and biological indices of C. viridis males (n = 20) and females (n = 14). Results are presented as mean ± SD. Asterisks indicate significant differences (p < 0.05) between males and females.
Table 2.
Biometric variables and biological indices of C. viridis males (n = 20) and females (n = 14). Results are presented as mean ± SD. Asterisks indicate significant differences (p < 0.05) between males and females.
| Parameters | Male (n = 20) | Max–Min | Female (n = 14) | Max–Min |
|---|---|---|---|---|
| Wet weight (K) | 2.200 ± 0.963 | 5.300–1.400 | 7.132 ± 1.610 * | 10.700–4.100 |
| Total length (M) | 0.632 ± 6 | 0.86–0.57 | 0.94 ± 0.04 * | 1.03–0.84 |
| K | 0.84 ± 0.15 | 1.10–0.54 | 0.83 ± 0.14 | 1.02–0.54 |
* represents differences between sexes. K: Fulton’s condition factor. Max–Min: represents minimum and maximum data values observed.
3.2. Proximate Analysis of Feeds
The nutritional analyses of the three feeds provided to the white snook are shown in Table 3.

Table 3.
Proximate profile of feeds used to maintain C. viridis broodstock (n = 3).
Regarding crude protein content, skipjack showed the highest value, and shrimp cephalothorax had the lowest value (p < 0.05). Crude lipid content was highest in shrimp cephalothorax and lowest in skipjack (p < 0.05). Ash content presented the highest values in shrimp cephalothorax, while skipjack showed the lowest (p < 0.0001). Moisture was highest in skipjack, followed by shrimp and semi-moist feed (p < 0.05) (Table 3).
3.3. Hematology
Hematological parameters of white snook are summarized in Table 4. The percentage of neutrophils, lymphocytes, monocytes, thrombocytes, leukocytes, RBC, WBC, HGB, MCH, and MCHC show no differences between sexes (p > 0.05). However, HCT and MCV show higher values in females (p < 0.05).

Table 4.
Blood parameters of C. viridis male (n = 20) and female (n = 14). Results are presented as mean ± SD. HCT = hematocrit; HGB = hemoglobin; RBC = red blood cell; MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; WBC = white blood cell. * denotes values that were significantly different between sexes. Max–Min: represent minimum and maximum data values observed. Asterisks indicate significant differences (p < 0.05) between male and female.
3.4. Blood Cell Size and Morphology
The measurements of cell dimensions are shown in Table 5. The cellular length and nucleus size of all cell types did not show differences between sexes (p > 0.05).

Table 5.
Blood cell size (μm) of white snook (Centropomus. viridis) male (n = 20) and female (n = 14) (n = 100 cells). Results are presented as mean ± SD. Asterisks indicate significant differences (p < 0.05) between male and female.
Blood cells were identified and characterized as erythrocytes, thrombocytes, and three types of leukocytes: lymphocytes (small and large lymphocytes), neutrophils, and monocytes (Figure 1). Erythrocytes were the most numerous cells, characterized by centric purple nuclei with light-gray cytoplasm (Figure 1a).
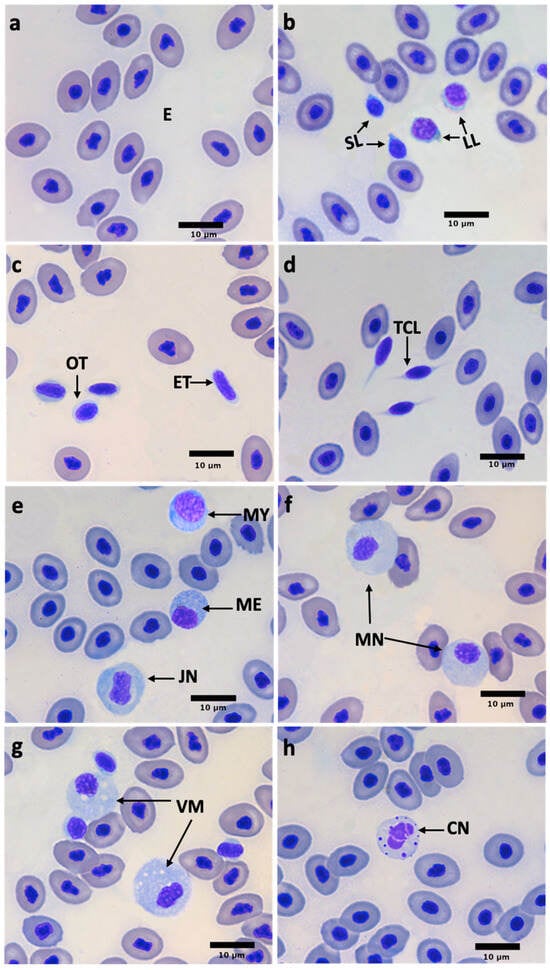
Figure 1.
Micrograph of white snook (Centropomus viridis) blood cells. (a) Erythrocytes (E); (b) small lymphocyte (SL), large lymphocyte (LL); (c) oval thrombocytes (OTs), elongate thrombocytes (ETs); (d) thrombocytes with cytoplasmic lengthening (TCL); (e) myelocyte (MY), metamyelocyte (ME), juvenile neutrophil (JN); (f) mature neutrophil (MN); (g) vacuolated monocyte (VM), (h) neutrophil–like cell (CN). Hemacolor stain; bar = 10 μm (×100 objective).
Thrombocytes were observed as single cells or in groups and were characterized as two types: ovoid or spindle-shape (Figure 1c). These cells presented a dark purple nucleus and rarely, a visible, blue–gray cytoplasm. Thrombocytes frequently showed flagellate-like cytoplasmic elongation (Figure 1d).
Lymphocytes are round, with a large, round, centrally located nucleus occupying most of the cell, where the nucleus of small lymphocytes stains dark purple compared to larger ones which have less complex chromatin and a violet nucleus. The cytoplasm is limited to a small area around the nucleus and is denoted by a blue–gray color. Some lymphocytes have cytoplasmic pseudopodia (Figure 1b).
Neutrophil morphology shows four developmental stages: (1) myelocyte: round cell with a round eccentric purple nucleus and an open chromatin pattern and a slightly blue cytoplasm, filled with small round granules (Figure 1e); (2) metamyelocyte: similar morphology to myelocyte, but with eccentric, reniform purple-painted nucleus (Figure 1e); (3) juvenile or banded neutrophil: a round cell with a centric-banded nucleus (Figure 1e); and (4) mature neutrophil: irregularly bordered cell with one or two purple-painted lobulated nuclei. The granulation of the cytoplasm is light-blue and blue–gray cytoplasm (Figure 1f).
Monocytes are round and irregular, characterized by a round or irregular eccentric nucleus. These cells exhibit a purple nucleus and blue–gray cytoplasm, and vacuoles often present unstained (Figure 1g).
In addition to erythrocytes, thrombocytes, and leukocytes, a cell type was identified which, for this study, will be referred to as the “neutrophil–like cell” (Figure 1h). It is mostly amorphous in morphology, although rounded shapes were also observed. The nucleus is irregular in shape, with multiple lobes connected by thin chromatin junctions. The cytoplasm is broad, pale blue, and contains several rounded vesicles or granules also stained purple, dispersedly distributed.
3.5. Blood Biochemistry
The blood biochemical parameters of the white snook are summarized in Table 6. Total protein showed no differences between sexes (p > 0.05). However, higher cholesterol, glucose, and triglyceride values were registered in females (p < 0.05).

Table 6.
Biochemical blood analysis of white snook (Centropomus viridis) male (n = 20) and female (n = 14). Results are presented as Mean ± SD.
4. Discussion
This is the first report of biochemical and hematological parameters in C. viridis broodstock under culture conditions, establishing a key physiological baseline for their management in captivity. The blood profiles of fish fed nutritious fresh and semi-moist diets reflected a stable and functional physiological state.
The nutritional value of the feed supplied to broodstock is essential to produce the quality of eggs and larvae. In marine fish aquaculture, it is common to use fresh and semi-moist feeds during the reproductive stage, since they promote well-being and good-quality spawning [30]. In this study, C. viridis broodstock was fed a semi-moist feed with high protein and lipid contents, supplemented with L. vannamei cephalothorax and K. pelamis muscle, whose nutritional profiles are consistent with previously reported values [31,32]. This feed is intended to cover the high requirements during reproduction.
In the present study, higher hematocrit (HCT) and mean corpuscular volume (MCV) values were observed in females compared to males. Although the number of erythrocytes was similar between sexes, the larger erythrocyte size in females suggests a blood volume adapted to their larger body size and greater metabolic demands [1,6,27,33]. In contrast, males present sufficient concentrations to meet their physiological requirements. These results support the idea that females are better adapted to maintain oxygen balance, probably due to their reproductive function and morphological differences [6,34].
The hematological indices of white snook were within the acceptable health ranges reported for fish [27]. A wide range of hematological indices is reported, highlighting the MCV (99 to 529 fL), MCH (72.6 to 55.74 pg), and MCHC (21.12 to 65 g/dL) [1,27,35,36,37,38,39,40,41]. Due to the wide range reported, finding values of C. viridis within these ranges can suggest that the fish maintain a healthy and stable physiological state, reflecting good blood function and adequate adaptations to captivity. However, this high inter-species variability highlights the need for species-specific studies.
RBCs possess an essential function in oxygen delivery to tissues. In the present study, the RBC values for the white snook are within the published values for other fishes (0.98–3.56 × 106 mm3) [14,27,42,43,44]. However, differences among various fish species are associated with physiological adaptations to respective environments that allow them to meet their metabolic needs [27,34]. These findings underscore the importance of performing species-specific hematological assessments to characterize their physiological and ecological characteristics.
When comparing the results obtained in this study with the values reported for juveniles of C. viridis (14–15 g) [24], it is observed that hematocrit (44–55%) and hemoglobin (18–24 g/dL) levels were higher in juveniles than in the adults analyzed in this work, clarifying that the adults were in the pre-spawning stage. This difference may be related to the accelerated growth rates in the juvenile stage, which demand an efficient oxygen supply to the tissues, and which could be reflected in higher HCT and HGB values [6,10].
The hematological parameters of fat snook (Centropomus parallelus) and C. viridis show a remarkable similarity, especially in the relative proportions of leukocytes, thrombocytes, and lymphocytes, suggesting a conserved physiology between species of the same genus. However, significant differences were observed in some erythrocyte indices, particularly in C. viridis females, which present higher values of HCT, HGB, and MCV, compared to the values recorded for C. parallelus [45]. These differences could be related to specific physiological requirements, such as higher tissue oxygenation demands due to size. Despite these variations, most parameters remain within similar physiological ranges, which supports the functional consistency of the hematopoietic system in species of the Centropomus genus.
In the present work, except for erythrocytes, no differences were found in the sizes of any other cell types between the sexes of C. viridis. The cell size of white snook erythrocytes found in the present work is within the published ranges for other freshwater and marine fishes, ranging between 8.6 and 11.7 μm [4,27,40,46]. In the case of lymphocytes, two sizes were identified (small lymphocytes: 4–6 μm and large lymphocytes: 6–8 μm) with similar morphological characteristics, reflecting results that are consistent with those reported for other fishes [27,44,45,46,47]. A cell’s size may reflect its functional status and maturity, as reported in other studies; however, further studies are needed to confirm this functionality [27,46,48].
Thrombocytes are involved in blood coagulation and tissue repair and expand the cell surface, making their phagocytosis function possible, while leukocytes are essential in immune defense and responsible for recognizing and eliminating pathogens [49]. In the case of thrombocytes, three morphologies were identified (oval, spindle-shape, and with cytoplasmic elongation), results that are consistent with those reported for other fishes [27,44,49]. The percentages of leukocytes and thrombocytes between sexes of C. viridis showed no differences. However, a higher percentage of lymphocytes than thrombocytes was found, consistent with reports on other fishes [14,46]. This pattern could indicate that the immune system of C. viridis prioritizes the leukocyte-mediated adaptive response, which could be related to their longevity in captivity [4,6].
Neutrophils were the second most abundant leukocyte type, which is consistent with their role in innate immunity, since they are the first cells to migrate to sites of infection or tissue damage, where they carry out phagocytic functions and release antimicrobial compounds [49,50,51]; these results agree with those found in other fishes [16,27]. Vacuolated monocytes were observed in this case, a typical feature of reactive cells [52]. Monocytes were third in abundance in white snook, characterized by being the largest blood cells with a fundamental role in the inflammatory response [52,53,54].
The presence of neutrophils and monocytes suggests a basal immune status, while eosinophils and basophils were not observed as previously reported in other fishes [27,52,55,56,57]. The absence of eosinophils and basophils could be attributed to the lack of parasitic infections, allergic processes, or chronic inflammation, indicating the good health status of the C. viridis broodstock under condition captive culture. However, it is also possible that failure to detect this cell type was due to limitations in the methodologies used for its identification. Therefore, their apparent absence should be interpreted with caution.
Females had higher cholesterol, triglycerides, and glucose values than males, which may be related to differences in metabolism or reproductive physiology [6]. Cholesterol plays a vital role during reproduction, as it participates in steroidogenesis as a precursor of steroid hormones [58], where cholesterol is converted to pregnenolone, then to progesterone, and finally to 17β-estradiol [18], a hormone that plays a key role initiating vitellogenesis in female, carrying out lipid and vitamin incorporation into oocytes [54,55]. The cholesterol concentrations (180–346 mg/dL) reported for C. viridis are within the ranges reported for other fish species [1,4,15,42]. In the case of triglycerides (161–371 mg/dL), the values from adults were within the range reported for juveniles of the same species [24]. In broodstock fish, triglycerides are associated with gonadal maturation, particularly during vitellogenesis [34,59].
The protein values found in C. viridis of both sexes are within the range reported for other fishes (39.36 to 75.45 g/L) [4,5,34,60]. The glucose values found for C. viridis broodstock were within those reported for other fish species (51.47–153.40 mg/dL). The blood biochemistry results obtained in this study denote differential values in protein and glucose levels compared to juvenile C. viridis (14–15 g), and they report ranges of total protein (57–72 g/L) and glucose (96–105 mg/dL) levels comparable to adults [24]. On the other hand, lower glucose levels could indicate a more efficient or sustained use of energy substrates in broodstock compared to juvenile fish [61].
This information is beneficial for designing and adjusting management programs in aquaculture systems, particularly in aspects such as nutrition, health monitoring, and improvement of reproductive performance. However, it is essential to consider that these results correspond exclusively to the specimens evaluated in this study under controlled culture conditions and specific nutritional management, so they should not be generalized to all individuals of the species. In this sense, each culture center should carry out analyses to establish specific reference values, considering that studies for specific immunological biomarkers in blood can be focused on, allowing for more precise monitoring of immunological status during cultivation, thus promoting more efficient and sustainable health management, adapted to the conditions of each system.
Recirculating aquaculture systems (RASs) elicit variable hematological responses among species, which is evidence that not all fish react to these conditions in the same way. In species such as tambaqui (Colossoma macropomum) [62] and European Catfish (Silurus glanis) [63], blood parameters remained stable, suggesting a good adaptation to RASs, while in Nile tilapia (Oreochromis niloticus) [64], cellular alterations associated with stress and higher parasite load were observed. In the case of C. viridis, although no parasitic challenges were detected during the study period, the absence of previous research precludes determining whether the RAS significantly influenced its hematological parameters. This uncertainty highlights the need for ongoing physiological monitoring or for comparisons with wild individuals to determine whether the controlled RAS environment induces subtle or cumulative changes in this species.
5. Conclusions
The preliminary values of the blood parameters for C. viridis broodstock show that females present higher levels of glucose, cholesterol, and triglycerides than males, due to a differential energy metabolism associated with their physiological demands of reproduction. Likewise, HCT, MCV, and erythrocyte cell size values were higher in females than males, influenced by their larger body size and higher oxygen transport demand.
The blood parameter values obtained in this study provide valuable information on captive C. viridis broodstock. The differences observed between sexes reflect particular physiological characteristics related to the specimens’ reproductive demands and body size. In addition, a functional basal immune system was identified in the organisms evaluated, indicating that the fish maintained good health under the culture conditions. These findings provide an essential basis for understanding the species’ physiology in controlled environments. However, the sample size of the present study limits its scope as a reference for the species. For this reason, it is necessary to implement a blood parameter sampling system to establish reference ranges that can serve as physiological markers for C. viridis.
Author Contributions
Conceptualization, I.A.H.-L. and J.M.M.-B.; methodology, I.A.H.-L. and V.P.D.-J.; validation, I.A.H.-L., O.B.D.R.-Z., V.P.D.-J., R.L.-O., R.M.M.-G., and J.M.M.-B.; formal analysis, I.A.H.-L., V.P.D.-J., R.L.-O., R.M.M.-G., and O.B.D.R.-Z.; investigation, I.A.H.-L.; resources, J.M.M.-B.; data curation, I.A.H.-L. and E.S.P.-M.; writing—original draft preparation, I.A.H.-L. and E.S.P.-M.; writing—review and editing, I.A.H.-L., E.S.P.-M., L.I.-C., O.B.D.R.-Z., and J.M.M.-B.; visualization, I.A.H.-L. and E.S.P.-M.; supervision, I.A.H.-L., J.M.M.-B., V.P.D.-J., R.L.-O., and R.M.M.-G.; project administration, J.M.M.-B. and I.A.H.-L.; funding acquisition, J.M.M.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
In Mexico, the use of fish in scientific research is not yet regulated explicitly under national animal welfare legislation (NOM-062-ZOO-1999), and ethical approval by an Institutional Animal Care and Use Committee is not mandatory for fish studies. However, all procedures in this study were conducted in accordance with internationally recognized ethical standards for the use of animals in research, including the Guidelines for the Use of Fishes in Research (American Fisheries Society, 2014), which ensures the humane treatment and welfare of the fish.
Informed Consent Statement
Not applicable.
Data Availability Statement
For access to the data presented in this study, please contact the corresponding author.
Acknowledgments
The authors thank Biol. Jesús Humberto Ochoa Campos, Biol. Jesús Rodolfo Monjaraz Camargo, and Biól. Jorge Alejandro Reyes Meza, technicians of the Marine Fish Hatchery of CIAD, Mazatlán, as well as Luz Estela Rodríguez Ibarra, researcher of the Reproduction Laboratory of CIAD, Mazatlán, for her valuable support in handling the organisms and in the whole process of obtaining samples.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IIO | Instituto de Investigaciones Oceanológicas |
| RBCs | Red Blood Cells |
| MCV | Mean Corpuscular Volume |
| MHC | Mean Corpuscular Hemoglobin |
| MCHC | Mean Corpuscular Hemoglobin Concentration |
| WBCs | White Blood Cells |
| AOAC | Association of Official Analytical Collaboration |
| UDK | Kjeldahl Distillation Unit |
| NFE | Nitrogen-free extract |
| CIAD | Centro de Investigación en Alimentación y Desarrollo S.A. |
| HGB | Hemoglobin |
| CMOS | Complementary Metal–Oxide Semiconductor |
| HCT | Hematocrit |
| DE | Standard Deviation |
| SL | Small Lymphocyte |
| LL | Large Lymphocyte |
| OT | Oval Thrombocyte |
| ET | Elongate Thrombocyte |
| TCL | Thrombocytes With Cytoplasmic Lengthening |
| MY | Myelocyte |
| ME | Metamyelocyte |
| JN | Juvenile Neutrophil |
| MN | Mature Neutrophil |
| VM | Vacuolated Monocyte |
| CN | Neutrophil-like cell |
| PSU | Practical Salinity Unit |
| RAS | Recirculating aquaculture systems |
| Nd | No determinate, cytoplasm almost null. |
References
- Gonzales, A.; Curto, G.; Fernández-Mendez, C. Hematological parameters of Brycon amazonicus (Bryconidae) breeders in culture. Rev. Investig. Vet. Perú 2019, 30, 133–142. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, G.A. Biological Interactions. In Physiology of Fish in Intensive Culture Systems; Springer: Boston, MA, USA, 1996; pp. 166–202. [Google Scholar]
- Gayatri, A.; Mohanty, P.K. Effect of sex on haemocyte biochemical profiling of silver tiger fish (Datnioides polota Hamilton, 1822). Comp. Clin. Pathol. 2018, 27, 1335–1342. [Google Scholar] [CrossRef]
- Ruiz-González, L.E.; Vega-Villasante, F.; Tintos-Gómez, A.; Del Río-Zaragoza, O.B.; Hernández-Rodríguez, M.; Patiño-Barragán, M.; Badillo-Zapata, D. Some hematology and blood chemistry parameters of the Pacific fat sleeper Dormitator latifrons (Richardson, 1844). Lat. Am. J. Aquat. Res. 2020, 48, 131–135. [Google Scholar] [CrossRef]
- Ahmed, I.; Reshi, Q.M.; Fazio, F. The influence of the endogenous and exogenous factors on hematological parameters in different fish species: A review. Aquacult. Int. 2020, 28, 869–899. [Google Scholar] [CrossRef]
- Clauss, T.M.; Dove, A.D.M.; Arnold, J.E. Hematologic disorders of fish. Fishes 2008, 11, 445–462. [Google Scholar] [CrossRef]
- Cnaani, A.; Tinman, S.; Avidar, Y.; Ron, M.; Hulata, G. Comparative study of biochemical parameters in response to stress in Oreochromis aureus, O. mossambicus and two strains of O. niloticus. Aquac. Res. 2004, 35, 1434–1440. [Google Scholar] [CrossRef]
- Parrino, V.; Cappello, T.; Costa, G.; Cannavà, C.; Sanfilippo, M.; Fazio, F.; Fasulo, S. Comparative study of haematology of two teleost fish (Mugil cephalus and Carassius auratus) from different environments and feeding habits. Eur. Zool. J. 2018, 85, 194–200. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Aragona, F.; Habib, S.S.; Fazio, F.; Zumbo, A.; Costa, A.; Riolo, K.; Giannetto, A.; Parrino, V. Morphometric, Nutritional, and Blood Analyses in Hybrid Striped Bass (Morone chrysops x Morone saxatilis, Walbaum 1972) Reared in a Recirculating Aquaculture System (RAS) Implant in Sicily, Italy. Fishes 2025, 10, 278. [Google Scholar] [CrossRef]
- Del Rio-Zaragoza, O.B.; Araújo, B.C.; Viana, M.T. Health status evaluation of striped bass (Morone saxatilis) exposed to low temperature in sea cage culture during the grow-out. Aquac. Res. 2021, 52, 2435–2445. [Google Scholar] [CrossRef]
- Ruiz-González, L.E.; Tafoya-Sánchez, D.J.; Tintos-Gómez, A.; Del Río-Zaragoza, O.B.; Vargas-Ceballos, M.A.; Vega-Villasante, F. Some blood parameters of Pacific fat sleepers, Dormitator latifrons (Richardson, 1844): A comparative study between male and female in two growth stages. Lat. Am. J. Aquat. Res. 2023, 51, 592–597. [Google Scholar] [CrossRef]
- Correa Negrete, J.C.; Garrido Correa, A.A.; Prieto Guevara, M.J.; Atencio García, V.J.; Pardo Carrasco, S.C. Caracterización de células sanguíneas y parámetros hematológicos en blanquillo Sorubim cuspicaudus. Zootec. Trop. 2009, 27, 393–405. [Google Scholar]
- Fazio, F.; Lanteri, G.; Saoca, C.; Laria, C.; Piccione, G.; Orefice, T.; Calabrese, E.; Vazzana, I. Individual variability of blood parameters in striped bass Morone saxatilis: Possible differences related to weight and length. Aquac. Int. 2020, 28, 1665–1673. [Google Scholar] [CrossRef]
- Sáez, G.; Chero, J.; Cruces, C.; Minaya, D.; Rodriguez, C.; Suyo, B.; Romero, S.; Guabloche, A.; Tuesta, E.; Alvariño, L.; et al. Hematological and blood biochemistry parameters in ten species of marine fish captured by artisanal fisheries in the Bay of Callao, Peru. Rev. Investig. Vet. Perú 2018, 29, 1161–1177. [Google Scholar] [CrossRef]
- Labastida-Che, A.; Núñez-Orozco, A.L.; Oviedo-Piamonte, J.A. Aspectos biológicos del robalo hocicudo Centropomus viridis, en el sistema lagunar Chantuto-Panzacola, Chiapas, México. Cienc. Pesq. 2013, 21, 21–28. [Google Scholar]
- Navarro-Flores, J.; Martínez-Brown, J.M.; Zavala-Leal, I.; Rojo-Cebreros, A.H.; Ibarra-Castro, L. Assessing the feasibility of exogenous 17β-estradiol for inducing sex change in white snook, C. viridis: From growth, resting and maturation studies. Aqua. Rep. 2023, 33, 101767. [Google Scholar] [CrossRef]
- Ibarra-Castro, L.; Navarro-Flores, J.; Sánchez-Téllez, J.L.; Martínez-Brown, J.M.; Ochoa- Bojórquez, L.A.; Rojo-Cebreros, A.H. Hatchery Production of Pacific White Snook at CIAD-Unity Mazatlan. Mex. World Aquac. 2017, 48, 25–29. [Google Scholar]
- Baldini, G.; Santamaría-Miranda, A.; Martínez-Brown, J.M.; Ibarra-Castro, L. Technical-economic viability of white snook Centropomus viridis culture in floating cages in a coastal lagoon in northwestern Mexico. Aquac. Rep. 2022, 23, 101048. [Google Scholar] [CrossRef]
- Montoya Ponce, C.O.; Santamaría Miranda, A.; Trigueros Salmerón, J.Á.; Apún Molina, J.P.; Valenzuela Orduño, F.G.; Lugo Gamboa, R.R. Bioeconomic Analysis of Snook Centropomus viridis, C. nigrescens, and C. medius for the Development of Mariculture in Northern Sinaloa. Fishes 2024, 9, 39. [Google Scholar] [CrossRef]
- Çelik, E.Ş.; Kaya, H.; Yılmaz, S.; Çakıcı, H. Effect of water temperature, salinity, season, reproduction, sex, size, and age on hematological parameters of horse mackerel (Trachurus trachurus). Kafkas Univ. Vet. Fak. Derg. 2012, 18, 551–558. [Google Scholar] [CrossRef]
- Suljević, D.; Mitrašinović-Brulić, M. The first record of brook trout (Salvelinus fontinalis, Salmonidae) blood cell characteristics and hematological profile: The influence of fish sex on leukocyte count. Aquac. Int. 2020, 28, 2505–2516. [Google Scholar] [CrossRef]
- Abdo-de la Parra, M.I.; Rodríguez-Ibarra, L.E.; Ibarra-Castro, L.; Martínez-Brown, J.M.; Álvarez-González, C.A.; Peña, E.; Velasco-Blanco, G.; Domínguez-Jiménez, P.; Rodríguez-Montes de Oca, G. Evaluation of different levels of dietary protein and lipids on the growth, feed efficiency, and biometric and hematological indexes of juvenile white snooks, Centropomus viridis. Cienc. Mar. 2023, 49, e3368. [Google Scholar] [CrossRef]
- [AOAC] Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; AOAC: Arlington, MA, USA, 2000; 684p. [Google Scholar]
- Abdo-de la Parra, M.I.; Rodríguez-Ibarra, L.E.; Ibarra-Castro, L.; Martínez-Brown, J.M.; Velasco-Blanco, G. Effects of frequency and feeding time on growth, food utilization, somatic indexes and survival of juvenile white snook Centropomus viridis. Cienc. Marinas. 2020, 46, 155–163. [Google Scholar] [CrossRef]
- Del Rio-Zaragoza, O.B.; Fajer-Ávila, E.J.; Almazán-Rueda, P.; Abdo de la Parra, M.I. Hematological characteristics of the spotted rose snapper Lutjanus guttatus (Steindachner, 1869) healthy and naturally infected by dactylogyrid monogeneans. Tiss. Cell 2011, 43, 137–142. [Google Scholar] [CrossRef]
- Del Rio-Zaragoza, O.B.; Hernández-Rodríguez, M.; Bückle-Ramirez, L.F. Thermal stress effect on tilapia Oreochromis mossambicus (Pisces: Cichlidae) blood parameters. Mar. Freshw. Behav. Physiol. 2008, 41, 135–145. [Google Scholar] [CrossRef]
- Natt, M.P.; Herrick, C.A. A new blood diluent for counting erythrocytes and leukocytes of the chicken. Poult. Sci. 1952, 31, 735–738. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Fernández-Palacios, H.; Tacon, A.G.J. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 2001, 197, 25–42. [Google Scholar] [CrossRef]
- Nurjanah, N.; Suseno, S.H.; Hidayat, T.; Arifianto, T.B. Changes in nutritional composition of skipjack (Katsuwonus pelamis) due to frying process. Int. Food Res. J. 2015, 22, 2093–2102. [Google Scholar]
- Dayakar, B.; Xavier, K.A.M.; Ngasotter, S.; Layana, P.; Balange, A.K.; Priyadarshini, B.; Nayak, B.B. Characterization of spray-dried carotenoprotein powder from Pacific white shrimp (Litopenaeus vannamei) shells and head waste extracted using papain: Antioxidant, spectroscopic, and microstructural properties. LWT 2022, 159, 113188. [Google Scholar] [CrossRef]
- Ranzani-Paiva, M.J.; Ishikawa, C.M. Haematological characteristics of freshwater-reared and wild mullet, Mugil platanus Günther (Osteichthyes, Mugilidae). Rev. Bras. Zool. 1996, 13, 561–568. [Google Scholar] [CrossRef]
- Chen, H.; Luo, D. Application of haematology parameters for health management in fish farms. Rev. Aquac. 2022, 15, 704–737. [Google Scholar] [CrossRef]
- Kim, S.G.; Kang, J.C. Effect of dietary copper exposure on accumulation, growth and hematological parameters of the juvenile rockfish, Sebastes schlegeli. Mar. Environ. Res. 2004, 58, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.M.; Baldwin, J.; Seymour, R.S.; Christian, K.; Brittain, T. Red blood cell function and haematology in two tropical freshwater fishes from Australia. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 141, 87–93. [Google Scholar] [CrossRef]
- Elahee, K.B.; Bhagwant, S. Hematological and gill histopathological parameters of three tropical fish species from a polluted lagoon on the west coast of Mauritius. Ecotoxicol. Environ. Saf. 2007, 68, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Kochinian, P.; Salati, A.P. The effect of sexuality on some haematological parameters of the yellowfin seabream, Acanthopagrus latus in Persian Gulf. Iran. J. Vet. Res. 2013, 14, 273–278. [Google Scholar] [CrossRef]
- Witeska, M. Erythrocytes in teleost fishes: A review. Zool. Ecol. 2013, 23, 275–281. [Google Scholar] [CrossRef]
- Sayed, A.E.H.; Mahmoud, U.M.; Muhammad, O.I. Comparative study of two carnivorous fish (Parupeneus forsskali and Thalassoma klunzingeri) from the Red Sea: Hemato-biochemical parameters and cellular characterization. Tissue Cell 2020, 63, 101316. [Google Scholar] [CrossRef]
- Habiba, M.M.; Hussein, E.E.; Ashry, A.M.; El-Zayat, A.M.; Hassan, A.M.; El-Shehawi, A.M.; Sewilam, H.; Van Doan, H.; Dawood, M.A.O. Dietary cinnamon successfully enhanced the growth performance, growth hormone, antibacterial capacity, and immunity of European Sea bass (Dicentrarchus labrax). Animals 2021, 11, 2128. [Google Scholar] [CrossRef]
- Satheeshkumar, P.; Ananthan, G.; Senthilkumar, D.; Khan, A.B.; Jeevanantham, K. Comparative investigation on haematological and biochemical studies on wild marine teleost fishes from Vellar estuary, southeast coast of India. Comp. Clin. Pathol. 2012, 21, 275–281. [Google Scholar] [CrossRef]
- Zhu, P.; Tang, Y.; Fan, J.; Fang, J.; Peng, X.; Cui, H. Hematological parameters and blood cell morphology of male and female Schizothorax (Racoma) davidi (Sauvage). J. World Aquac. Soc. 2017, 48, 821–830. [Google Scholar] [CrossRef]
- Nabi, N.; Ahmed, I.; Wani, G.B. Hematological and serum biochemical reference intervals of rainbow trout, Oncorhynchus mykiss cultured in Himalayan aquaculture: Morphology, morphometrics and quantification of peripheral blood cells. Saudi J. Biol. Sci. 2022, 29, 2942–2957. [Google Scholar] [CrossRef]
- Santos, A.A.; Ranzani-Paivad, M.J.T.; Leite da Veigae, M.; Faustinof, L.; Egami, M.I. Hematological parameters and phagocytic activity in fat snook (Centropomus parallelus) bred in captivity. Fish Shellfish Immunol. 2012, 30, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Yao, H.; Qiang, L.; Chen, X.; Gao, Y. Comparative study of peripheral blood cells in two marine fishes (Synechogobius hasta and Sebastes schlegelii): Morphological and cytochemical characterization. Tissue Cell 2021, 73, 101633. [Google Scholar] [CrossRef]
- Imagawa, T.; Hashimoto, Y.; Kitagawa, H.; Kon, Y.; Kudo, N.; Sugimura, M. Morphology of blood cells in carp (Cyprinus carpio L.). Nihon Juigaku Zasshi 1989, 51, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qiang, L.; Wu, N.; Wang, H.; Hao, Y. Morphological and cytochemical characterization of the peripheral blood cells in Paralichthys olivaceus. Heliyon 2024, 10, e37011. [Google Scholar] [CrossRef]
- Zhu, W.; Su, J. Immune functions of phagocytic blood cells in teleost. Rev. Aquac. 2021, 14, 630–646. [Google Scholar] [CrossRef]
- Havixbeck, J.J.; Barreda, D.R. Neutrophil development, migration, and function in teleost fish. Biology 2015, 4, 715–734. [Google Scholar] [CrossRef]
- Buchmann, K. Neutrophils and aquatic pathogens. Parasite Immunol. 2022, 44, e12915. [Google Scholar] [CrossRef]
- Megarani, D.V.; Hardian, A.B.; Arifianto, D.; Santosa, C.M.; Salasia, S.I.O. Comparative morphology and morphometry of blood cells in zebrafish (Danio rerio), common carp (Cyprinus carpio carpio), and tilapia (Oreochromis niloticus). J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 673–680. [Google Scholar] [CrossRef]
- Rowley, A.F.; Hunt, T.C.; Page, M.; Mainwaring, G. Fish. In Vertebrate Blood Cells; Rowley, A.F., Ratcliffe, N.A., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 19–127. [Google Scholar]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Tavares-Dias, M.; Barcellos, J.F.M.; Marcon, J.L.; Menezes, G.C.; Ono, E.A.; Affonso, E.G. Hematological and biochemical parameters for the pirarucu Arapaima gigas Schinz, 1822 (Osteoglossiformes, Arapaimatidae) in net cage culture. Electron. J. Ichthyol. 2007, 2, 61–68. [Google Scholar] [CrossRef]
- Hrubec, T.C.; Cardinale, J.L.; Smith, S.A. Haematology and plasma chemistry reference intervals for cultured tilapia (Oreochromis hybrid). Vet. Clin. Pathol. 2000, 29, 7–12. [Google Scholar] [CrossRef]
- Fajer-Ávila, E.J.; Guzman-Beltran, L.; Zárate-Rodríguez, W.C.; Del Río-Zaragoza, O.B.; Almazan-Rueda, P. Pathology caused by adult Pseudochondracanthus diceraus (Copepoda: Chondracanthidae), a parasite of bullseye puffer fish Sphoeroides annulatus. Rev. Biol. Mar. Oceanogr. 2011, 46, 293–302. [Google Scholar] [CrossRef]
- Nieto-Vera, M.T.; Rodríguez-Pulido, J.A.; Góngora-Orjuela, A. ¿Qué sabemos de los esteroides sexuales y las gonadotropinas en la reproducción de teleósteos neotropicales? Orinoquia 2020, 24, 52–63. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.; Fiocchi, E.; Toomey, L.; Boscarato, M.; Manfrin, A.; Dimitroglou, A.; Papaharisis, L.; Passabi, E.; Stefani, A.; Lembo, G.; et al. Comparative analysis of blood protein fractions in two Mediterranean farmed fish: Dicentrarchus labrax and Sparus aurata. BMC Vet. Res. 2024, 20, 322. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Petillo, E.C.; Ferreira, A.d.C.; Oliveira CPFd Brandão, L.V.; Marinho-Pereira, T.; Cavero, B.A.S. Tambaqui (Colossoma macropomum) in RAS Technology: Zootechnical, Hematological, Biochemical and Kn Profiles at Different Stocking Densities During the Initial Grow-Out Phase. Aquac. J. 2025, 5, 1. [Google Scholar] [CrossRef]
- Barbacariu, C.-A.; Rimbu, C.M.; Burducea, M.; Dirvariu, L.; Miron, L.-D.; Boiangiu, R.S.; Dumitru, G.; Todirascu-Ciornea, E. Comparative Study of Flesh Quality, Blood Profile, Antioxidant Status, and Intestinal Microbiota of European Catfish (Silurus glanis) Cultivated in a Recirculating Aquaculture System (RAS) and Earthen Pond System. Life 2023, 13, 1282. [Google Scholar] [CrossRef]
- Stallbohm, R.; Owatari, M.S.; Zaniboni-Filho, E.; Martins, M.L. Recirculating aquaculture systems affects hematological parameters and increases ectoparasite susceptibility in Nile tilapia Oreochromis niloticus. Mar. Fish. Sci. (MAFIS) 2024, 37, 609–618. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).