1. Introduction
Sea urchin roe (gonads), highly regarded as gourmet, is particularly popular in some countries and regions, including Asia, the Mediterranean, and the Americas [
1] Nevertheless, the rapidly increasing international demand for gonads has led to the overexploitation of natural populations of edible sea urchins [
2]. In this context, echinoculture is regarded as an advantageous countermeasure to meet the scarcity of wild resources [
3]. In 1989,
Strongylocentrotus intermedius was first imported to China from Japan by our research group [
4].
S. intermedius has become lucrative in northern China [
5]. Sea urchins naturally live mainly on fresh macroalgae. However, the availability and nutrition of macroalgae vary at different seasons all year round, which has limited the massive use for large-scale aquaculture [
6]. The gonadal color of sea urchins is an important determinant of their commercial value [
7], with bright yellow and yellow-orange widely accepted as premium colors by consumers. Compared to kelp, formulated feeds usually produce sea urchins with pale gonads [
1,
8]. Thus, it becomes critically imperative to clarify the underlying mechanisms and develop efficient feed optimization strategies to enhance the gonadal color of sea urchins.
Sea urchin gonadal color is attributed to the accumulation of carotenoids [
9]. Echinenone has proven to correlate positively with the occurrence of gonads with bright yellow and yellow-orange color [
10,
11,
12]. Sea urchins have been reported to have the ability to synthesize echinenone by oxidizing β-carotene [
11,
12]. Previous studies have showed that β-carotene at an addition level of 100–250 mg/kg can effectively provide a more commercially desirable color to the gonads of sea urchin
S. droebachiensis [
13]. Additionally, with the advancement of high-throughput sequencing technology, a growing number of researchers are using transcriptome analysis to investigate molecular mechanisms [
14]. New color-related genes were discovered by sequencing color-different aquatic animal samples and combining them with biochemical analyses [
15,
16,
17]. Two apolipoprotein genes involved in carotenoid transport and storage were identified by the transcriptome analysis of Pacific oyster
Crassostrea gigas fed different levels of β-carotene [
16]. These results have significantly enhanced our understanding of pigmentation processes and their regulatory mechanisms in aquatic animals. However, from what we know, no studies have been published on the impact of dietary β-carotene on the distribution of major carotenoids and the associated mechanisms about echinenone biosynthesis in
S. intermedius.
Therefore, the aims of this research are to determine (i) how β-carotene affects the distribution of major carotenoids in S. intermedius and (ii) the specific genes and pathways that participate in the echinenone biosynthesis in the gonads of S. intermedius. These results could help provide some basic clues for producing gonads with market-favored colors of S. intermedius.
2. Materials and Methods
2.1. Diet Preparation and Feeding Experiment
Kelp (
Saccharina japonica) and three iso-proteinic (20.13%) and iso-lipidic (5.92%) formulated feeds were used for the feeding experiment. The formulated feeds were produced with the addition of β-carotene at different concentrations (0 mg/kg, 150 mg/kg, 300 mg/kg) (
Table 1), which were named C0, C150, and C300, respectively.
All powder ingredients (<150 μm) of each feed were accurately weighed and mixed. Subsequently, the oils were blended with the mixture. After that, 30% water was added before the diets were processed into pellets using a pelletizer (DES-TS1280, Jinan, China). Feed pellets were dried, sealed, and placed in a refrigerator (−20 °C).
After acclimation to the experimental conditions, 120 healthy sea urchins (9.33 ± 0.12 g) were randomly divided among 12 floating cages (30 cm × 15 cm × 45 cm). Then, three cages of sea urchins were randomly fed one of the test diets until they showed obvious satiation thrice daily (8:00, 12:00, and 18:00) for 60 days. On average, 30% of water was replaced daily by siphoning the remaining dietary residues and excrement from the bottom of the tank. During the feeding period, the water temperature, pH, salinity, and dissolved oxygen were kept at 13 ± 1 °C, 8.0 ± 0.1, 33 ± 1‰, and above 8.0 mg/L.
2.2. Sampling
After the feeding test ended, the experimental animals were starved overnight before being counted and weighed separately to obtain the survival rate (SR) and weight gain rate (WGR). Then, sea urchins were individually dissected and sampled to obtain digestive tracts, gonads, and skeletons. The weights of the wet body mass, digestive tract, and gonads for every individual sea urchin were taken to compute the digestive tract index (DTI) and the gonadosomatic index (GSI). Subsequently, the digestive tracts of the sea urchins were preserved at −80 °C for subsequent analysis of carotenoid levels and gene expression levels. The gonads of each sea urchin were separated into four sections: one section for the histological observation; two sections for color measurement and carotenoid determination; one section for transcriptome analysis; and one section for gene expression. Finally, the skeletons of each sea urchin were comminuted into fine powder and kept at −80 °C for carotenoid determination.
2.3. Gonad Color Measurement
The gonad color was measured using a Color Cue 2 Colorimeter (PANTONE, Carlstadt, NJ, USA). ΔE denoted the distinction between the actual color value and normal color levels. The standards for ΔE1 (L* = 68.9, a* = 28.7, b* = 60.4) and ΔE2 (L* = 74.6, a* = 28.7, b* = 66.1) were reported by McBride [
18].
2.4. Determination of Carotenoids
The procedures for extraction and carotenoid analysis were in line with Baião et al. [
19]. The contents of β-carotene in the diets and those of echinenone in the sea urchin tissues were determined by a high-performance liquid chromatograph (HPLC) (model Agilent-1290-6470 brand Agilent) (Agilent Technologies, Santa Clara, CA, USA) and a mass spectrometer (model QQQ brand Agilent). The carotenoid extracts of 0.05 mg lyophilized samples were suspended in 0.2 mL of methanol/methyl tert-butyl ether (1:1), and 5 μL was injected into the Agilent C18 column (2.1 mm × 100 mm, 1.8 μm). Elution agents include solvent A (methanol/acetonitrile = 3:1) and solvent B (methyl tertiary-butyl ether). The 0.3 mL/min flow rate was prescribed, the column temperature was stabilized at 30 °C, and the acquisition mode was established as APCI. The mobile phase gradient ratio was 95%A + 5%B for the first 0–1 min, and then the gradient ratio was linearly changed to 5%A + 95%B for 1–3 min and maintained for 1 min. After that, the mobile phase was linearly changed to 95%A + 5%B for 4–4.5 min and held for 1.5 min. Finally, the gradient ratio was linearly changed to 70%A + 30%B for 6–9 min. The atmospheric pressure chemical ion source for mass spectrometry was set as follows: the atomization temperature of vaporization was 325 °C; the gas flow speed was 4 L/min; the pressure of the nebulizer was 20 psi; and the voltage of the capillary voltage was 4500 V. The retention time of each carotenoid was recorded by comparison with pure products of β-carotene (Tan mo, Changzhou City, Jiangsu Province, China) and echinenone (CaroteNature, Münsingen, Switzerland). The specific contents of β-carotene and echinenone were calculated by the calibration curves. The data were expressed as μg/kg dry weight.
2.5. Histological Analysis
Gonadal sections were prepared based on the methodology defined by Santos et al. [
20]. To sum up briefly, the fixed tissues were subjected to ethanol treatment for the purpose of dehydration and then embedded in paraffin before being sectioned into thin slices. These sections were stained by immersion in hematoxylin and eosin. Eventually, the slices were analyzed microscopically. For the stages of gonadal maturation (stages I–VI), refer to Santos et al. [
20].
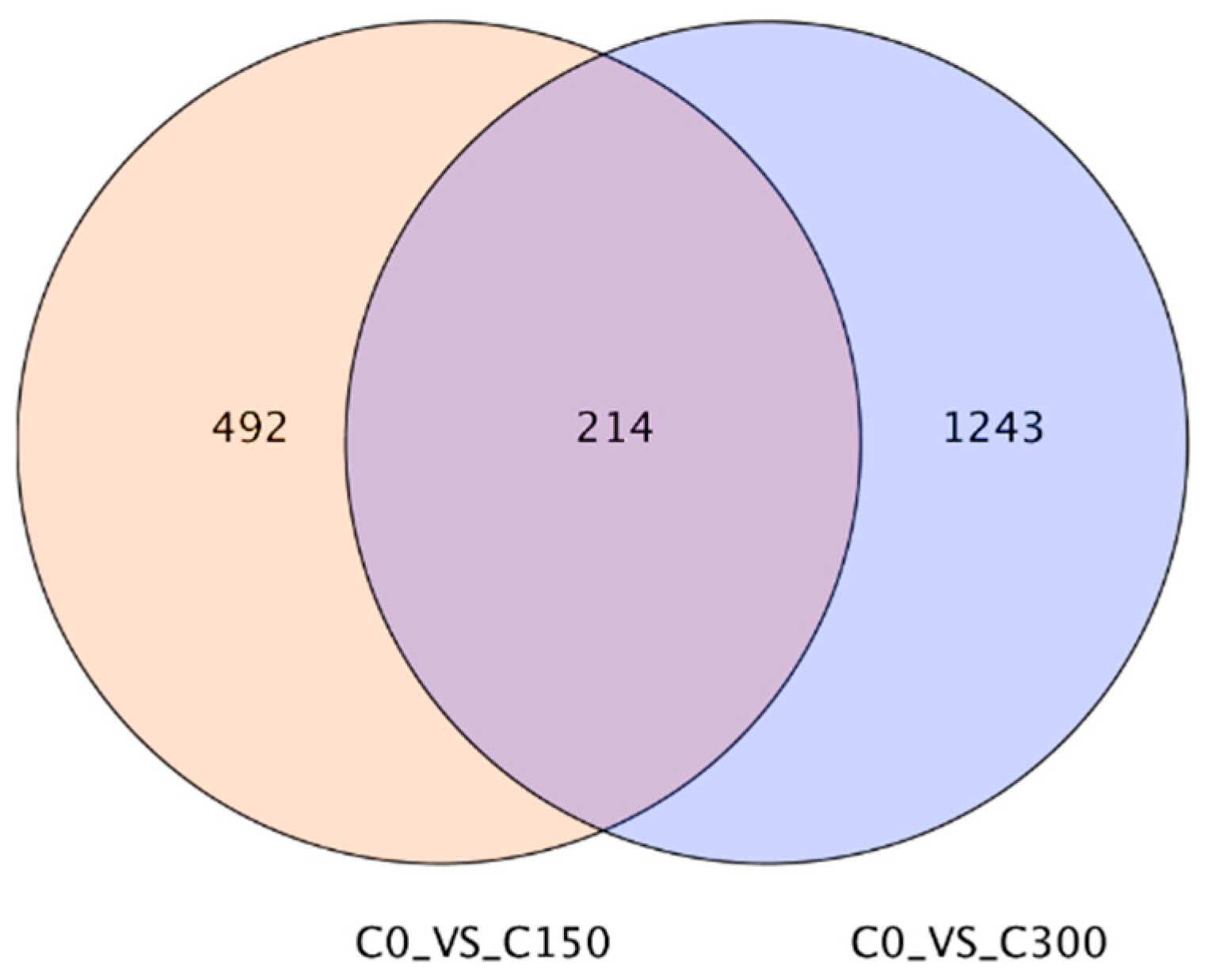
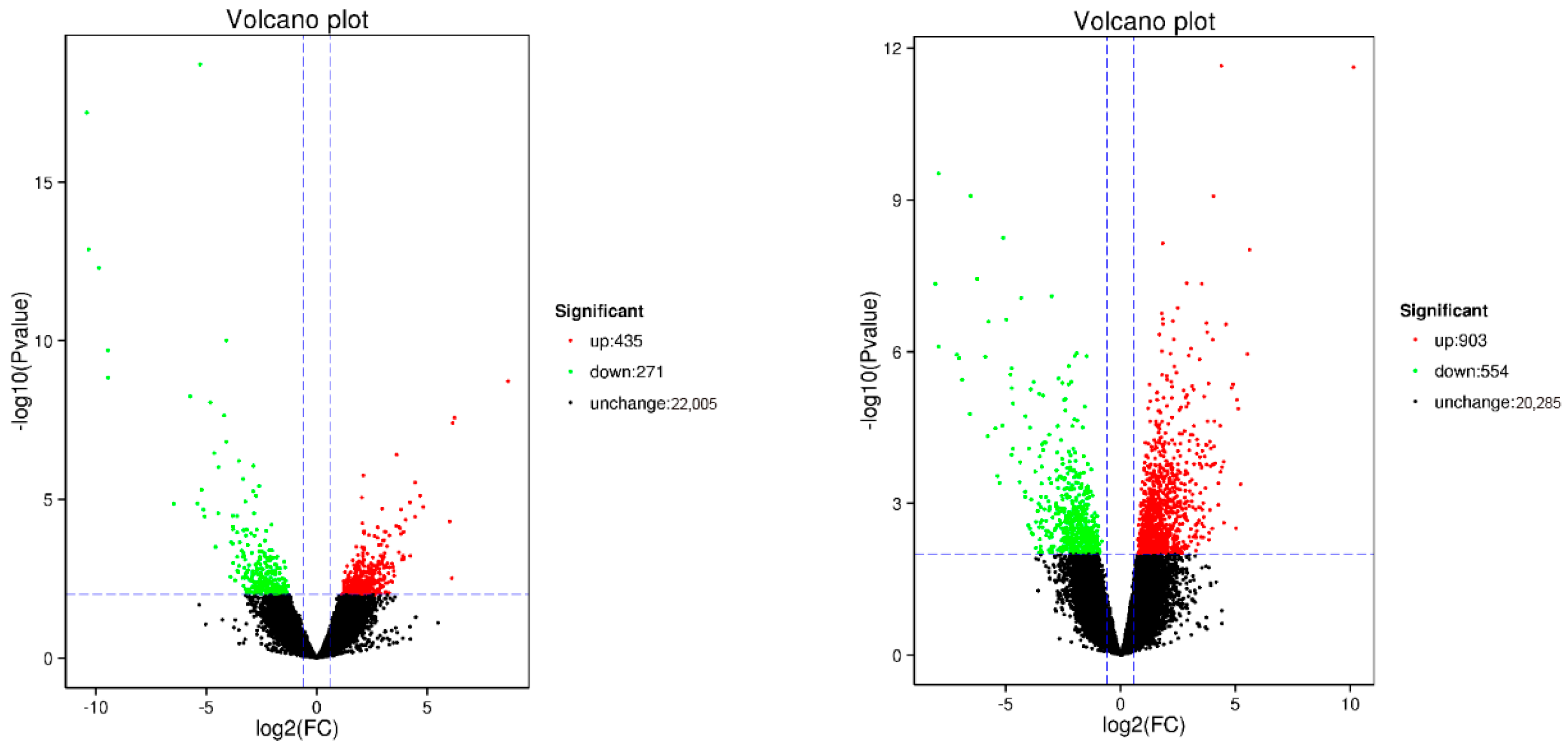
2.6. RNA-Seq and Transcriptome Analyses
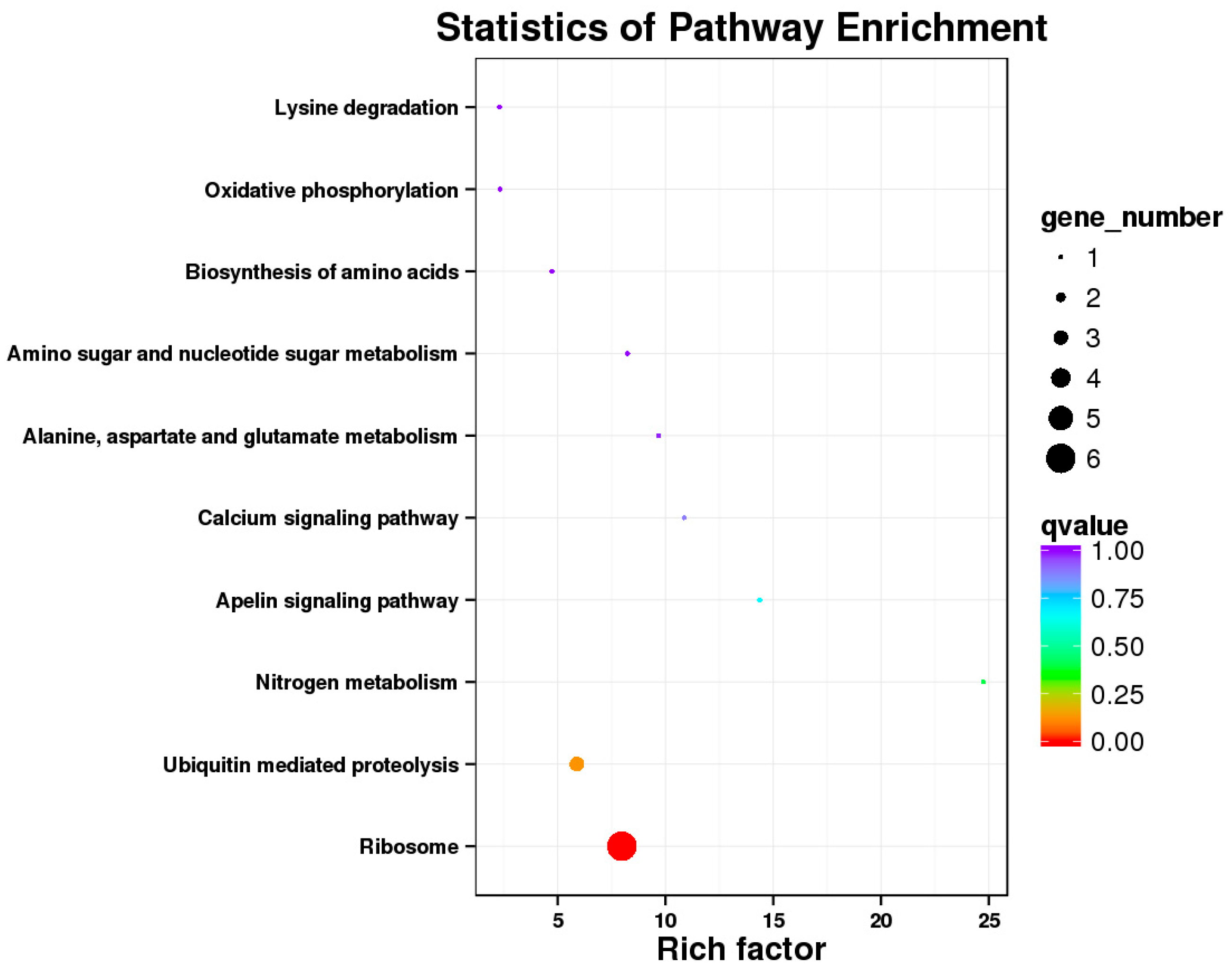
The Illumina Nova seq 6000 was used to sequence the gonadal transcriptomes of sea urchins of different dietary groups. Trinity was used to complete the transcriptome assembly [
21]. DIAMOND was used to annotate the assembled genes based on multiple databases, such as the NCBI non-redundant (Nr) database, Swiss-Prot, KOG/COG (protein homology groups), GO (gene ontology), and Pfam (large collection of protein families) [
22]. To annotate potential pathways of metabolism, each gene was aligned to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. The reads generated by sequencing were aligned with the Unigene library by using Bowtie [
23]. On the basis of the findings of the comparison, an expression level estimate was taken in combination with RSEM [
22]. Gene sets exhibiting differential expression were identified in distinct samples using the DESeq2 software (version 1.36.0) [
24]. To ascertain the differentially expressed genes (DEGs) between samples, the thresholds of multiple changes ≥ 1.5 and
p < 0.01 were set.
2.7. Real-Time Quantitative PCR of Different Expression Genes
The specific description of the operations has been provided by Li et al. [
25]. RNA was first extracted to meet the quality of the qPCR. A FastKing gDNA Dispelling RT SuperMix kit (TIANGEN, Beijing, China) was used for the generation of the cDNA. Finally, the cDNA templates were dissolved fivefold before being used for qPCR.
A FastReal qPCR PreMix (SYBR Green) kit (TIANGEN, Beijing, China) was used for the qPCR by following the instructions. The qPCR was accomplished with the application of the LightCycler
® 96 real-time PCR system(Roche Diagnostics, Basel, Switzerland). The reaction settings were as follows: 95 °C for 120 s was set first, and then 40 cycles of 5 s at 95 °C and 15 s at 60 °C followed. The relative gene transcriptional level was computed using the formula 2
−ΔΔCT. The sequences of the primers can be found in
Table 2.
2.8. Calculations and Statistical Analyses
N
i stands for the initial quantity of sea urchins, while N
f stands for the final quantity; IW and FW denote the initial and final average weight of sea urchin in every cage; GW and DW are the final wet weights of sea urchin gonads and digestive tract of every sea urchin sampled; FPKM (Fragments Per Kilobase of transcript per Million mapped reads) is the number of reads per kilobase of the length of a gene compared to a gene in a million reads [
27].
The SPSS software (version 22.0) was employed to conduct the data analysis. The results were compared between the different dietary groups using one-way analysis of variance (ANOVA). Duncan’s multiple range test was conducted to assess mean differences between the diets if a significant difference was found. Independent t-tests were used to evaluate data between sexes within the identical dietary group. p < 0.05 was regarded as statistically different. The mean ± standard error of the mean (SEM) was adopted for expressing the results.
4. Discussion
Jintasataporn and Yuangsoi [
28] have highlighted that carotenoids, as sensitive organic compounds, are easily damaged by water, oxygen, and high temperatures. Baião et al. [
19] further confirmed the vulnerability of carotenoids to degradation during feed processing. In this study, to minimize the loss of carotene activity, a series of measures have been adopted such as rapid mixing, low-temperature drying, sealing, and freezing storage during feed production. However, the degradation of carotenoids was not avoided based on the fact that the actual values were markedly lower than the targeted values. Therefore, the incorporation method of carotenoids should be improved in the following studies, such as microencapsulating carotenoids, adding higher contents or more potent antioxidants, and using vacuum freeze-drying methods.
In this experiment, the WGR of
S. intermedius demonstrated a rising trend as the β-carotene addition level increased. Similar results have been obtained in other aquatic animals. Compared to the control diet, specific growth rate and weight gain were obviously higher in Nile tilapia
Oreochromis niloticus fed a diet containing 50 mg/kg β-carotene [
29]. White shrimp
Litopenaeus vannamei fed β-carotene at a concentration of 300–400 mg/kg had obviously better growth performance [
30]. A higher specific growth rate and weight gain were found in juvenile pacu fish
Piaractus mesopotamicus fed diets supplemented with β-carotene in comparison with those fed extruded feeds without β-carotene [
31]. Dietary β-carotene can improve the developmental performance of juvenile green sea urchins
Strongylocentrotus droebachiensis [
32] and the larvae of the sea urchin
Paracentrotus lividus [
33]. However, the elevation in growth performance was not observed in adult
P. lividus [
19]. This difference could result from the larger initial body weight (44.0 g) of the sea urchins used by Baião et al. [
19]. In this study, the gonadosomatic index (GSI) and gonadal development were not affected by β-carotene addition. These results were in line with the discoveries of some earlier investigations, which found that dietary β-carotene did not impact the gonad growth and maturation of the sea urchin
Lytechinus variegatus [
34] and
P. lividus [
19,
35].
Gonads with bright yellow and bright orange colors are more acceptable by consumers and thus have higher market values [
5,
18]. The redness (a*) and yellowness (b*) of gonads are caused by the accumulation of carotenoids and metabolism [
2,
11]. Since most animals are unable to produce carotenoids de novo [
36], supplementing with β-carotene in the diet is an effective way to obtain carotenoids and thereby enhance the deposition of pigments in sea urchin gonads [
10]. The addition of β-carotene, including both natural and artificial sources, to feed successfully has turned out to be an effective way to improve sea urchin gonadal color [
10,
13,
37,
38]. In this study, the favorable color frequency coupled with a* and b* values of the sea urchin gonads enhanced with the increasing addition of β-carotene; female sea urchins exhibited higher a* and b* values than males. Studies have demonstrated that when fed identical diets, female
Paracentrotus lividus consistently achieve better coloration than males [
19]. While the compositional profiles of carotenoids in the gonads of female and male Australian sea urchins and red sea urchins are similar, the carotenoid levels in ovaries are markedly higher than those in testes. During gametocyte development, carotenoids are transferred to developing eggs along with other nutrients, leading to a higher carotenoid concentration in mature oocytes compared to testes [
9], a phenomenon not reported in male sea urchins. In addition, according to the unpublished research of our team, this may also be related to the decreased ability of carotenoid absorption, transportation, and metabolism caused by excessive inflammation in the digestive tract and gonads of male sea urchins. Echinenone, as the predominant carotenoid in most sea urchin gonads, is proved to be closely associated with the formation of characteristic color, especially redness [
10]. Sea urchins could possess a certain capacity to synthesize echinenone from the substrate β-carotene [
11,
12]. In the current investigation, the concentrations of β-carotene and echinenone in the digestive tract and gonads of sea urchins were elevated with the increase in β-carotene in diets. Since the experimental feeds did not contain echinenone, the echinenone in the digestive tract and gonads could be synthesized through a series of steps, including the oxidation of β-carotene by
S. intermedius.
In this study, the variation between the levels of β-carotene and β-carotene was more pronounced in the gonads, which was nearly ten times higher than that in the digestive tract. This indicated that gonad could be the main synthetic site of echinenone in sea urchins. It could also be inferred that the echinenone synthesis could be regulated by steroids because the coloration and echinenone contents of gonads are different between male and female individuals. The synthesis of β-carotene to echinenone requires hydroxylation and then ketonization [
9]. However, to our knowledge, there are no reports of β-carotene hydroxylases and ketonases in sea urchins. A large proportion of β-carotene oxidases in other animals belong to the CYP450 family [
39], where an electron donor is needed for catalyzing the reaction, usually NADH or NADPH. These electron donors pass electrons through specific reductases (e.g., CYP450 reductases) to the CYP450 enzymes [
40]. In this process, NADH or NADPH is oxidized and the CYP450 enzyme is reduced so that it can accept the substrate and carry out the oxidation reaction. The findings of this study indicated that the expression of
ND1,
ND2, and
ND4 demonstrated a rising trend with the increase in β-carotene. The
ND1,
ND2, and
ND4 genes encode the core subunits of the mitochondrial respiratory chain complex I. The proteins
ND1,
ND2, and
ND4 are subunits of the enzyme NADH dehydrogenase, which is situated in the inner membrane of the mitochondrion and is one of the five largest complexes in the electron transport chain. Their functions mainly include the initiation of NADH dehydrogenase (ubiquinone) activity, participation in mitochondrial electron transport, NADH to ubiquinone, and mitochondrial respiratory chain complex I assembly [
41]. Thus, increased NADH enzyme activity provides electrons for the transformation of β-carotene to echinenone in the gonad, thereby increasing the amount of echinenone in the gonad.
Furthermore, some echinenone in the gonads was probably transported from the digestive tract, which functions as an important organ for storing lipids and lipid-soluble substances. Carotenoids are mostly lipid-soluble substances and have been reported to cross membranes mainly through diffusion with the help of specific carrier proteins and membrane fluidity [
42]. This is a way for substances to pass through cell membranes that depends on specific carrier proteins in the membrane. The fluidity of the membrane allows the carrier proteins to move freely across the membrane. Cholesterol content is an important factor that is negatively correlated with the fluidity and permeability of cell membranes [
43]. In this experiment, the supplementation of β-carotene increased the expression of
cholesterol 25-hydroxylase (
CH25H) and decreased the expression of the
24-dehydrocholesterol reductase (
DHCR24). The
CH25H could limit the activation of
sterol-regulatory element binding protein-2 (
SREBP2) and thereby reduce cellular cholesterol synthesis [
44]. The
DHCR24 protein is the final step of intracellular cholesterol synthesis by converting desmosterol to cholesterol [
45]. Therefore, the addition of β-carotene could inhibit cholesterol synthesis by increasing the expression of
CH25H and decreasing the expression of
DHCR24, which in turn increases the fluidity and permeability of the cell membranes. This could increase the transport efficiency of β-carotene and echinenone from the digestive tract to the gonads. However, the cholesterol contents of cell membrane were not assayed in the present study. Future studies are needed to find more direct evidence to support our hypothesis.