Short-Term Anesthesia with Clove Oil and Propofol: Physiological Responses in Persian Sturgeon (Acipenser persicus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Acclimation in Laboratory
2.2. Anesthetic Agents
2.3. Experimental Design
2.4. Blood Sampling and Hematological Assays
2.5. Serum Biochemical Analysis
2.6. Statistical Analysis
3. Results
3.1. Induction and Anaesthetic Recovery
3.2. Hematological Indices
3.3. Serum Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vasilyeva, L.M.; Astafyeva, S.S.; Elhetawy, A.I.G.; Sudakova, N.V. History, current status and prospects of sturgeon aquaculture in Russia. Aquac. Res. 2019, 50, 979–993. [Google Scholar] [CrossRef]
- Williot, P.; Arlati, G.; Chebanov, M.; Gulyas, T.; Kasimov, R.; Kirschbaum, F.; Patriche, N.; Pavlovskaya, L.P.; Poliakova, L.; Pourkazemi, M.; et al. Status and management of Eurasian sturgeon: An overview. Int. Rev. Hydrobiol. 2002, 87, 483–506. [Google Scholar] [CrossRef]
- Nazari, S.; Pourkazemi, M.; Khoshkholgh, M. Analysis of the genetic structure of the Persian sturgeon (Acipenser persicus) populations: Comparison of control region sequencing and PCR-RFLP analysis of mitochondrial DNA. Iran. J. Fish. Sci. 2020, 19, 3201–3220. [Google Scholar] [CrossRef]
- Adel, M.; Sadegh, A.B.; Yeganeh, S.; Adel, M.; Movafagh, A.N.; Saoud, I.P. Anesthetic efficacy of clove oil, propofol, 2-phenoxyethanol, and ketamine hydrochloride on Persian Sturgeon, Acipenser persicus, juveniles. J. World Aquac. Soc. 2016, 47, 812–819. [Google Scholar] [CrossRef]
- Chebanov, M.; Rosenthal, H.; Gessner, J.; van Anrooy, R.; Doukakis, P.; Pourkazemi, M.; Williot, P. Sturgeon Hatchery Practices and Management for Release Guidelines; FAO Fisheries and Aquaculture Technical Paper No. 570; FAO: Ankara, Turkey, 2011; pp. 12–17. [Google Scholar]
- Kim, E.J.; Nam, Y.K. Anesthetic protocol for microinjection-related handling of Siberian sturgeon (Acipenser baerii; Acipenseriformes) prolarvae. PLoS ONE, 2018; 13, e0209928. [Google Scholar] [CrossRef]
- Avazeh, A.; Elmdust, A.A.; Mirvaghefi, A.; Soltani, M. Comparing the efficiency of anesthesia with clove, propofol and electric method on the duration and return from anesthesia and its effect on some immune and physiological responses in the beluga (Huso huso). J. Fish 2023, 76, 209–221. [Google Scholar] [CrossRef]
- Akbulut, B.; Çakmak, E.; Aksungur, N.; Çavdar, Y. Effect of exposure duration on time to recovery from Anaesthesia of clove oil in juvenile of Russian sturgeon. Turk. J. Fish. Aquat. Sci. 2011, 11, 463–467. [Google Scholar] [CrossRef]
- Gomułka, P.; Dągowski, J.; Własow, T.; Szczepkowski, M.; Czerniak, E.; Ziomek, E.; Szczerbowski, A.; Łuczyński, M.; Szkudlarek, M. Haematological and biochemical blood profile in Russian sturgeon following propofol and eugenol Anaesthesia. Turk. J. Fish. Aquat. Sci. 2015, 15, 13–17. [Google Scholar] [CrossRef]
- Fenn, C.M.; Glover, D.C.; Small, B.C. Efficacy of AQUI-S 20E as a sedative for handling and cortisol suppression in Pallid Sturgeon. N. Am. J. Fish. Manag. 2013, 33, 1172–1178. [Google Scholar] [CrossRef]
- Arani, M.M.; Shahbaz, S.; Safari, O. A study on the anesthetization of Persian sturgeon (Acipenser persicus) fingerlings in three weight groups; using clove (Eugenia caryophyllata) oil. Int. Res. J. Appl. Basic Sci. 2013, 5, 1454–1460. [Google Scholar]
- Imanpoor, M.R.; Bagheri, T.; Hedayati, S.A.A. The anesthetic effects of clove essence in Persian sturgeon, Acipenser persicus. World J. Fish Mar. Sci. 2010, 2, 29–36. [Google Scholar]
- Gomulka, P.; Własow, T.; Velíšek, J.; Svobodová, Z.; Chmielinska, E. Effects of eugenol and MS-222 anaesthesia on Siberian sturgeon Acipenser baerii Brandt. Acta Vet. Brno 2008, 77, 447–453. [Google Scholar] [CrossRef][Green Version]
- Hamáčková, J.; Kouřil, J.; Kozák, P.; Lepič, P.; Lepičová, A.; Mikodina, J.V.; Sedova, M.A.; Stupka, Z.; Stupka, Z.; Vachta, R. Anesthesie of sterlet (Acipenser ruthenus) with clove oil in different water temperature. In Let Rybářské Specializace Na; Spurný, P., Ed.; MZLU v Brně: Brno, Czech Republic, 2004; Volume 55, pp. 105–113. [Google Scholar]
- Taylor, P.W.; Roberts, S.D. Clove oil: An alternative anaesthetic for aquaculture. N. Am. J. Aquac 1999, 61, 150–155. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and prospects for the application of eugenol—A review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhou, M.; Wei, S. Progress on the antimicrobial activity research of clove oil and eugenol in the food antisepsis field. J. Food Sci. 2018, 83, 1476–1483. [Google Scholar] [CrossRef]
- Da Silva, F.F.M.; Monte, F.J.Q.; de Lemos, T.L.G.; Do Nascimento, P.G.G.; de Medeiros Costa, A.K.; De Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef]
- Anderson, W.G.; McKinley, R.S.; Colavecchia, M. The use of clove oil as an anesthetic for rainbow trout and its effects on swimming performance. N. Am. J. Fish. Manag 1997, 17, 301–307. [Google Scholar] [CrossRef]
- Kaur, K.; Kaushal, S.; Rani, R. Chemical composition, antioxidant and antifungal potential of clove (Syzygium aromaticum) essential oil, its major compound and its derivatives. J. Essent. Oil Bear. Plants 2019, 22, 1195–1217. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove essential oil (Syzygium aromaticum L. Myrtaceae): Extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Guénette, S.; Uhland, F.; Hélie, P.; Beaudry, F.; Vachon, P. Pharmacokinetics of eugenol in rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 266, 262–265. [Google Scholar] [CrossRef]
- Kahn, J.; Mohead, M. A Protocol for Use of Shortnose, Atlantic, Gulf, and Green Sturgeons; US. Department of Commerce: Canada, USA, 2010. [Google Scholar]
- Obirikorang, K.A.; Asante-Tuoh, D.T.; Agbo, N.W.; Amponsah, A.K.; Skov, P.V. Anaesthetic potential of propofol for nile tilapia (Oreochromis niloticus): Effect of anaesthetic concentration and body weight. Sci. Afr. 2020, 10, e00595. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E.; Rizk, A. Comparison propofol and eugenol anesthetics efficacy and effects on general health in Nile Tilapia. Aquaculture 2021, 534, 736251. [Google Scholar] [CrossRef]
- Gomułka, P.; Czerniak, E.; Dągowski, J.; Łuczyński, M.; Szczerbowski, A.; Szkudlarek, M. Effects of propofol and carbon dioxide on acid-base balance in Siberian sturgeon. Pol. J. Vet. Sci. 2015, 18, 267–272. [Google Scholar] [CrossRef][Green Version]
- Gomulka, P.; Fornal, E.; Berecka, B.; Szmagara, A.; Ziomek, E. Pharmacokinetics of propofol in rainbow trout following bath exposure. Pol. J. Vet. Sci. 2015, 18, 147–152. [Google Scholar] [CrossRef]
- Gomułka, P.; Wlasow, T.; Szczepkowski, M.; Misiewicz, L.; Ziomek, E. The effect of propofol anaesthesia on haematological and biochemical blood profile of European whitefish. Turk. J. Fish. Aquat. Sci. 2014, 14, 331–337. [Google Scholar] [CrossRef]
- GholipourKanani, H.; Ahadizadeh, S. Use of propofol as an anesthetic and its efficacy on some haematological values of ornamental fish Carassius auratus. SpringerPlus 2013, 2, 76. [Google Scholar] [CrossRef]
- Miller, S.; Mitchell, M.; Heatley, J.; Avian, T.W.; Lapuz, F.; Lafortune, M.; Smith, J. Clinical and cardiorespiratory effects of propofol in the spotted bamboo shark (Chylloscyllium plagiosum). J. Zoo Wildl. Med. 2005, 36, 673–676. [Google Scholar] [CrossRef]
- Fleming, G.J.; Heard, D.J.; Floyd, R.F.; Riggs, A. Evaluation of propofol and medetomidine–ketamine for short-term immobilization of Gulf of Mexico sturgeon (Acipenser oxyrinchus de soti). J. Zoo Wildl. Med. 2003, 34, 153–158. [Google Scholar] [CrossRef]
- Adel, M.; Riyahi Cholicheh, H.; Gholamhosasoosiseini, A.; Bigham Sadegh, A.; Zorriehzahra, M. A comparison between sedation using propofol and clove oil in Levantine scraper (Capoeta damascina). Iran. J. Fish. Sci. 2020, 19, 2893–2900. [Google Scholar]
- Félix, L.; Correia, R.; Sequeira, R.; Ribeiro, C.; Monteiro, S.; Antunes, L.; Silva, J.; Venâncio, C.; Valentim, A. MS-222 and propofol sedation during and after the simulated transport of Nile tilapia (Oreochromis niloticus). Biology 2021, 10, 1309. [Google Scholar] [CrossRef]
- Weber, R.; Peleteiro, J.; Martín, L.G.; Aldegunde, M. The efficacy of 2-phenoxyethanol, metomidate, clove oil and MS-222 as anaesthetic agents in the Senegalese sole (Solea senegalensis Kaup 1858). Aquaculture 2009, 288, 147–150. [Google Scholar] [CrossRef]
- Ross, L.G.; Ross, B. Anaesthetic and Sedative Techniques for Aquatic Animals; John Wiley & Sons: Chichester, United Kindom, 2009. [Google Scholar]
- Blaxhall, P.; Daisley, K. Routine haematological methods for use with fish blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Rashmeei, M.; Shekarabi, S.P.H.; Mehrgan, M.S.; Paknejad, H. Assessment of dietary chaste tree (Vitex agnuscastus) fruit extract on growth performance, hemato-biochemical parameters, and mRNA levels of growth and appetite-related genes in goldfish (Carassius auratus). Aquac. Fish. 2022, 7, 296–303. [Google Scholar] [CrossRef]
- Shaluei, F.; Hedayati, A.; Jahanbakhshi, A.; Kolangi, H.; Fotovat, M. Effect of subacute exposure to silver nanoparticle on some haematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix). Hum. Exp. Toxicol. 2013, 32, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Marking, L.L.; Meyer, F.P. Are better anesthetics needed in fisheries? Fisheries 1985, 10, 2–5. [Google Scholar] [CrossRef]
- Bell, G.R. A Guide to the Properties, Characteristics and Users of Some General Anaesthetics for Fish; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1964. [Google Scholar]
- Ghanawi, J.; Monzer, S.; Saoud, I.P. Anaesthetic efficacy of clove oil, benzocaine, 2-phenoxyethanol and tricaine methanesulfonate in juvenile marbled spinefoot (Siganus rivulatus). Aquac. Res. 2013, 44, 359–366. [Google Scholar] [CrossRef]
- Matter, F.D.L.; da Silva, E.; Deschamps, G.T.; Santos, F.T.R.; Terra, J.P.; Martins, C.E.N.; Weber, R.A. Exploring the anesthetic potential of propofol in Ictalurus punctatus (Rafinesque, 1818). Aquac. Int. 2024, 32, 6887–6901. [Google Scholar] [CrossRef]
- Leonardi, F.; Costa, G.L.; Interlandi, C.D.; Rosa, J.; Ghidelli, A.; Musicò, M. Immersion anaesthesia in goldfish (Carassius auratus) with three concentrations of alfaxalone. Veter. Anaesth. Analg. 2019, 46, 79–83. [Google Scholar] [CrossRef]
- Ahmed, I.; Reshi, Q.M.; Fazio, F. The influence of the endogenous and exogenous factors on haematological parameters in different fish species: A review. Aquac. Int. 2020, 28, 869–899. [Google Scholar] [CrossRef]
- Banaee, M.; Beitsayah, A.; Zeidi, A.; Haghi, B.N.; Piccione, G.; Faggio, C.; Multisanti, C.R.; Impellitteri, F. Toxicity of cigarette butts (CBs) leachate on Nile tilapia (Oreochromis niloticus): Blood biochemical parameters, oxidative stress biomarkers, and metabolic profile. Ecotoxicol. Environ. Saf. 2024, 279, 116514. [Google Scholar] [CrossRef]
- Multisanti, C.R.; Riolo, K.; Impellitteri, F.; Zicarelli, G.; Vazzana, I.; Cafeo, G.; Faggio, C.; Giannetto, A. Bergamot (Citrus bergamia) as a potential anti-stress agent: Counteracting cellular and physiological changes by sodium lauryl sulphate in Mytilus galloprovincialis. Environ. Pollut. 2025, 371, 125939. [Google Scholar] [CrossRef]
- Impellitteri, F.; Multisanti, C.R.; Riolo, K.; Zicarelli, G.; Porretti, M.; Cafeo, G.; Russo, M.; Dugo, P.; Di Bella, G.; Piccione, G.; et al. Bergamot (Citrus bergamia): A Potential New Nutraceutical Against Cellular and Physiological Alterations Induced by Emerging Contaminants in Sentinel Organisms. Antioxidants 2025, 14, 539. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.H.; Ghosh, S.; Adhurjya, D.; Chatterjee, P.; Samajdar, I.; Mukherjee, D.; Dhara, K.; Saha, N.C.; Piccione, G.; Multisanti, C.R.; et al. Exploring the impact of zinc oxide nanoparticles on fish and fish-food organisms: A review. Aquac. Rep. 2024, 36, 102038. [Google Scholar] [CrossRef]
- Mousaviyon, Z.; Pourkhabbaz, H.R.; Banaee, M.; Khodadoust, S.; Pourkhabbaz, A.R.; Trivedi, A.; Faggio, C.; Multisanti, C.R. Toxicity of Crude Oil Wastewater Treated with Nano-ZnO as a Photocatalyst on Labeo rohita: A Biochemical and Physiological Investigation. J. Xenobiot. 2025, 15, 25. [Google Scholar] [CrossRef]
- Sadeghi, S.; Mousavi-Sabet, H.; Hedayati, A.; Zargari, A.; Multisanti, C.R.; Faggio, C. Copper-oxide nanoparticles effects on goldfish (Carassius auratus): Lethal toxicity, haematological, and biochemical effects. Vet. Res. Commun. 2024, 48, 1611–1620. [Google Scholar] [CrossRef]
- Summerfelt, R.C.; Smith, L.S. Anesthesia, surgery, and related techniques. In Methods for Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 213–272. [Google Scholar]
- Trapani, G.; Altomare, C.; Sanna, E.; Biggio, G.; Liso, G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr. Med. Chem. 2000, 7, 249–271. [Google Scholar] [CrossRef]
- Esmaeili, M. Blood performance: A new formula for fish growth and health. Biology 2021, 10, 1236. [Google Scholar] [CrossRef]
- Regan, M.D.; Brauner, C.J. The evolution of Root effect hemoglobins in the absence of intracellular pH protection of the red blood cell: Insights from primitive fishes. J. Comp. Physiol. B 2010, 180, 695–706. [Google Scholar] [CrossRef]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef]
- Ainsworth, A.; Dexiang, C.; Waterstrat, P. Changes in peripheral blood leukocyte percentages and function of neutrophils in stressed channel catfish. J. Aquat. Anim. Health 1991, 3, 41–47. [Google Scholar] [CrossRef]
- Davis, A.; Maney, D.; Maerz, J. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Velíšek, J.; Svobodova, Z.; Piačková, V. Effects of clove oil anaesthesia on rainbow trout (Oncorhynchus mykiss). Acta Vet. Brno 2005, 74, 139–146. [Google Scholar] [CrossRef]
- Velisek, J.; Svobodova, Z.; Piackova, V.; Groch, L.; Nepejchalova, L. Effects of clove oil anaesthesia on common carp (Cyprinus carpio L.). Vet. Med. 2005, 50, 269–275. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Mechanisms of cortisol action in fish hepatocytes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 199, 136–145. [Google Scholar] [CrossRef]
- Laiz-Carrión, R.; Sangiao-Alvarellos, S.; Guzmán, J.M.; Martín del Río, M.P.; Míguez, J.M.; Soengas, J.L.; Mancera, J.M. Energy metabolism in fish tissues related to osmoregulation and cortisol action. Fish Physiol. Biochem. 2002, 27, 179–188. [Google Scholar] [CrossRef]
- Iversen, M.; Finstad, B.; McKinley, R.S.; Eliassen, R.A. The efficacy of metomidate, clove oil, Aqui-S™ and Benzoak® as anaesthetics in Atlantic salmon (Salmo salar L.) smolts, and their potential stress-reducing capacity. Aquaculture 2003, 221, 549–566. [Google Scholar] [CrossRef]
- Ferreira, J.M.; Jorge, S.; Félix, L.; Morello, G.M.; Olsson, I.A.S.; Valentim, A.M. Behavioural aversion and cortisol level assessment when adult zebrafish are exposed to different anaesthetics. Biology 2022, 11, 1433. [Google Scholar] [CrossRef]
- Taghavizadeh, M.; Shekarabi, S.P.H.; Mehrgan, M.S.; Islami, H.R. Efficacy of dietary lysophospholipids (Lipidol™) on growth performance, serum immuno-biochemical parameters, and the expression of immune and antioxidant-related genes in rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 525, 735315. [Google Scholar] [CrossRef]
- Parodi, T.V.; Cunha, M.A.; Becker, A.G.; Zeppenfeld, C.C.; Martins, D.I.; Koakoski, G.; Barcellos, L.G.; Heinzmann, B.M. Anesthetic activity of the essential oil of Aloysia triphylla and effectiveness in reducing stress during transport of albino and gray strains of silver catfish, Rhamdia quelen. Fish Physiol. Biochem. 2014, 40, 323–334. [Google Scholar] [CrossRef]
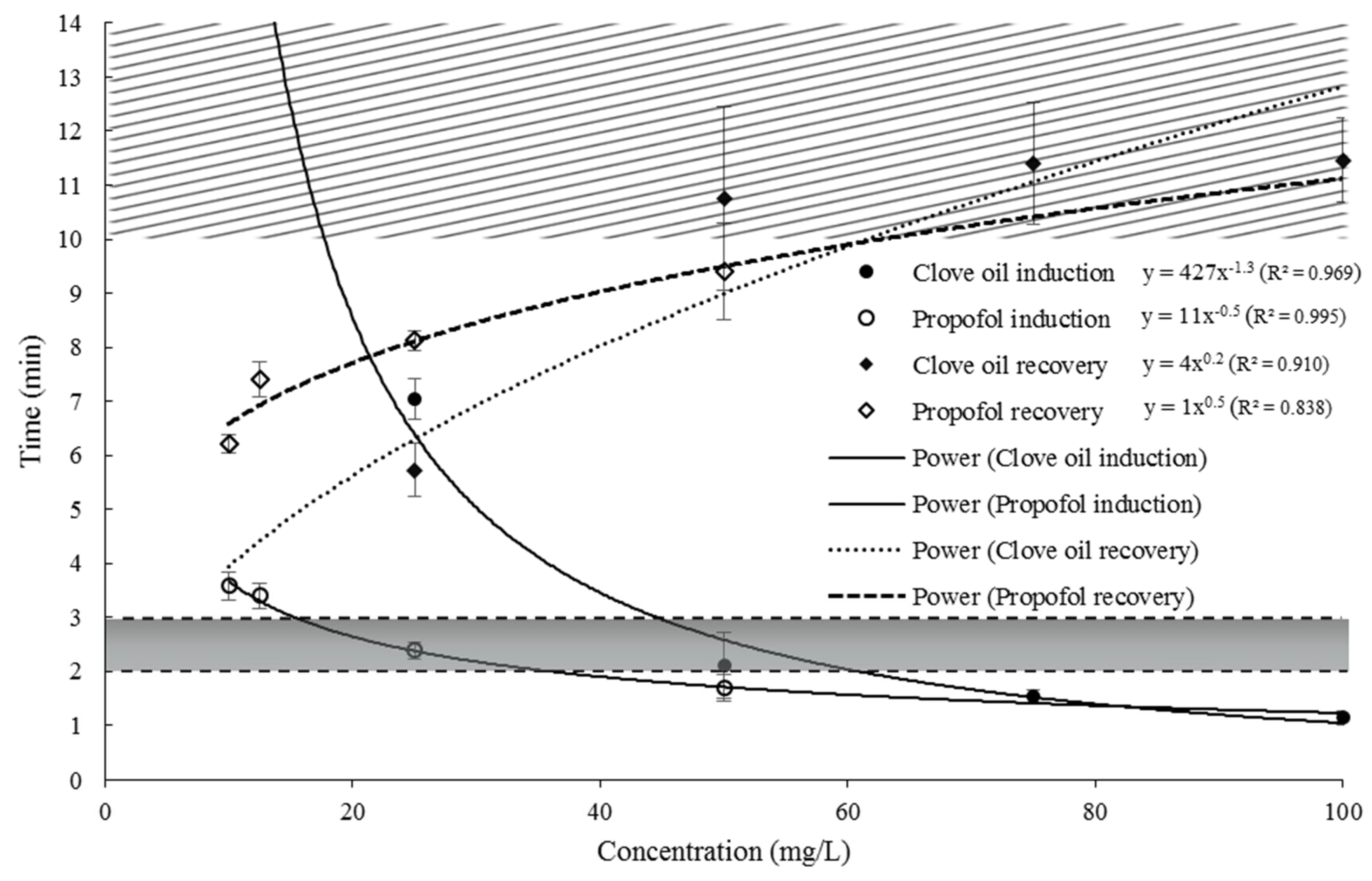
| Concentration (mg/L) | Induction Time (min) | Recovery Time (min) | ||
|---|---|---|---|---|
| Clove Oil | Propofol | Clove Oil | Propofol | |
| 10 | — | 3.56 ± 0.21 | — | 6.23 ± 0.30 |
| 12.5 | — | 3.39 ± 0.18 | — | 7.45 ± 0.42 |
| 25 | 7.08 ± 0.44 | 2.38 ± 0.16 | 5.75 ± 0.27 | 8.10 ± 0.38 |
| 50 | 2.15 ± 0.15 | 1.71 ± 0.13 | 10.74 ± 0.40 | 9.37 ± 0.33 |
| 75 | 1.61 ± 0.12 | — | 11.38 ± 0.48 | — |
| 100 | 1.11 ± 0.09 | — | 11.41 ± 0.52 | — |
| Indicator | Clove Oil Concentration (mg L−1) | ||||
|---|---|---|---|---|---|
| 0 (Control) | 25 | 50 | 75 | 100 | |
| RBC (×106 mL−1) | 0.86 ± 0.08 a | 0.87 ± 0.09 a | 0.84 ± 0.06 a | 0.85 ± 0.07 a | 0.83 ± 0.10 a |
| Hb (g L−1) | 6.72 ± 0.44 a | 6.60 ± 0.32 a | 6.48 ± 0.40 a | 6.25 ± 0.38 ab | 5.94 ± 0.25 b |
| HCT (%) | 21.2 ± 0.7 a | 21.1 ± 0.4 a | 21.4 ± 0.5 a | 20.3 ± 0.4 a | 20.5 ± 0.8 a |
| WBC (×103 mL−1) | 23.16 ± 0.26 a | 22.84 ± 0.22 a | 22.26 ± 0.18 b | 22.34 ± 0.15 b | 22.20 ± 0.16 b |
| Lymphocytes (%) | 83.8 ± 1.1 a | 83.4 ± 1.0 ab | 82.9 ± 0.8 ab | 81.5 ± 1.2 b | 81.8 ± 0.9 b |
| Neutrophils (%) | 9.1 ± 0.6 b | 9.5 ± 0.7 ab | 11.0 ± 0.9 a | 11.6 ± 0.9 a | 11.2 ± 0.9 a |
| Monocytes (%) | 4.2 ± 0.5 a | 4.0 ± 0.6 a | 4.3 ± 0.4 a | 4.1 ± 0.5 a | 3.9 ± 0.6 a |
| Eosinophil (%) | 1.9 ± 0.2 a | 1.7 ± 0.3 a | 1.8 ± 0.1 a | 2.0 ± 0.4 a | 2.1 ± 0.3 a |
| Indicator | Propofol Concentration (mg L−1) | ||||
|---|---|---|---|---|---|
| Control | 10.0 | 12.5 | 25.0 | 50.0 | |
| RBC (×106 mL−1) | 0.85 ± 0.07 a | 0.87 ± 0.07 a | 0.90 ± 0.09 a | 0.86 ± 0.06 a | 0.80 ± 0.08 a |
| Hb (g L−1) | 6.70 ± 0.45 a | 6.87 ± 0.62 a | 7.23 ± 0.40 a | 7.02 ± 0.48 a | 6.64 ± 0.65 a |
| HCT (%) | 21.2 ± 0.2 a | 21.3 ± 0.3 a | 22.0 ± 0.6 a | 20.7 ± 0.8 a | 20.9 ± 0.7 a |
| WBC (×103 mL−1) | 23.2 ± 0.3 a | 23.3 ± 0.4 a | 23.1 ± 0.2 a | 22.9 ± 0.3 a | 22.0 ± 0.2 b |
| Lymphocytes (%) | 83.5 ± 1.1 a | 83.2 ± 1.0 a | 82.8 ± 1.2 a | 82.6 ± 0.9 a | 80.6 ± 0.8 b |
| Neutrophils (%) | 9.4 ± 0.7 b | 9.7 ± 0.6 b | 10.2 ± 0.8 b | 10.0 ± 0.7 b | 11.5 ± 0.9 a |
| Monocytes (%) | 3.9 ± 0.4 a | 3.8 ± 0.5 a | 4.0 ± 0.7 a | 4.2 ± 0.6 a | 4.0 ± 0.3 a |
| Eosinophil (%) | 2.0 ± 0.3 a | 2.1 ± 0.4 a | 2.2 ± 0.5 a | 1.9 ± 0.3 a | 2.1 ± 0.6 a |
| Indicator | Clove Oil Concentration (mg L−1) | ||||
|---|---|---|---|---|---|
| Control | 25 | 50 | 75 | 100 | |
| Glucose (mmol L−1) | 2.49 ± 0.15 b | 2.79 ± 0.36 b | 3.58 ± 0.25 a | 3.31 ± 0.22 a | 3.45 ± 0.23 a |
| TP (g L−1) | 25.8 ± 2.4 a | 25.2 ± 1.9 a | 23.4 ± 1.8 a | 23.8 ± 2.0 a | 20.4 ± 1.3 b |
| TGs (mmol L−1) | 66.0 ± 9.8 a | 69.2 ± 10.6 a | 65.4 ± 9.1 a | 70.1 ± 10.4 a | 68.3 ± 10.6 a |
| ALT (U L−1) | 11.58 ± 1.2 a | 11.74 ± 1.0 a | 11.62 ± 1.2 a | 11.43 ± 1.5 a | 11.87 ± 0.9 a |
| AST (U L−1) | 218.3 ± 26.2 b | 274.5 ± 30.6 b | 320.6 ± 34.9 a | 282.1 ± 32.3 ab | 308.4 ± 35.0 a |
| LDH (U L−1) | 1342 ± 119 a | 1376 ± 125 a | 1369 ± 120 a | 1394 ± 132 a | 1329 ± 111 a |
| Cortisol (ng mL−1) | 18.32 ± 1.8 c | 27.42 ± 2.9 b | 25.82 ± 2.3 b | 29.87 ± 4.7 b | 38.92 ± 5.1 a |
| Indicator | Propofol Concentration (mg L−1) | ||||
|---|---|---|---|---|---|
| Control | 10.0 | 12.5 | 25.0 | 50.0 | |
| Glucose (mmol L−1) | 2.49 ± 0.19 b | 2.67 ± 0.25 ab | 2.79 ± 0.21 ab | 3.40 ± 0.44 a | 2.92 ± 0.33 ab |
| TP (g L−1) | 26.0 ± 1.4 a | 26.4 ± 1.6 a | 25.7 ± 1.3 a | 26.8 ± 1.9 a | 25.6 ± 1.2 a |
| TGs (mmol L−1) | 66.0 ± 10.4 a | 67.4 ± 12.3 a | 70.9 ± 13.6 a | 69.2 ± 13.4 a | 70.3 ± 14.5 a |
| ALT (U L−1) | 11.72 ± 1.0 a | 11.86 ± 1.4 a | 11.52 ± 1.7 a | 11.64 ± 1.5 a | 12.04 ± 1.6 a |
| AST (U L−1) | 219.7 ± 20.8 a | 228.5 ± 24.0 a | 235.1 ± 30.2 a | 242.3 ± 27.6 a | 240.2 ± 28.5 a |
| LDH (U L−1) | 1376 ± 128.2 a | 1392 ± 135.4 a | 1435 ± 140.0 a | 1412 ± 135.8 a | 1490 ± 156.6 a |
| Cortisol (ng mL−1) | 17.92 ± 1.6 c | 22.18 ± 2.3 c | 20.76 ± 2.7 c | 28.14 ± 3.9 b | 36.48 ± 3.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adel, M.; Shekarabi, S.P.H.; Gomułka, P.; Amiri, A.B.; Multisanti, C.R.; Faggio, C. Short-Term Anesthesia with Clove Oil and Propofol: Physiological Responses in Persian Sturgeon (Acipenser persicus). Fishes 2025, 10, 286. https://doi.org/10.3390/fishes10060286
Adel M, Shekarabi SPH, Gomułka P, Amiri AB, Multisanti CR, Faggio C. Short-Term Anesthesia with Clove Oil and Propofol: Physiological Responses in Persian Sturgeon (Acipenser persicus). Fishes. 2025; 10(6):286. https://doi.org/10.3390/fishes10060286
Chicago/Turabian StyleAdel, Milad, Seyed Pezhman Hosseini Shekarabi, Piotr Gomułka, Alireza Babaalian Amiri, Cristiana Roberta Multisanti, and Caterina Faggio. 2025. "Short-Term Anesthesia with Clove Oil and Propofol: Physiological Responses in Persian Sturgeon (Acipenser persicus)" Fishes 10, no. 6: 286. https://doi.org/10.3390/fishes10060286
APA StyleAdel, M., Shekarabi, S. P. H., Gomułka, P., Amiri, A. B., Multisanti, C. R., & Faggio, C. (2025). Short-Term Anesthesia with Clove Oil and Propofol: Physiological Responses in Persian Sturgeon (Acipenser persicus). Fishes, 10(6), 286. https://doi.org/10.3390/fishes10060286








