1. Introduction
Fish is a key source of animal protein and an essential part of a balanced human diet. It has been widely accepted as a nutritious source of protein which can help support a healthy body [
1,
2]. Amino acids in fish proteins are critical to determining protein nutritional quality. They serve as precursors for the synthesis of important biological substances such as peptide hormones and neurotransmitters. Additionally, they are vital for cell signaling, nutrient transport, metabolic disease prevention and treatment, cellular metabolism, and innate and cell-mediated immune responses [
3,
4,
5]. Fish is also recognized as an important source of essential polyunsaturated fatty acids [
6,
7]. DHA and EPA are crucial indicators for evaluating nutritional quality, given their substantial role in preventing diverse diseases including diabetes, chronic cardiovascular disease, cancer, and age-related degenerative diseases. A high Σn-3/Σn-6 polyunsaturated fatty acids (∑n-3/∑n-6 PUFA) ratio is crucial for human health, as n-3 PUFAs are known for their anti-inflammatory effects, while n-6 PUFAs promote inflammation [
8,
9,
10,
11,
12]. Moreover, a high ∑n-3/∑n-6 PUFAs ratio helps prevent the onset of cardiogenic diseases, and the recommended ratio in the diet is higher than 0.25. The demand for high-quality seafood is surging with the rapid socioeconomic development.
Aquaculture systems have emerged as a critical component of sustainable food production, serving as a sustainable mechanism to supply bioavailable animal-derived proteins that are fundamental for optimal ontogenetic development, neurocognitive function, and cardiovascular health maintenance in human populations. The
C. myriaster is an economically important eel species and an important marine resource for the fish processing industry in East Asian countries, which is mainly distributed in the Bohai, Yellow, and East China Seas, the coastal waters of the Korean Peninsula, and the coast of Japan from southern Hokkaido to northern Okinawa [
13,
14]. In recent years,
C. myriaster populations have declined drastically due to intensive fishing, so it has become important to protect this species [
15,
16]. As a result, many countries are regulating
C. myriaster fishing in order to manage this resource [
17,
18]. Despite the implementation of robust conservation strategies aimed at enhancing
C. myriaster population recovery, persistent demographic monitoring indicates that additional adaptive management interventions are required to effectively mitigate ongoing population declines. Presently, the practice of captive breeding
C. myriaster is emerging along the Yellow Sea coast of China [
19,
20,
21]. Moreover, high-quality genome assembly provides a robust foundation for developing molecular markers and advancing conservation and aquaculture efforts for
C. myriaster [
22]. The experimental aquaculture techniques for
C. myriaster include pond culture, industrialized open-flowing water culture, and industrialized recirculating aquaculture [
13]. In recent years, a number of studies have been conducted to investigate the relationship between morphological traits, proximate composition, amino acid and fatty acid contents, and antioxidant enzyme activity [
23,
24,
25,
26,
27]. However, comparisons of the differences between different regions of
C. myriaster from different regions have not been reported.
The growth performance of eels is species-specific and related to the growing environment [
28]. In recent years, there has been a lot of attention on
C. myriaster due to its significant nutritional value, including its rich protein content and substantial quantities of unsaturated fatty acids [
29]. It has been shown that the farmed
C. myriaster has higher contents of lipids, total amino acids, essential amino acids (EAAs), DHA, EPA, n-3 PUFAs, and total polyunsaturated fatty acids (total PUFAs) compared to wild fish [
3]. However, comparisons of artificially cultured
C. myriaster among the different regions have not been reported. Since all artificially cultured
C. myriaster require wild fry captured from offshore waters, artificially cultured
C. myriaster from different regions may also exhibit differentiation due to genetic factors. Therefore, a comparative evaluation can help us to better understand the overall quality and characteristics of farmed
C. myriaster from different regions. In view of this, in this study,
C. myriaster produced under the same culture environments were selected to determine the differences in morphology and quality of farmed
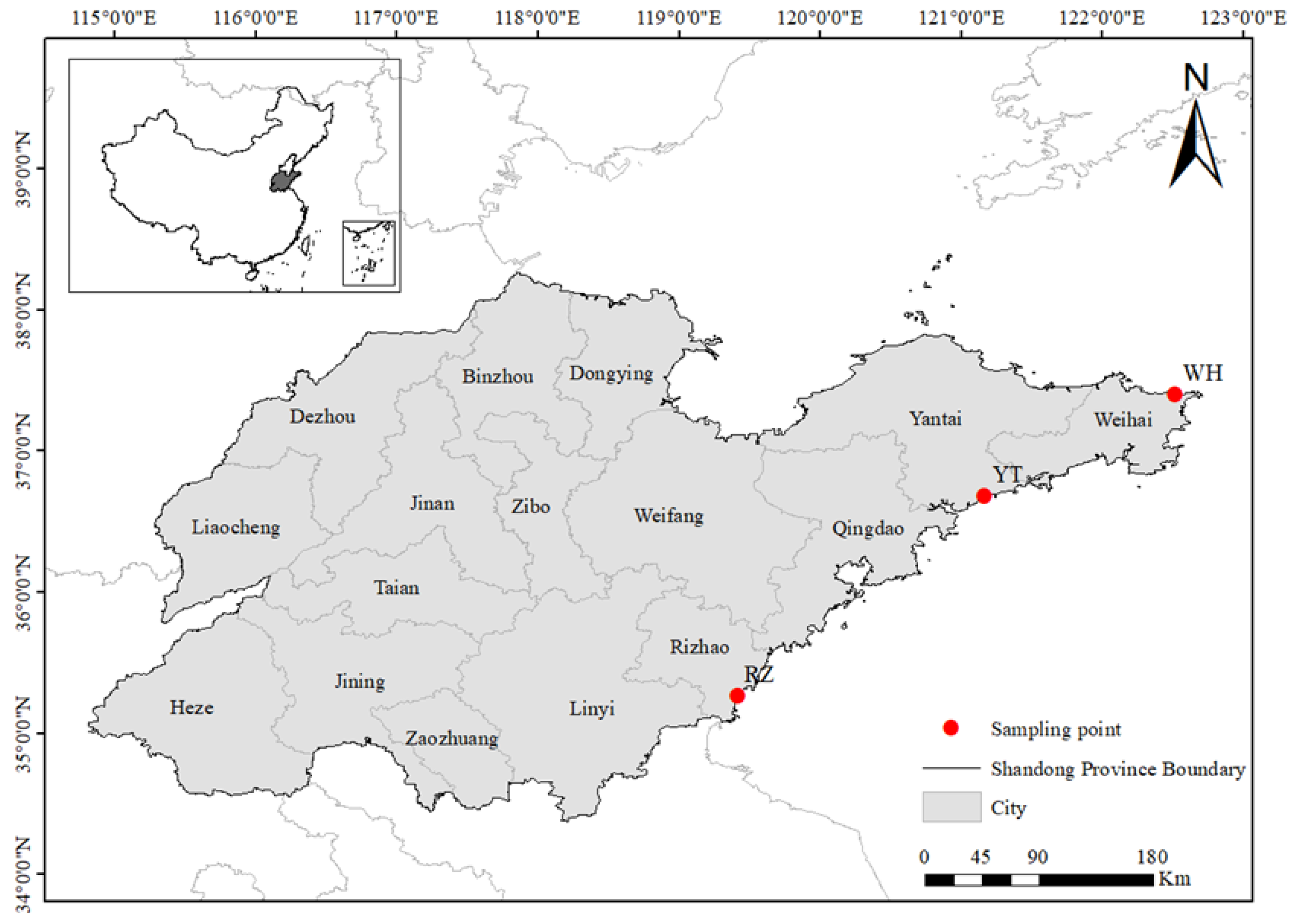
C. myriaster from different regions in China (namely Yantai, Shandong province (YT, 121.758 E, 36.411 N), Rizhao, Shandong province (RZ, 119.272 E, 35.253 N), and Weihai, Shandong province (WH, 122.754 E, 37.302 N)). By comparing their morphological traits, hepatosomatic index, gonadosomatic index, condition factor, proximate composition, amino acid composition, fatty acid composition, and antioxidant capacity, we can obtain important information for further investigating the genetic variations in populations of
C. myriaster in different regions.
4. Discussion
Length and weight are two critical morphometric traits strongly affected by species, ontogenetic changes, and environmental conditions [
35]. The relationship between weight and length was used to develop CF [
36]. In fish, CF serves as an indicator of the physiological and health status of fish and can be used to compare their relative health, with larger values indicating increased fat mass [
37,
38]. This study found that there were no significant differences in the body weights of samples from YT, RZ, and WH. The body length of WH was significantly higher than that of YT and RZ, and the body length of RZ was significantly higher than that of YT. Comparison of CF revealed that it was significantly higher in YT than in WH and RZ. In addition, the crude fat content of YT was significantly higher than that of RZ and WH. In a related study on European eel
Anguilla anguilla, it was noted that higher CF and fat contents indicated a better fish condition [
39]. The liver is an important organ for storing energy and is often the first site for lipid storage in many fish species. Therefore, HSI is used as an indirect measure of body condition to assess energy reserves and metabolic activity [
40]. A high-fat diet (HFD) has been widely used in modern aquaculture because of its low cost, reduced nitrogen emissions, and protein-saving effects [
41]. It has been observed that the intake of an HFD in the rice field eel
Monopterus albus caused the deposition of fat in the liver, which increased the HSI [
42]. In this study, all the collected
C. myriaster samples were fed with an HFD; however, the HSIs of RZ and WH were higher than that of YT. This may be related to the level of lipid metabolism in the liver. This suggests that the
C. myriaster population of YT might have been in a better health condition and made good use of diet resources in terms of energy acquisition. In summary, the
C. myriaster population of YT was in a better condition and able to access energy resources in their diet more effectively, with less lipid deposition and higher metabolic levels in the liver. The AMPK/PPAR signaling pathway is a major regulatory pathway of lipid catabolism [
43], and the expression characteristics of key genes affecting this pathway in
C. myriaster from different regions still need to be further investigated. Future work is expected to explore the differences caused by genetic variation in
C. myriaster among geographically distinct populations.
Fish muscles are a major source of nutrition in the human diet and contain fats, proteins, and minerals that are of great value to human health [
44]. The proximate composition of fish muscles can provide information about its physiological condition, energetic adaptation, habits, nutritional value, and commercial applications [
45]. In this study, we determined the moisture, crude protein, crude lipid, crude ash, and total sugar contents in the muscle of
C. myriaster. There was no significant difference in the moisture contents of RZ and WH (
p > 0.05), but they were significantly higher than that in YT (
p < 0.05). On the contrary, YT had a significantly higher lipid content than RZ and WH (
p < 0.05). In fish, the lipid content is usually inversely proportional to the moisture content [
46]. The results of the present study show that there may be a negative correlation between lipid and moisture content in
C. myriaster. There is a higher intake of protein and lipid from fish muscle, as these macronutrients are important sources of energy and play a vital role in bodily functions [
47]. The results of this study showed that the crude protein contents of YT and RZ were significantly higher than that of WH (
p < 0.05). Moreover, a higher crude lipid content was observed in the muscles of
C. myriaster from YT compared to those from RZ and WH. Therefore,
C. myriaster from YT may have a superior nutritional quality and value. Interestingly, the crude ash content of YT was significantly lower than that of RZ and WH (
p < 0.05); this suggests that
C. myriaster from RZ and WH may contain a higher mineral content compared to YT. The reasons for these differences can be explored in depth by testing the elemental content of
C. myriaster in each region, with the aim of analyzing the intrinsic mechanisms between proximate composition.
High-quality proteins have a high utilization rate and contain EAAs in quantities that correspond to human requirements [
48]. This study indicated that the 12 kinds of EAAs were found in the muscle samples from the three regions, with abundant lysine acid, leucine acid, arginine acid, and valine acid, which is consistent with the findings reported by Zhang et al. [
3]. Met has a growth-promoting effect, which can improve protein synthesis and enhance antioxidant capacity [
49]. In this study, the Met content of RZ was significantly higher than that of YT and WH. However, there were no significant differences in T-AOC, SOD, and CAT of YT, RZ, and WH in this study. In addition, according to the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) criteria, good-quality proteins not only contain the complete range of essential amino acids but also have an appropriate essential amino acid-to-total amino acid ratio [
50]. The ratios of essential amino acids to total amino acids for YT, RZ, and WH were 43.9%, 45.4%, and 43.9%, which were in accordance with the FAO/WHO ideal model standard (40%). The ratios of essential amino acids to non-essential amino acids were 96.2%, 102.3%, and 96.0%, respectively, which far exceed FAO/WHO standards (60%). This demonstrates that the proteins in
C. myriaster muscle in YT, RZ, and WH exhibit a well-balanced essential amino acid composition and were all of a high quality. Lys is the most abundant free amino acid in
C. myriaster from YT, RZ, and WH; it crucially regulates metabolism and growth in fish [
51]. The proteins in
C. myriaster protein from YT, RZ, and WH heavily feature Glu, which plays important physiological roles in protein synthesis and muscle flesh quality [
52]. Glu is the most abundant free amino acid in artificially cultured yellow croaker
Larimichthys crocea, as well as in African catfish
Clarias gariepinus [
53,
54], while Lys is the most abundant free amino acid in Japanese eel
Anguilla japonica [
55]. The amino acid content of crayfish
Procambarus clarkii from different regions has been determined, and it was found that the most common component was Glu [
29]. Comparison of the amino acid content of male Chinese mitten crab
Eriocheir sinensis cultured in different regions showed that there were fewer individual differences between regions [
30]. It can be concluded that the differences in free amino acid content in artificially farmed fish from different regions were minor, indicating that geographical differences are not an important factor in determining the amino acid content in fish. On the other hand, the differences in free amino acids between different fish were substantial, which may be related to genetic factors.
The fatty acid composition of fish muscle is an important indicator for assessing nutritional value [
56]. The Σn-3 and Σn-6 can serve as important indicators for assessing the nutritional quality because n-3 polyunsaturated fatty acids have an anti-inflammatory effect, whereas n-6 polyunsaturated fatty acids are pro-inflammatory [
8,
9,
10,
11,
12]. In this study, the Σn-3 of
C. myriaster from YT was significantly higher than that from WH and RZ, and the Σn-6 of
C. myriaster from RZ was significantly higher than that from YT and WH. It has been shown that the amounts and types of fatty acids in tissues were often affected by the maturity period, different seasons, geographical locations, and size and age of the fish [
57]. The ΣMUFA and ΣPUFA of
C. myriaster were significantly higher in YT than in RZ and WH, and this difference may be related to geographical location. DHA and EPA are dominant unsaturated fatty acids in fish oil which have potential therapeutic benefits in the treatment of a variety of human diseases including autoimmune disorders, inflammatory diseases, cardiovascular disease, and diabetes mellitus [
58]. In addition, DHA and EPA are essential for fish reproduction and gonadal development [
59]. In this study, DHA and EPA in
C. myriaster from YT were significantly higher than in samples from RZ and WH. As a result, the
C. myriaster of YT may be healthier and have a higher nutritional value.
5. Conclusions
In this study, the morphological traits, body composition, amino acid composition, fatty acid composition, and antioxidant enzyme activity of artificially cultured C. myriaster from YT, RZ, and WH were comparatively analyzed. Significant differences were found in total length, body length, vertical eye diameter, the vertical dimension from rostral side to orbit trailing edge, and fatty acid C12:0, C16:1, C18:2n6c, C20:1, C20:2 contents between fish from among the three regions. Preliminary evidence suggests that the C. myriaster population of YT is able to access energy resources in their diet more effectively and may have superior nutritional quality and value. This suggests that differences in geographic location may affect the growth and development level, as well as the nutritional value, of artificially cultured C. myriaster. Further studies can be carried out on the ultrastructure of muscle fibers, muscle mineral elements, and taste substances. However, C. myriaster from YT, RZ, and WH differed significantly only in Met content, suggesting that geographical differences are not a critical factor in determining the amino acid content, but it can may be determined genetically. In the future, genetic factors will be explored by combining data from genomic resources such as whole-genome sequencing (WGS) and 10 × Genomics analysis from the C. myriaster populations of various regions. Our study provides a foundation for the subsequent identification of the specific factors affecting C. myriaster in different geographic locations and provides theoretical ideas for how geographic location variations cause alterations in the genetic factors of farmed C. myriaster.