Effect of Berberine Hydrochloride on Disposition Characteristics of Ciprofloxacin Hydrochloride and Its Mechanism in Yellow Catfish (Pelteobagrus fulvidraco) Following Combined Oral Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Administration and Sample Collection of Yellow Catfish
2.3. Equipment
2.3.1. Chromatographic Conditions
2.3.2. Mass Spectrometry Conditions
2.4. Sample Pretreatment
2.5. Method Validation
2.6. Detection of Gene Expression in Yellow Catfish
2.7. Data Processing and Analysis
3. Results
3.1. Validation of HPLC-MS/MS Method
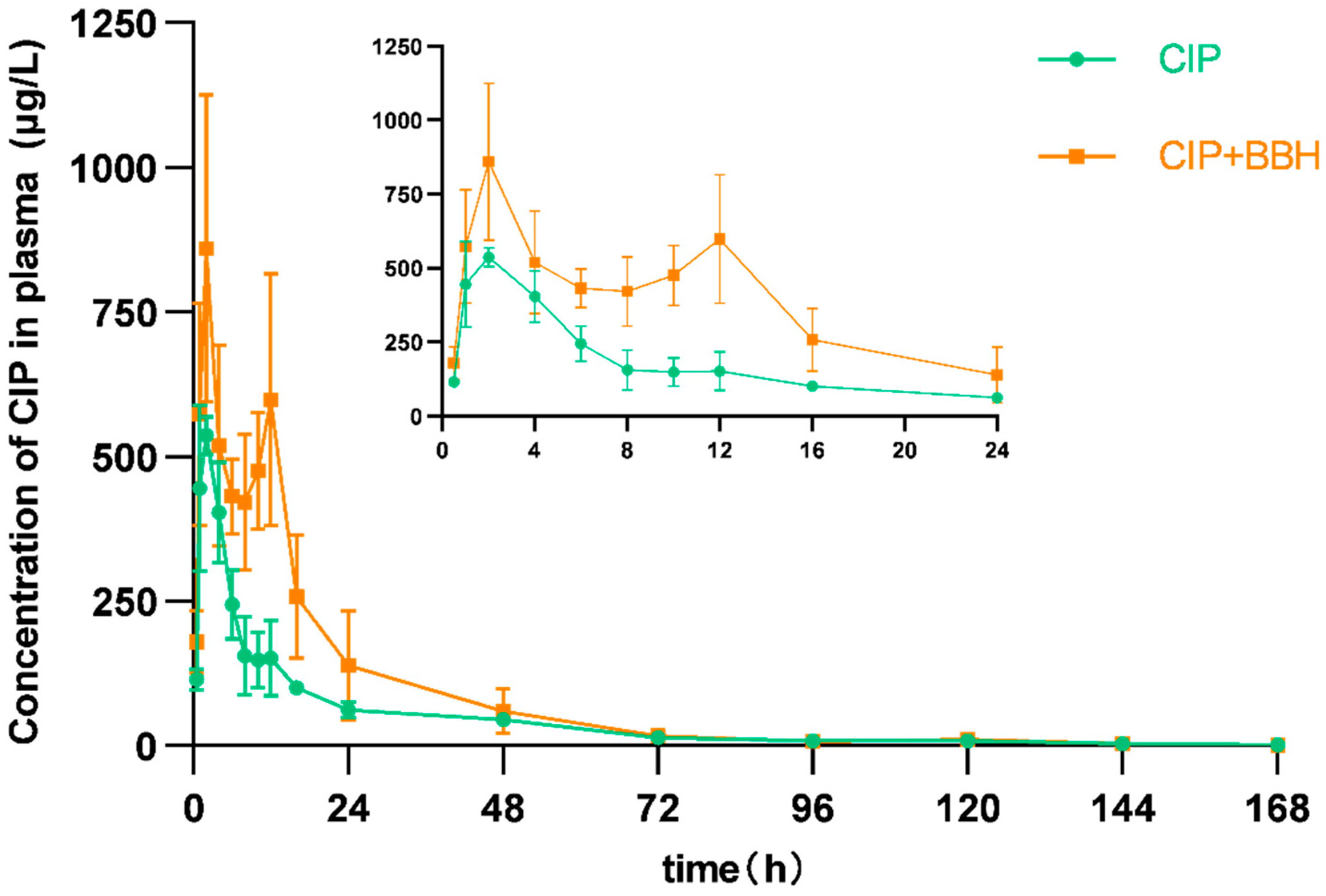
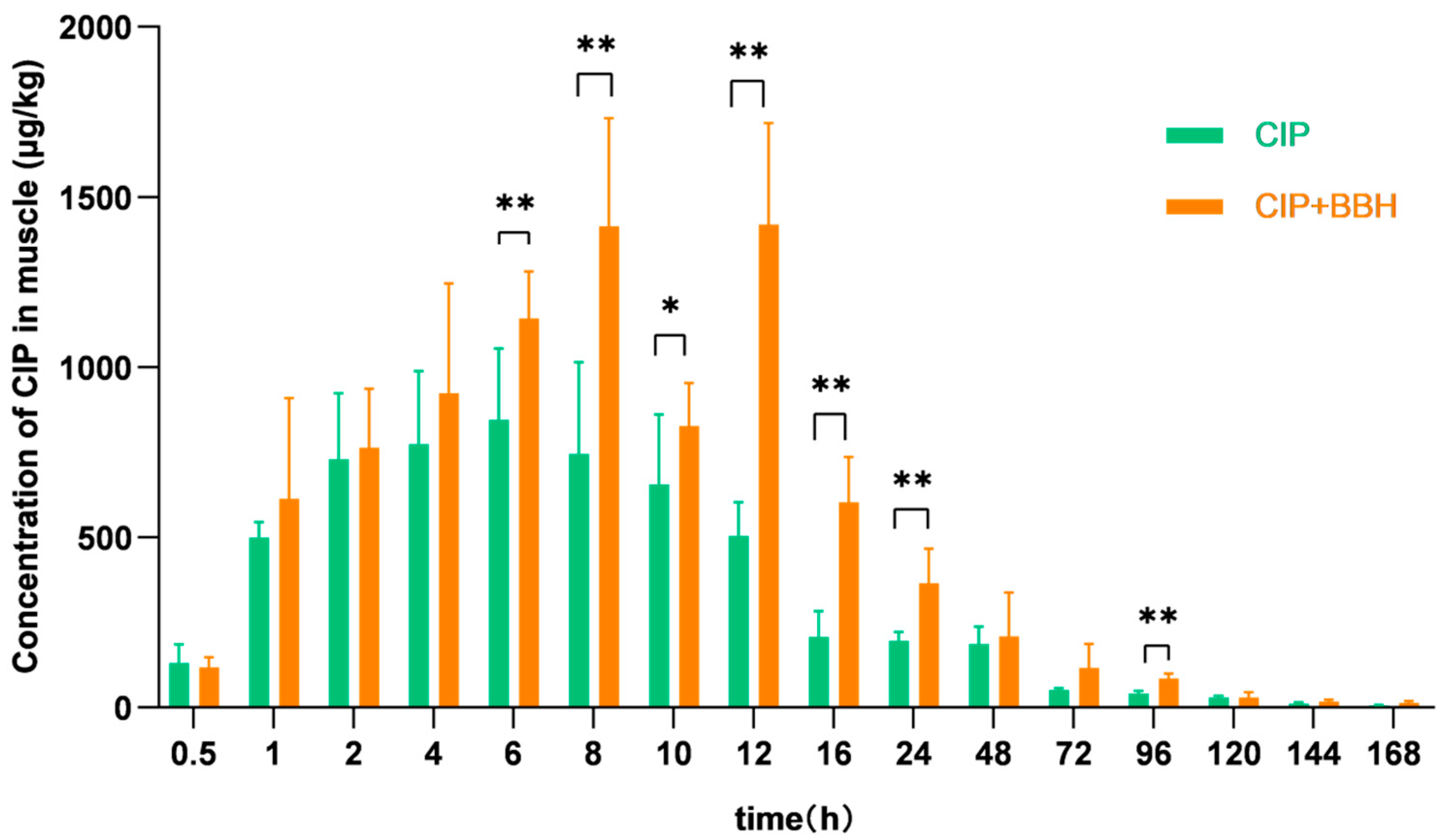
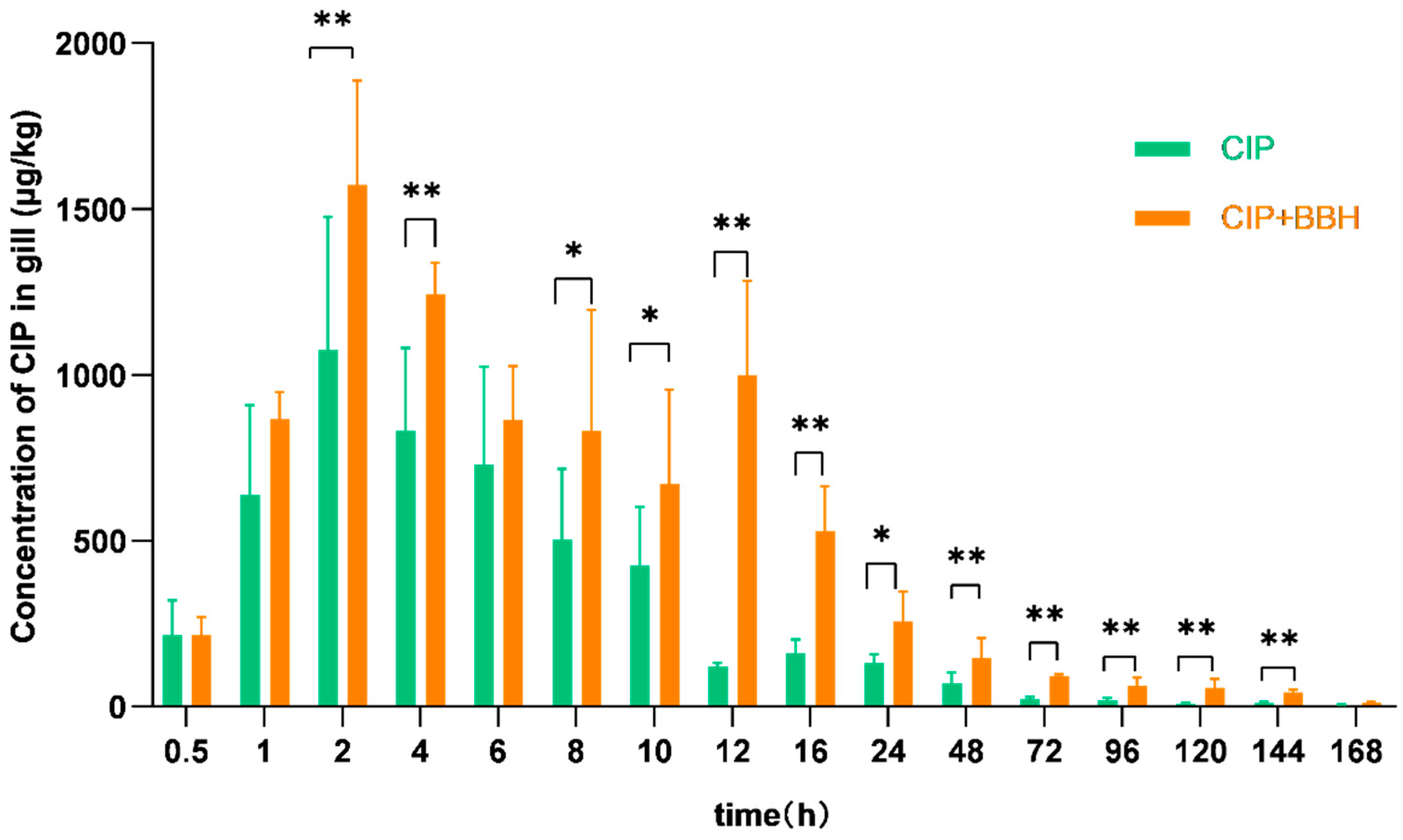
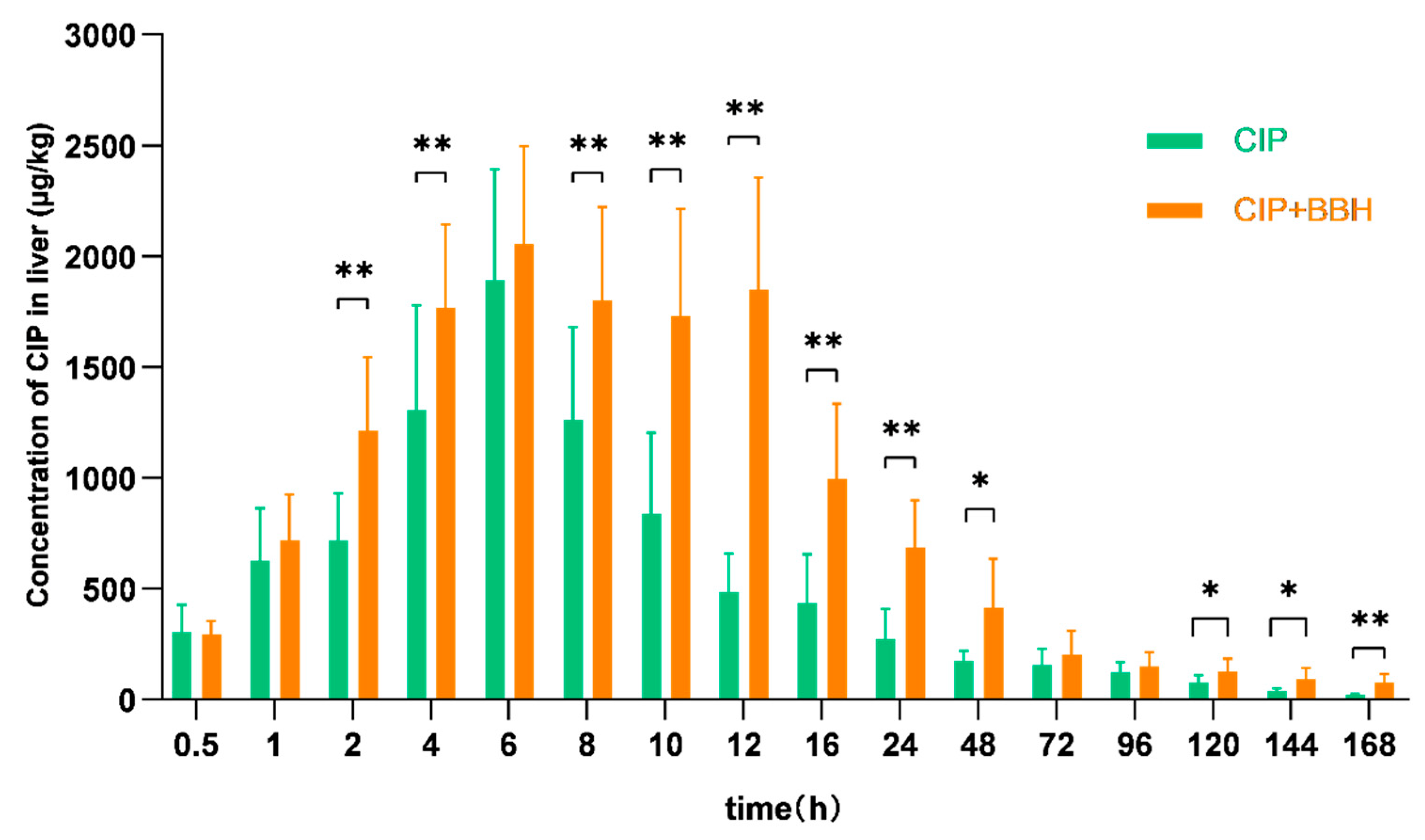
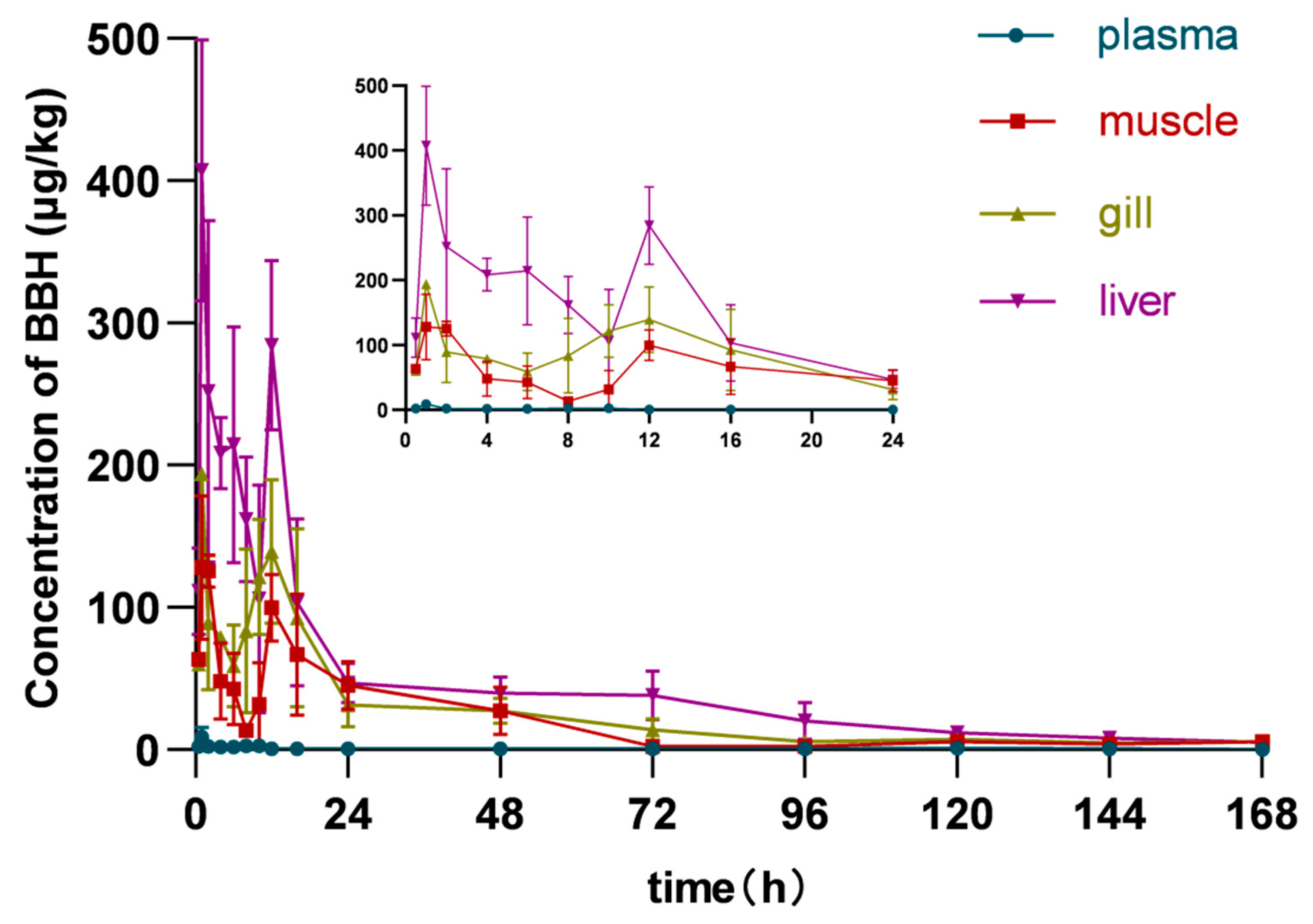
3.2. Analysis of Pharmacokinetic Parameters in Plasma and Tissues of Yellow Catfish
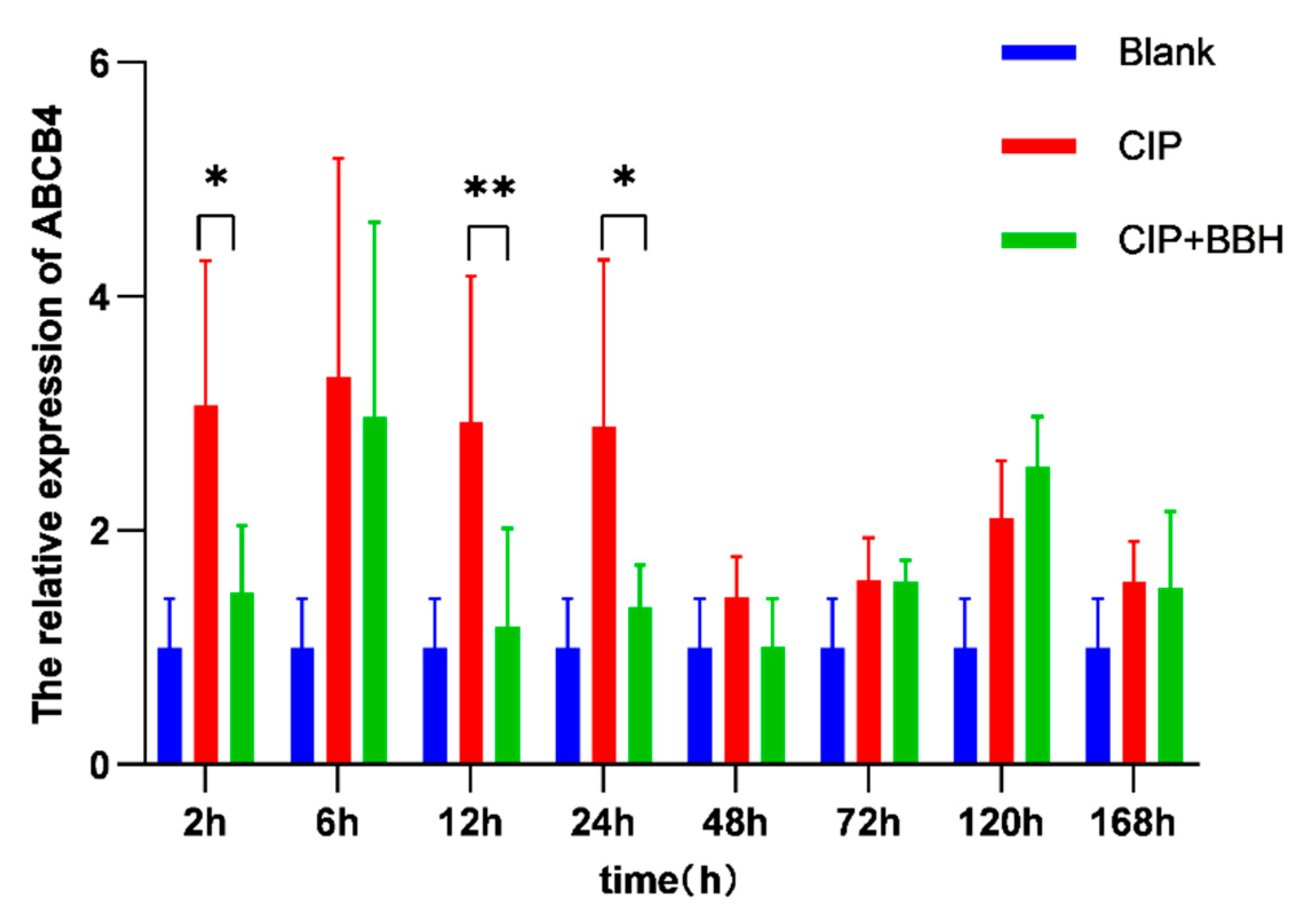
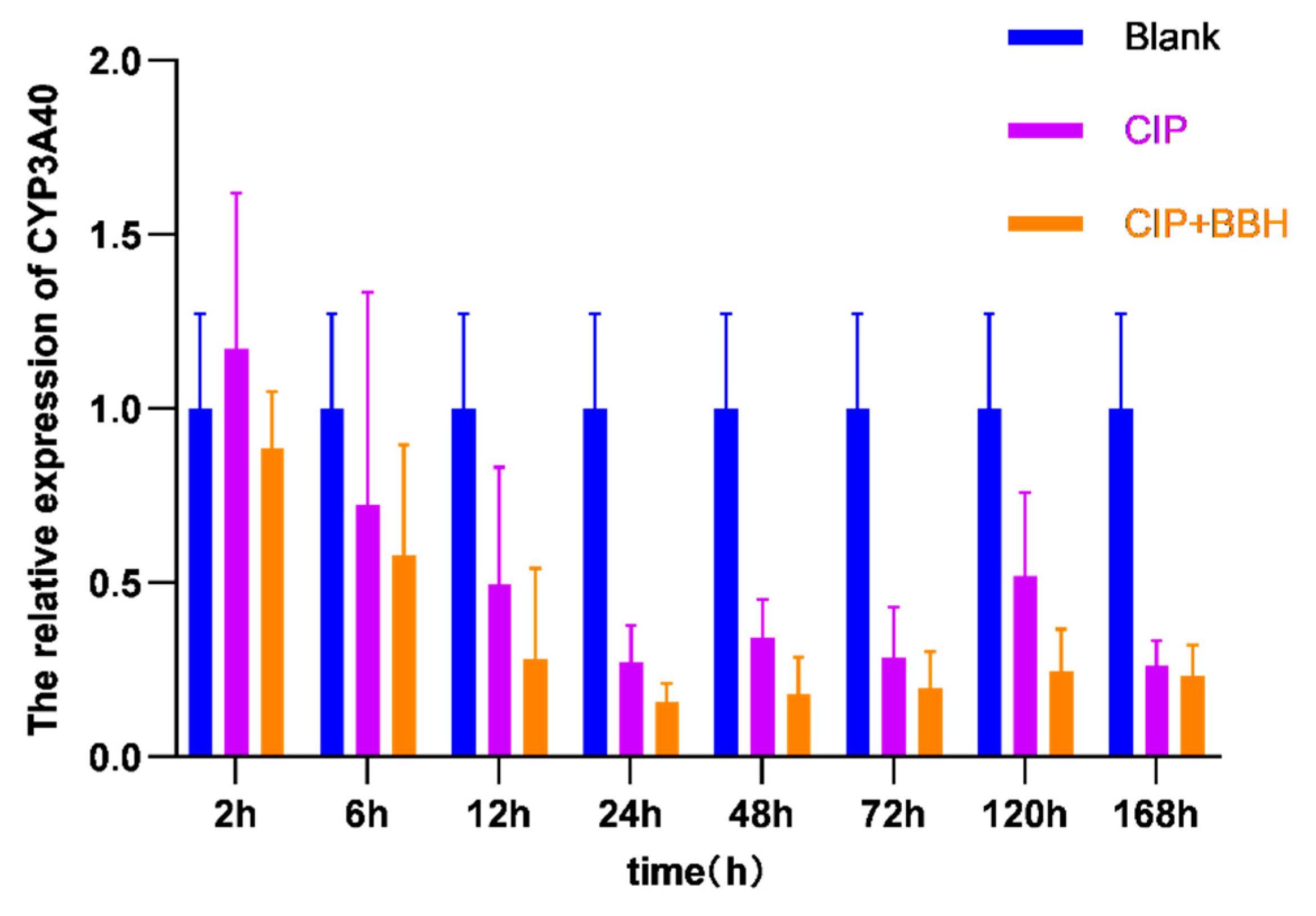
3.3. Analysis of Gene Expression Level in Yellow Catfish
4. Discussion
4.1. Effect of BBH on the Pharmacokinetics of CIP
4.2. The Effect of BBH on the Expression of Genes Related to Drug Absorption and Metabolism
4.3. Effect of Combined Administration on Efficacy
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CCFA. China Fisheries Statistics Yearbook; China Agriculture Press: Beijing, China, 2024. [Google Scholar]
- Guo, M.; Sun, R.; Wu, Z.; Li, A.; Wang, Q.; Zhao, Z.; Liu, H.; Wang, B.; Xiao, K.; Shi, Z.; et al. A comparative study on the immune response in the head and trunk kidney of yellow catfish infected with Edwardsiella ictaluri. Fish Shellfish Immunol. 2024, 154, 109895. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Liu, D.; Han, S.; Zhou, Y.; Wang, K.Y.; Huang, X.L.; Chen, D.F.; Peng, X.; Lai, W.M. Outbreaks of vibriosis associated with Vibrio mimicus in freshwater catfish in China. Aquaculture 2014, 433, 82–84. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J. Pharmacology, 9th ed.; People’s Medical Publishing House: Beijing, China, 2018. [Google Scholar]
- Millanao, A.R.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Hidalgo, A.A. Biological Effects of Quinolones: A Family of Broad-Spectrum Antimicrobial Agents. Molecules 2021, 26, 7153. [Google Scholar] [CrossRef]
- Jiang, Y.; Yao, L.; Li, F.; Tan, Z.; Zhai, Y.; Wang, L. Characterization of antimicrobial resistance of Vibrio parahaemolyticus from cultured sea cucumbers (Apostichopus japonicas). Lett. Appl. Microbiol. 2014, 59, 147–154. [Google Scholar] [CrossRef]
- Lukkana, M.; Jantrakajorn, S.; Wongtavatchai, J. Antimicrobial susceptibility and enrofloxacin resistance of streptococcal bacteria from farmed Nile tilapia, Oreochromis niloticus (Linnaeus 1758) in Thailand. Aquac. Res. 2016, 47, 3136–3144. [Google Scholar] [CrossRef]
- Chokmangmeepisarn, P.; Thangsunan, P.; Kayansamruaj, P.; Rodkhum, C. Resistome characterization of Flavobacterium columnare isolated from freshwater cultured Asian sea bass (Lates calcarifer) revealed diversity of quinolone resistance associated genes. Aquaculture 2021, 544, 737149. [Google Scholar] [CrossRef]
- Tomova, A.; Ivanova, L.; Buschmann, A.H.; Godfrey, H.P.; Cabello, F.C. Plasmid-Mediated Quinolone Resistance (PMQR) Genes and Class 1 Integrons in Quinolone-Resistant Marine Bacteria and Clinical Isolates of Escherichia coli from an Aquacultural Area. Microb. Ecol. 2017, 75, 104–112. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Chen, J.; Ma, J.; Si, H.; Xia, D. Inhibition of inflammation by berberine: Molecular mechanism and network pharmacology analysis. Phytomedicine 2024, 128, 155258. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, X.; Liu, N.; Ren, M.; Wang, Z.; Li, M.; Chen, N.; Li, S. Effects of dietary berberine hydrochloride inclusion on growth, antioxidant capacity, glucose metabolism and intestinal microbiome of largemouth bass (Micropterus salmoides). Aquaculture 2022, 552, 738023. [Google Scholar] [CrossRef]
- Sun, Q.; Tao, Q.; Ming, T.Q.; Tang, S.; Zhao, H.; Liu, M.L.; Yang, H.; Ren, S.; Lei, J.R.; Liang, Y.J.; et al. Berberine is a suppressor of Hedgehog signaling cascade in colorectal cancer. Phytomedicine 2023, 114, 154792. [Google Scholar] [CrossRef]
- Zhang, D.; Li, A.; Xie, J.; Ji, C. In vitro antibacterial effect of berberine hydrochloride and enrofloxacin to fish pathogenic bacteria. Aquac. Res. 2010, 41, 1095–1100. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, C.; Gong, H.; Chen, J.; Tang, B.; Yang, M.; Qin, Q.; Wei, S. Berberine inhibits SGIV replication by suppressing inflammatory response and oxidative stress. Fish Shellfish Immunol. 2024, 149, 109522. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, T.; Yang, F.; Wang, M.; Liu, X.; Li, P.; Ling, F.; Wang, G. Mitochondrial apoptotic pathway-mediated suppression of largemouth bass virus proliferation by berberine. Aquaculture 2024, 588, 740881. [Google Scholar] [CrossRef]
- Feng, X.; Wang, K.; Cao, S.; Ding, L.; Qiu, F. Pharmacokinetics and Excretion of Berberine and Its Nine Metabolites in Rats. Front. Pharmacol. 2020, 11, 594852. [Google Scholar] [CrossRef]
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef]
- Wu, F.; Cui, M.; Wang, S.; Yu, C.; Yin, W.; Li, J.; Yan, X. Effect of berberine on pharmacokinetics and pharmacodynamics of atorvastatin in hyperlipidemia rats. Xenobiotica 2023, 53, 644–652. [Google Scholar] [CrossRef]
- Xin, H.W.; Tang, X.; Ouyang, M.; Zhong, J.X.; Li, W.L. Effects of berberine on pharmacokinetics of midazolam and rhodamine 123 in rats in vivo. SpringerPlus 2016, 5, 380. [Google Scholar] [CrossRef]
- Li, S.; Wang, B.; Zhang, M.; Yuan, D.; Li, J.; Li, X.; Liang, G. Effects of berberine on the pharmacokinetics of florfenicol and levels of cytochrome P450 3A37, multidrug resistance 1, and chicken xenobiotic-sensing orphan nuclear receptor mRNA expression in broilers. Vet. Med. Sci. 2022, 8, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin-resistant breast cancer cells. Mol. Med. Rep. 2019, 19, 5162–5168. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, Y.; Luo, L.; You, Z.; Chen, H.; Wang, J.; Zhang, F.; Liu, Y.; Ke, Y. Glioblastoma stem cells deliver ABCB4 transcribed by ATF3 via exosomes conferring glioblastoma resistance to temozolomide. Cell Death Dis. 2024, 15, 318. [Google Scholar] [CrossRef]
- Luckenbach, T.; Burkhardt-Medicke, K. Differing temperature dependencies of functional homologs zebrafish Abcb4 and human ABCB1. Front. Pharmacol. 2024, 15, 1426040. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.; Alabdalla, L.; Mishra, S.; McHaourab, H. Generation and characterization of a zebrafish knockout model of abcb4, a homolog of the human multidrug efflux transporter P-glycoprotein. Hum. Genom. 2023, 17, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Tian, F.; Zhang, S.Q.; Jin, H. Advances in Pharmacokinetic Mechanisms of Transporter-Mediated Herb-Drug Interactions. Pharmaceuticals 2022, 15, 1126. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, G.; Xiong, T.; Peng, Y.; Ruan, T.; Wang, G.; Sun, J. Research progress of P-glycoprotein induction. J. China Pharm. Univ. 2018, 49, 26–33. [Google Scholar] [CrossRef]
- Wei, Y.; Palazzolo, L.; Ben Mariem, O.; Bianchi, D.; Laurenzi, T.; Guerrini, U.; Eberini, I. Investigation of in silico studies for cytochrome P450 isoforms specificity. Comput. Struct. Biotechnol. J. 2024, 23, 3090–3103. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Benet, L.Z. The gut as a barrier to drug absorption—Combined role of cytochrome P450 3A and P-glycoprotein. Clin. Pharmacokinet. 2001, 40, 159–168. [Google Scholar] [CrossRef]
- CVPC. Quality Standards for Veterinary Drugs, 2017 ed.; China Agriculture Press: Beijing, China, 2017. [Google Scholar]
- Liu, Y.; Han, G.; Dong, J.; Yang, Y.; Yang, Q.; Xu, N.; Zhou, S.; Ai, X. Sex-related differences in disposition of sulfamethoxazole, N-acetyl-sulfamethoxazole and trimethoprim in yellow catfish (Pelteobagrus fulvidraco) following a single oral administration. Aquaculture 2021, 531, 735869. [Google Scholar] [CrossRef]
- EC. Commission Decision of 12 August 2002 Implementing Council Directive 96/23/Ec Concerning the Performance of Analytical Methods and the Interpretation of Results (2002/657/Ec). L221, 8–36: Official Journal of the European Communities. 2002. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002D0657&from=EN (accessed on 11 May 2024).
- Alnaqeeb, M.; Mansor, K.A.; Mallah, E.M.; Ghanim, B.Y.; Idkaidek, N.; Qinna, N.A. Critical pharmacokinetic and pharmacodynamic drug-herb interactions in rats between warfarin and pomegranate peel or guava leaves extracts. BMC Complement. Altern. Med. 2019, 19, 29. [Google Scholar] [CrossRef]
- Qin, X.; Li, J.; Wang, S.; Chen, X.; Huang, M.; Bi, H. Co-administration of Wuzhi tablet (Schisandra sphenanthera extract) alters tacrolimus pharmacokinetics in a dose- and time-dependent manner in rats. J. Ethnopharmacol. 2020, 263, 113233. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, G.; Yang, Y.; Zhou, S.; Dong, J.; Yang, Q.; Xu, N.; Ai, X. Artemisinin alters disposition regularities of enrofloxacin and its metabolite ciprofloxacin in channel catfish (Ictalurus punetaus) following combined oral administration. Aquaculture 2023, 572, 739466. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, L.; Ma, X.; Gong, Z.; Li, Y.; Chen, S.; Pan, J.; Wang, A.; Zheng, L.; Huang, Y. Herb-drug interaction: The effect of Polygonum capitatum extract on pharmacokinetics of levofloxacin in rats. J. Pharm. Biomed. Anal. 2021, 195, 113832. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, L.; Chen, X.; Yuan, J.; Tian, D.; Jiang, F.; Wu, Z. Pharmacokinetics of enrofloxacin in Procambarus clarkiiat different temperatures. J. Huazhong Agric. Univ. 2021, 40, 112–119. [Google Scholar] [CrossRef]
- Ibarra, M.; Troconiz, I.F.; Fagiolino, P. Enteric reabsorption processes and their impact on drug pharmacokinetics. Sci. Rep. 2021, 11, 5794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, L.; Huang, J.; Sun, Y.; He, F.; Zloh, M.; Wang, L. Inhibitory Effect of Berberine on Broiler P-glycoprotein Expression and Function: In Situ and In Vitro Studies. Int. J. Mol. Sci. 2019, 20, 1966. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.; Fang, W.; Yang, X.; Hu, L.; Zhou, S.; Zhou, J. Inhibition of CYP450 1A and 3A by berberine in crucian carp Carassius auratus gibelio. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 360–366. [Google Scholar] [CrossRef]
- LeCluyse, E.L. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur. J. Pharm. Sci. 2001, 13, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.Y.; Jaiswal, S.; Sharma, A.; Shukla, M.; Lal, J. Role of enterohepatic recirculation in drug disposition: Cooperation and complications. Drug Metab. Rev. 2016, 48, 281–327. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Fan, R.; Lv, H.; Sun, H.; Xie, H.; Tang, T.; Luo, J.; Xia, Z. The effects of ferulic acid on the pharmacokinetics of warfarin in rats after biliary drainage. Drug Des. Devel Ther. 2016, 10, 2173–2180. [Google Scholar] [CrossRef]
- Stieger, B.; Meier, P.J. Pharmacogenetics of Drug Transporters in the Enterohepatic Circulation. Pharmacogenomics 2011, 12, 611–631. [Google Scholar] [CrossRef]
- Wright, D.H.; Brown, G.H.; Peterson, M.L.; Rotschafer, J.C. Application of fluoroquinolone pharmacodynamics. J. Antimicrob. Chemother. 2000, 46, 669–683. [Google Scholar] [CrossRef]
- Liu, F.; Meng, L.; Yang, S.; Shi, Y.; Meng, D.; Yi, X. The study of yellow catfish muscle decay and its therapy technique. Chin. Fish. Qual. Stand. 2016, 6, 63–70. [Google Scholar]
- Akinbowale, O.L.; Peng, H.; Barton, M.D. Antimicrobial resistance in bacteria isolated from aquaculture sources in Australia. J. Appl. Microbiol. 2006, 100, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.L.; del Castillo, J.R.; Bousquet-Melou, A. The pharmacokinetic-pharmacodynamic approach to a rational dosage regimen for antibiotics. Res. Vet. Sci. 2002, 73, 105–114. [Google Scholar] [CrossRef] [PubMed]
- St-Hilaire, S.; Chan, S.C.H.; Lim, K.Z.; MacKinnon, B.; Cheng, T.H.; Cheng, K.P.F.; Leung, A.C.F.; Lam, S.H.Y.; Bhardwaj, V.; Chan, O.S.K. Subsidized veterinary extension services may reduce antimicrobial resistance in aquaculture. Sci. Rep. 2023, 13, 10118. [Google Scholar] [CrossRef]
- Rairat, T.; Hsieh, C.Y.; Thongpiam, W.; Sung, C.H.; Chou, C.C. Temperature-dependent pharmacokinetics of florfenicol in Nile tilapia (Oreochromis niloticus) following single oral and intravenous administration. Aquaculture 2019, 503, 483–488. [Google Scholar] [CrossRef]
| Time (min) | A (V/V, %) | B (V/V, %) | Flow Rate (μL/min) |
|---|---|---|---|
| 0 | 80 | 20 | 300 |
| 3 | 80 | 20 | 300 |
| 4 | 10 | 90 | 300 |
| 5 | 80 | 20 | 300 |
| 9 | 80 | 20 | 300 |
| Component | Parent Ion (m/z) | Fragment Ion (m/z) | CE (eV) |
|---|---|---|---|
| CIP | 332 | 288.3 | 18 |
| 314.1 * | 22 | ||
| BBH | 336 | 292.1 | 31 |
| 320.4 * | 29 | ||
| CIP-D8 | 340 | 322.5 * | 26 |
| Gene | GenBank Number | Sequence (5′–3′) | Base | Product (bp) |
|---|---|---|---|---|
| ABCB4 | XM_027172914.2 | F: GCAGCCCTGGATAAGGTGAG | 20 | 116 |
| R: ATTTCTGCGACTTCCCCGTT | 20 | |||
| CYP3A40 | XM_027170875.2 | F: CTCTCAGACACACTACAGTAACG | 23 | 178 |
| R: CAGTACCCATACAGCAGGAC | 20 | |||
| β-actin | XM_027148463.2 | F: TCTATGAAGGTTATGCTCTGCCCC | 24 | 130 |
| R: ATTTCCCTCTCAGCTGTGGTAGTG | 24 |
| Tissues | Analytes | Concentration (μg/L or μg/kg) | Average Recoveries (%) | Precisions (CV, %) | |
|---|---|---|---|---|---|
| Intra-Day | Inter-Day | ||||
| CIP | plasma | 1 | 110.7 | 7.8 | 4.3 |
| 20 | 87.2 | 8.9 | 9.1 | ||
| 200 | 92.1 | 1.1 | 3.1 | ||
| muscle | 1 | 93.4 | 7.4 | 5.8 | |
| 20 | 81.7 | 4.7 | 3.9 | ||
| 200 | 87.9 | 1.3 | 5.6 | ||
| gill | 1 | 90.6 | 1.2 | 2.1 | |
| 20 | 84.2 | 3.0 | 6.9 | ||
| 200 | 92.1 | 4.0 | 5.1 | ||
| liver | 1 | 96.8 | 1.2 | 7.9 | |
| 20 | 85.7 | 2.1 | 2.3 | ||
| 200 | 95.1 | 1.4 | 7.1 | ||
| BBH | plasma | 1 | 84.9 | 2.8 | 3.4 |
| 20 | 90.6 | 4.6 | 4.9 | ||
| 200 | 83.6 | 3.8 | 6.7 | ||
| muscle | 1 | 86.5 | 4.7 | 4.3 | |
| 20 | 81.0 | 2.2 | 3.7 | ||
| 200 | 88.8 | 1.8 | 3.8 | ||
| gill | 1 | 89.2 | 1.7 | 5.3 | |
| 20 | 94.7 | 4.0 | 2.2 | ||
| 200 | 90.7 | 3.3 | 4.7 | ||
| liver | 1 | 85.4 | 1.1 | 4.2 | |
| 20 | 108.5 | 9.3 | 2.8 | ||
| 200 | 117.9 | 4.5 | 1.7 | ||
| Plasma | Muscle | Gill | Liver | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CIP | CIP + BBH | CIP | CIP + BBH | CIP | CIP + BBH | CIP | CIP + BBH | ||
| Tmax | h | 2 | 2 | 6 | 12 | 2 | 2 | 6 | 6 |
| Cmax | μg/L (μg/kg) | 537.37 ± 32.67 | 859.83 ± 265.45 * | 844.64 ± 209.95 | 1418.80 ± 298.23 ** | 1073.92 ± 401.42 | 1572.09 ± 314.10 ** | 1892.26 ± 500.07 | 2056.71 ± 439.02 |
| AUC0–168 | h·μg/L | 7109.94 | 13,479.46 | 21,224.55 | 35,130.89 | 13,786.01 | 30,330.60 | 34,788.00 | 64,469.70 |
| AUC0–∞ | h·μg/L | 7169.71 | 13,513.24 | 21,519.86 | 35,573.68 | 13,945.92 | 30,909.73 | 35,769.58 | 72,228.07 |
| λz | L/h | 0.032 | 0.036 | 0.025 | 0.024 | 0.024 | 0.023 | 0.024 | 0.010 |
| t1/2 | h | 21.80 | 19.38 | 27.67 | 29.26 | 28.87 | 29.66 | 29.23 | 70.05 |
| Vz/F | L/kg | 43.85 | 20.67 | - | - | - | - | - | - |
| CL/F | L/h/kg | 1.39 | 0.74 | - | - | - | - | - | - |
| MRT0–168 | h | 27.02 | 21.63 | 32.63 | 31.90 | 24.40 | 34.32 | 40.34 | 39.66 |
| MRT0–∞ | h | 28.46 | 22.07 | 35.07 | 34.10 | 26.54 | 37.60 | 45.00 | 64.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, T.; Du, X.; Chen, Y.; Liu, Y. Effect of Berberine Hydrochloride on Disposition Characteristics of Ciprofloxacin Hydrochloride and Its Mechanism in Yellow Catfish (Pelteobagrus fulvidraco) Following Combined Oral Administration. Fishes 2025, 10, 245. https://doi.org/10.3390/fishes10060245
Zhong T, Du X, Chen Y, Liu Y. Effect of Berberine Hydrochloride on Disposition Characteristics of Ciprofloxacin Hydrochloride and Its Mechanism in Yellow Catfish (Pelteobagrus fulvidraco) Following Combined Oral Administration. Fishes. 2025; 10(6):245. https://doi.org/10.3390/fishes10060245
Chicago/Turabian StyleZhong, Tianfu, Xiangxuan Du, Yueyan Chen, and Yongtao Liu. 2025. "Effect of Berberine Hydrochloride on Disposition Characteristics of Ciprofloxacin Hydrochloride and Its Mechanism in Yellow Catfish (Pelteobagrus fulvidraco) Following Combined Oral Administration" Fishes 10, no. 6: 245. https://doi.org/10.3390/fishes10060245
APA StyleZhong, T., Du, X., Chen, Y., & Liu, Y. (2025). Effect of Berberine Hydrochloride on Disposition Characteristics of Ciprofloxacin Hydrochloride and Its Mechanism in Yellow Catfish (Pelteobagrus fulvidraco) Following Combined Oral Administration. Fishes, 10(6), 245. https://doi.org/10.3390/fishes10060245






