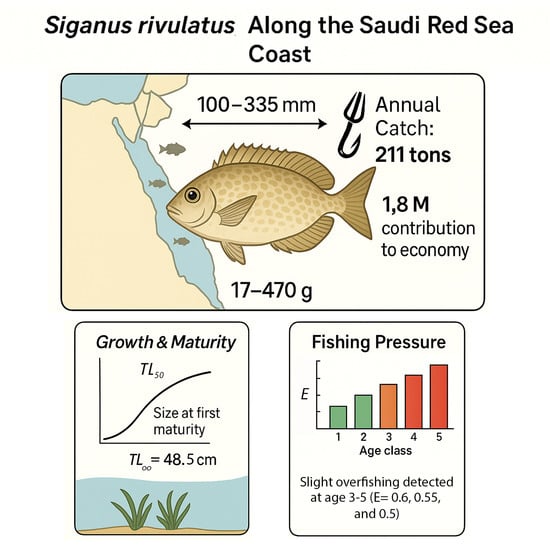
Observations on the Biology and Fishery of the Marbled Spinefoot (Siganus rivulatus Forsskål & Niebuhr, 1775) in the Eastern Red Sea
Abstract
1. Introduction
2. Materials and Methods
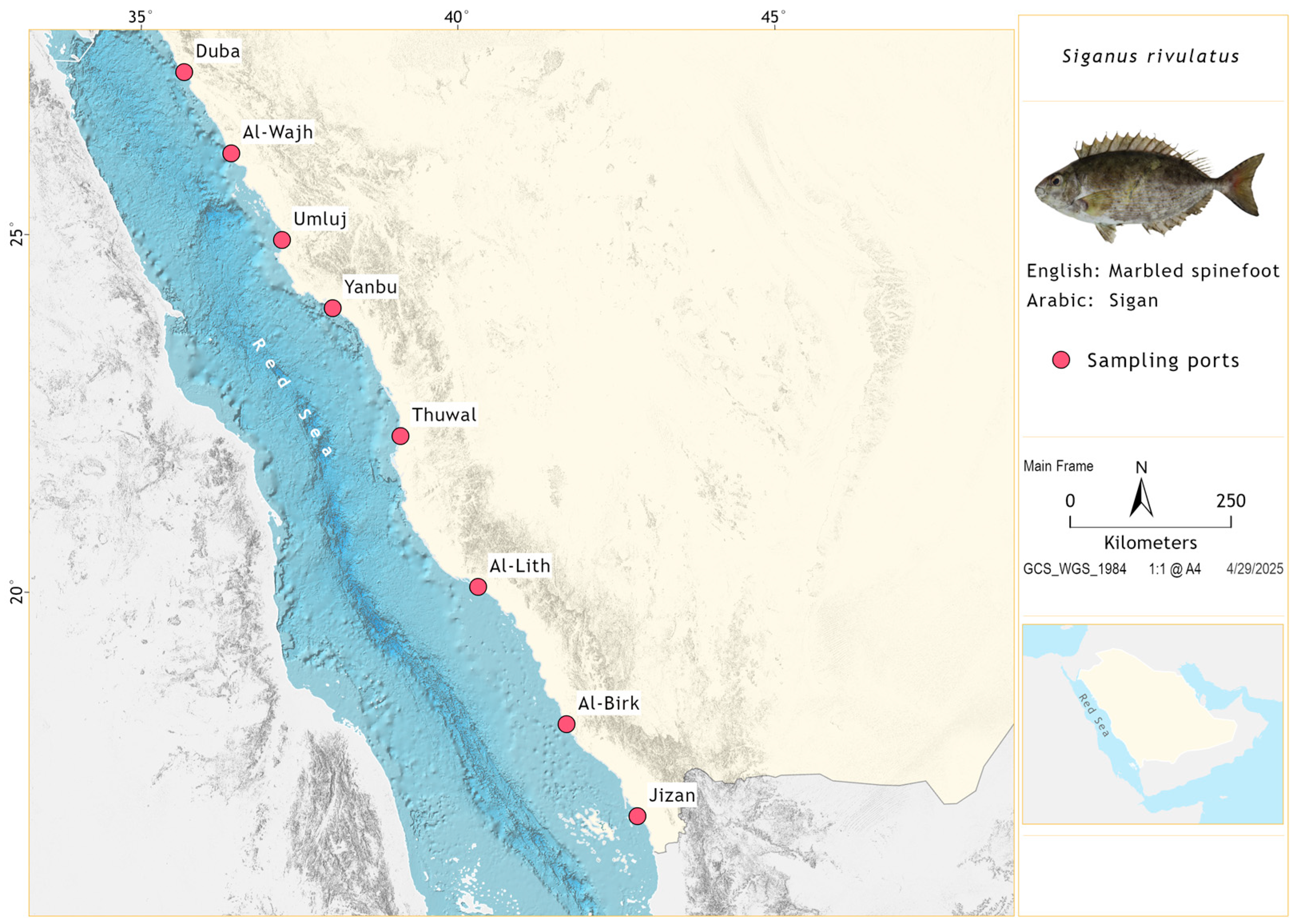
2.1. Study Area and Biological Data Collection
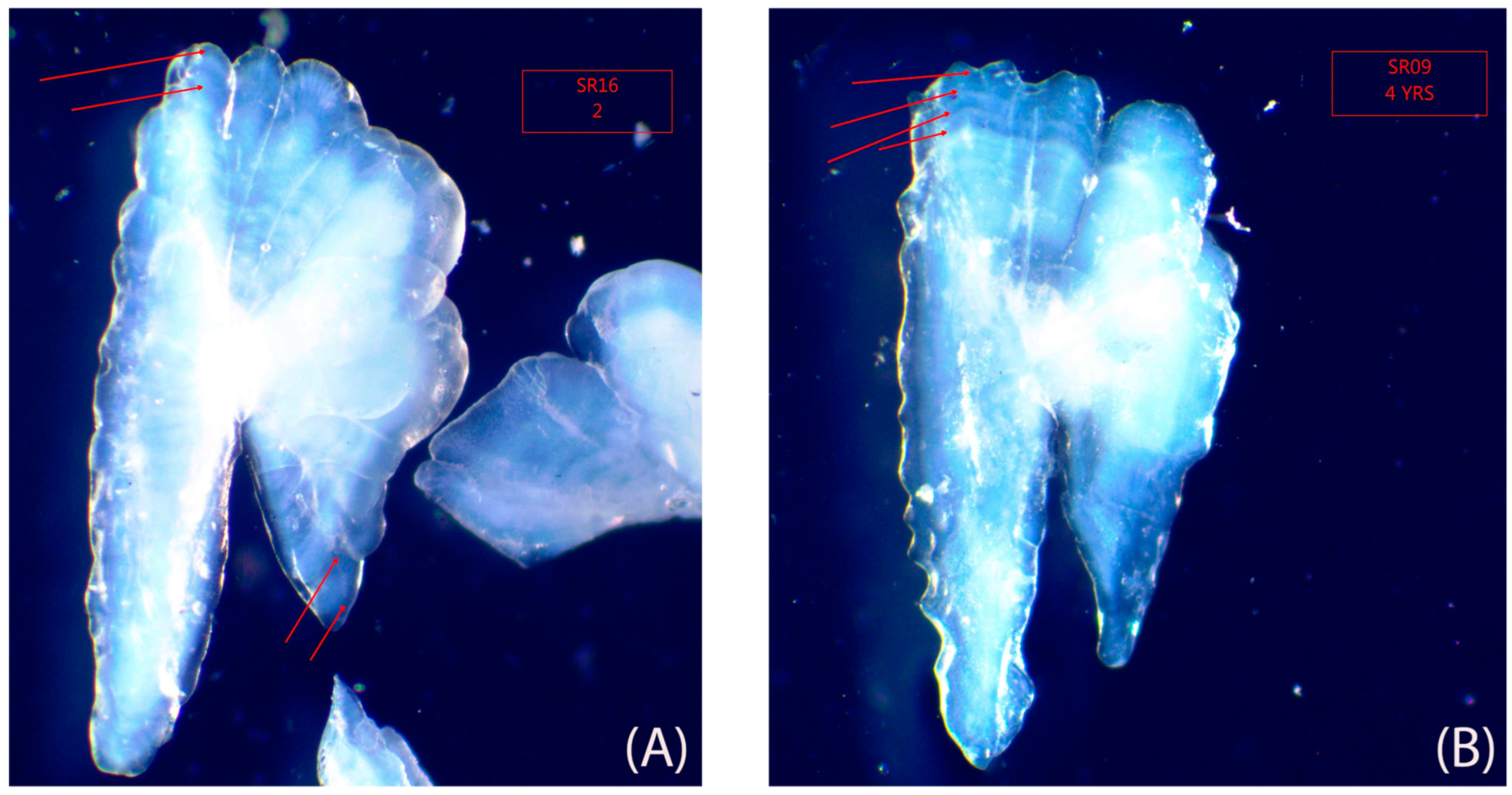
2.2. Otolith Extraction and Reading
2.3. Data Analysis
3. Results
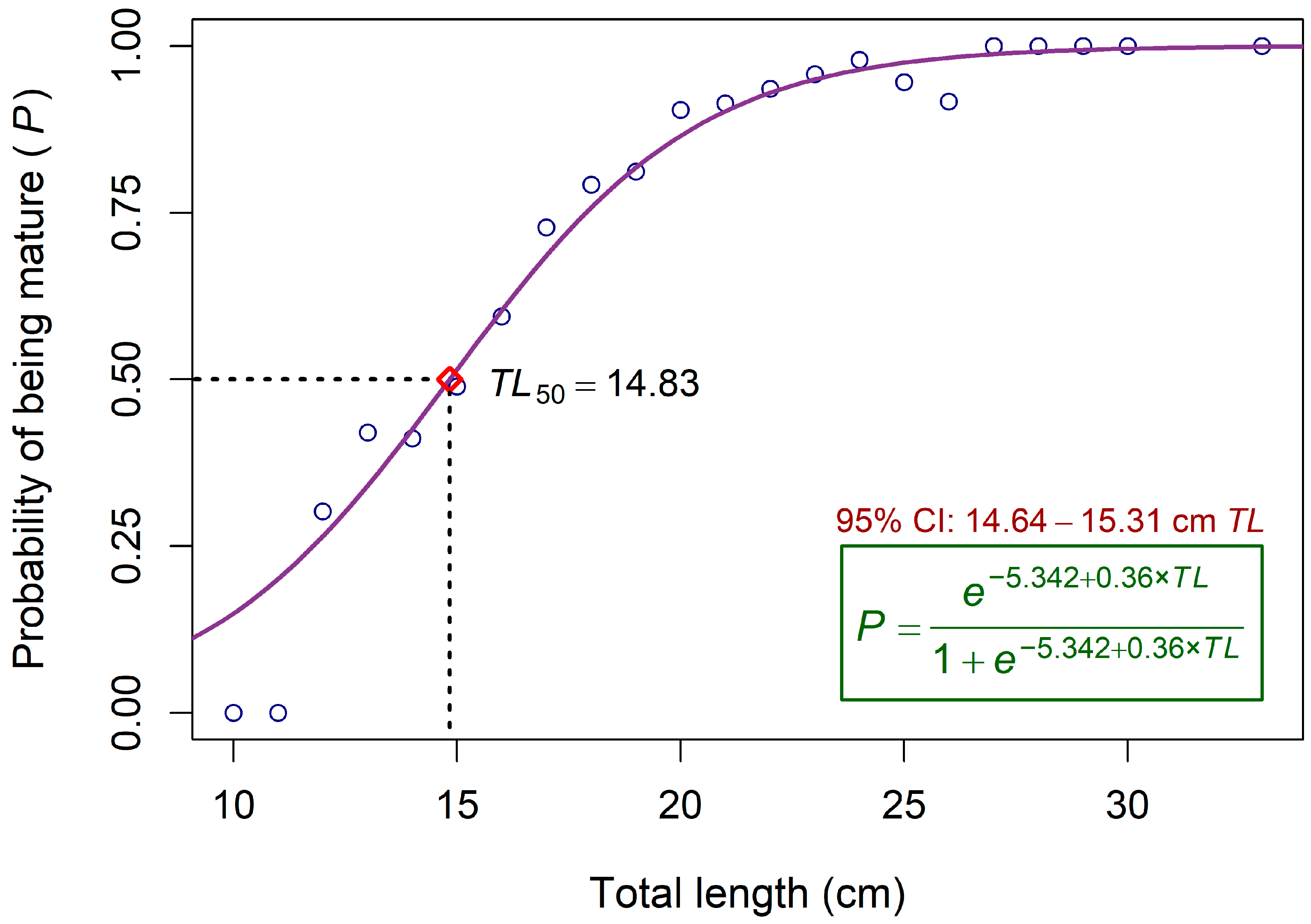
3.1. Maturity
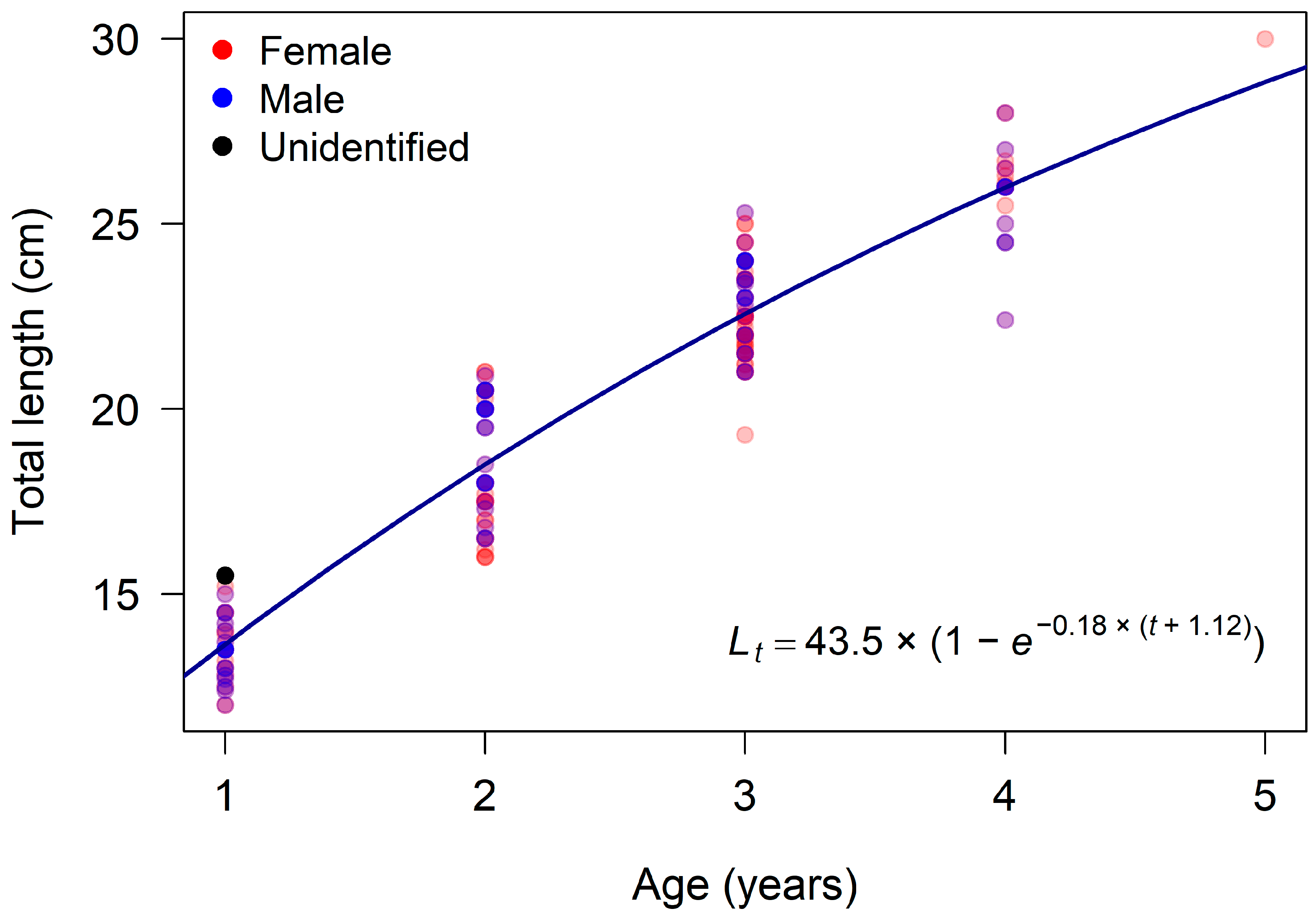
3.2. Age and Growth
3.3. Natural Mortality
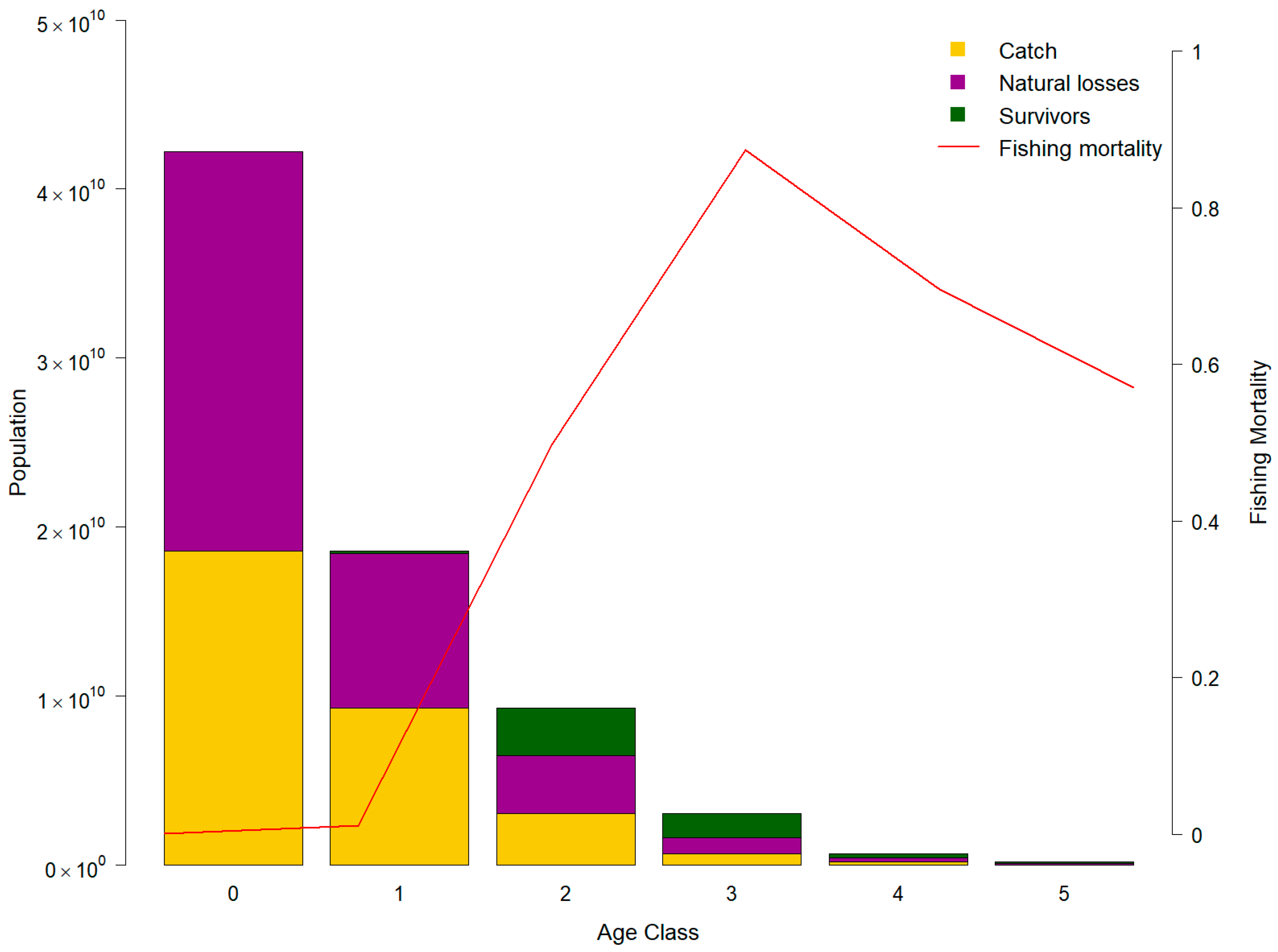
3.4. Stock Assessment
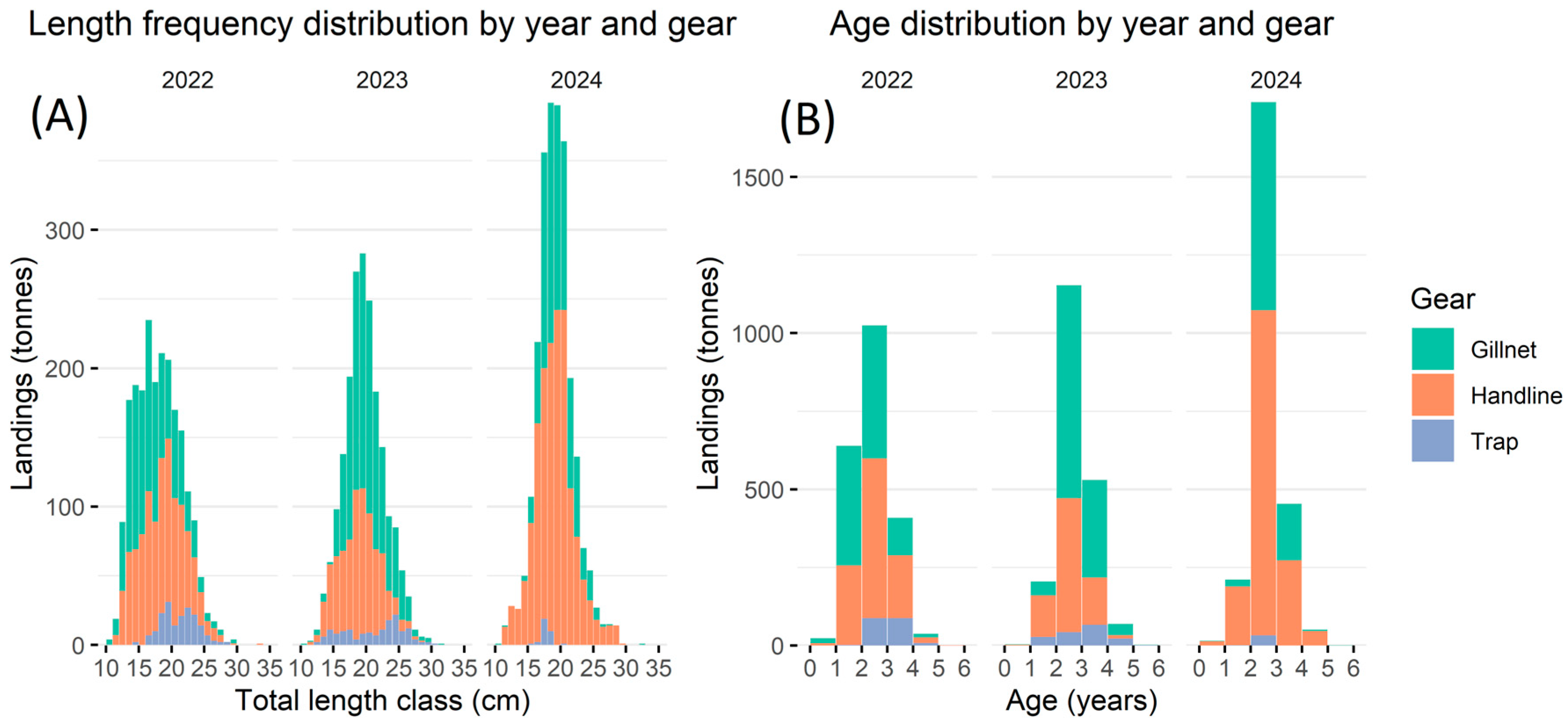
3.5. Fishery Characteristics
4. Discussion
| Growth | ||||||
|---|---|---|---|---|---|---|
| N | TL∞ (cm) | K (y−1) | t0 (y) | Region | Reference | |
| 781 | 31.89 | 0.23 | −1.31 | Mediterranean, Lebanon | [30] | |
| 2176 | 38.10 | 0.28 | −0.24 | Red Sea, Saudi Arabia | [31] | |
| 251 | 32.85 | 0.47 | −0.09 | Red Sea, Egypt | [29] | |
| 521 | 22.30 | 0.28 | −0.50 | Mediterranean, Türkiye | [33] | |
| 1672 | 35.0 | 0.16 | −1.04 | Mediterranean, Libya | [32] | |
| 425 | 37.1 | 0.397 | −0.186 | Red Sea, Egypt | [5] | |
| 2000 | 34.44 | 0.38 | −0.41 | Red Sea, Egypt | [8] | |
| 1116 | 22.8 | 0.341 | −1.376 | Mediterranean, Egypt | [39] | |
| 2904 | 43.50 | 0.18 | −1.22 | Red Sea, Saudi Arabia | Present study | |
| Mortality | ||||||
| Dates | Z (y−1) | M (y−1) | F (y−1) | E | Region | Reference |
| 2013–2014 | 2.04 | 0.41 | 1.63 | 0.80 | Red Sea, Saudi Arabia | [31] |
| 2017–2018 | 2.46 | 0.64 | 1.82 | 0.74 | Red Sea, Egypt | [8] |
| 2000–2001 | 1.27 | 0.26 | 1.01 | 0.80 | Red Sea, Egypt | [5] |
| 2022–2024 | 1.29 | 0.62 | 0.67 | 0.52 | Red Sea, Saudi Arabia | Present study |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodland, D.J. Zoogeography of the Siganidae (Pisces): An interpretation of distribution and richness patterns. Bull. Mar. Sci. 1983, 33, 713–717. [Google Scholar]
- Golana, D.; Ben-Tuvia, A. Lessepsian migration and the Mediterranean fisheries of Israel. In Conditions of the World’s Aquatic Habitats: Proc. World Fisheries Congress Theme I; Armantrout, N.B., Ed.; Oxford and IBH Pub. Co. Pvt. Ltd.: New Delhi, India, 1995; pp. 279–289. [Google Scholar]
- Kuiter, R.H.; Debelius, H. Surgeonfishes, Rabbitfishes and Their Relatives: A Comprehensive Guide to Acanthuroidei; TMC Publishing: Chorley, UK, 2001. [Google Scholar]
- Fricke, R. Siganus rivulatus (Errata Version Published in 2017). The IUCN Red List of Threatened Species. 2010: E.T155025A115261652. 2010. Available online: https://dx.doi.org/10.2305/IUCN.UK.2010-4.RLTS.T155025A4703643.en (accessed on 3 April 2023).
- Mehanna, S.F.; Abdallah, M. Population dynamics of the Rabbitfish Siganus rivulatus from the Egyptian sector of the Red Sea. J. King Abdulaziz Univ. Mar. Sci. 2002, 13, 161–170. [Google Scholar] [CrossRef]
- Bariche, M.; Harmelin-Vivien, M.; Quignard, J.P. Reproductive cycles and spawning periods of two Lessepsian siganid fishes on the Lebanese coast. J. Fish Biol. 2003, 62, 129–142. [Google Scholar] [CrossRef]
- Popper, D.; Pitt, R.; Zohar, Y. Experiments on the propagation of Red Sea Siganids and some notes on their reproduction in nature. Aquaculture 1979, 16, 177–181. [Google Scholar] [CrossRef]
- Mehanna, S.F.; Osman, A.G.M.; Farrag, M.M.S.; Osman, Y.A.A. Age and Growth of Three Common Species of Goatfish Exploited by Artisanal Fishery in Hurghada Fishing Area, Egypt. J. Appl. Ichthyol. 2018, 34, 917–921. [Google Scholar] [CrossRef]
- Edwards, F.J. Climate and Oceanography. In Red Sea; Edwards, A.J., Head, S.M., Eds.; Pergamon Press: Oxford, UK, 1987; pp. 45–69. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. 2025. Available online: www.fishbase.org (accessed on 30 December 2024).
- Tesfamichael, D.; Pauly, D. The Red Sea Ecosystem and Fisheries; Springer: Berlin/Heidelberg, Germany, 2016; Volume 7, ISBN 978-94-017-7433-8. [Google Scholar]
- Kattan, A.; Coker, D.J.; Berumen, M.L. Reef fish communities in the central Red Sea show evidence of asymmetrical fishing pressure. Mar. Biodivers. 2017, 47, 1227–1238. [Google Scholar] [CrossRef]
- Abdelhak, E.M.; Madkour, F.F.; El Ganainy, A.A.; Abu El-Regal, M.A.; Ahmed, M.I. Reproductive biology of Siganus rivulatus (Forsskal, 1775) in the Red Sea, Suez Canal and the Mediterranean Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 117–134. [Google Scholar] [CrossRef]
- Shellem, C.T.; Ellis, J.I.; Coker, D.J.; Berumen, M.L. Red Sea fish market assessments indicate high species diversity and potential overexploitation. Fish. Res. 2021, 239, 105922. [Google Scholar] [CrossRef]
- Alshaikhi, A.; Alshaye, K.; Alharbi, B.; Almohsen, I.; Guemes, P. Fish stocks situation Saudi Arabia 2022. Brochure FAO/MEWA, 11p. 2023. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/67a3bc5b-0597-43ec-9a40-6f84108ab3f9/content (accessed on 29 November 2024).
- MEWA. The Statistical Book. 2023; pp. 184–203. Available online: https://www.mewa.gov.sa/ar/InformationCenter/Researchs/Reports/Pages/default.aspx (accessed on 29 November 2024).
- Gunderson, D.R. Surveys of Fisheries Resources; John Wiley and Sons: Hoboken, NJ, USA, 1993. [Google Scholar]
- Carbonara, P.; Follesa, M.C. (Eds.) Handbook on Fish Age Determination: A Mediterranean Experience; Studies and Reviews. No. 98; FAO: Rome, Italy, 2019; p. 192. [Google Scholar]
- Quinn, T.J.; Deriso, R.B. Quantitative Fish Dynamics; Oxford University Press: New York, NY, USA, 1999; p. 542. [Google Scholar]
- von Bertalanffy, L. Quantitative Laws in Metabolism and Growth. Q. Rev. Biol. 1957, 32, 217–231. [Google Scholar] [CrossRef]
- Aydın, C.M.; Tıraşın, E.M. Information on the deep-water giant red shrimp Aristaeomorpha foliacea (Risso, 1827) (Crustacea, Decapoda, Aristeidae) population in Antalya Bay (Eastern Mediterranean Sea, South of Turkey) based on the MEDITS protocol. Reg. Stud. Mar. Sci. 2023, 60, 102885. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall/CRC: New York, NY, USA, 1993. [Google Scholar]
- Hamel, O.S.; Cope, J.M. Development and considerations for application of a longevity-based prior for the natural mortality rate. Fish. Res. 2022, 256, 106477. [Google Scholar] [CrossRef]
- Then, A.Y.; Hoenig, J.M.; Hall, N.G.; Hewitt, D.A. Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J. Mar. Sci. 2015, 72, 82–92. [Google Scholar] [CrossRef]
- Abella, A.; Caddy, J.F.; Serena, F. Estimation of the Parameters of the Caddy Reciprocal M-at-Age Model for the Construction of Natural Mortality Vectors. In Dynamique des Populations Marines; Lleonart, J., Ed.; CIHEAM: Zaragoza, Spain, 1998; Volume 35, pp. 191–200. [Google Scholar]
- Martiradonna, A. Modelli di Dinamica Delle Popolazioni Ittiche: Stima dei Fattori di Incremento e Decremento Dello Stock. Master’s Thesis, Università di Bari, Dipartimento di Matematica, Bari, Italy, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.4.2; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 20 December 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: London, UK, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 18 October 2024).
- El-Gammal, F.I. Age, growth and mortality of the rabbitfish Siganus rivulatus (Forsk., 1775) from the Red Sea. Bull. Inst. Oceanogr. Fish. 1988, 14, 13–21. [Google Scholar]
- Bariche, M. Age and growth of Lessepsian rabbitfish from the eastern Mediterranean. J. Appl. Ichthyol. 2005, 21, 141–145. [Google Scholar] [CrossRef]
- Gabr, M.H.; Bakaili, A.S.; Mal, A.O. Growth, mortality and yield per recruit of the rabbit fish Siganus rivulatus (Forsskål 1775) in the Red Sea coast of Jeddah, Saudi Arabia. Int. J. Fish. Aquat. Stud. 2018, 6, 87–96. [Google Scholar]
- Shakman, E.; Winkler, H.; Oeberst, R.; Kinzelbach, R. Morphometry, Age and Growth of Siganus luridus Rüppell, 1828 and Siganus rivulatus Forsskål, 1775 (Siganidae) in the Central Mediterranean (Libyan Coast). Rev. Biol. Mar. Oceanogr. 2008, 43, 521–529. [Google Scholar] [CrossRef]
- Bilecenoglu, M.; Kaya, M. Growth of marbled spinefoot Siganus rivulatus Forsskal, 1775 (Teleostei: Siganidae) introduced to Antalya Bay, eastern Mediterranean Sea (Turkey). Fish. Res. 2002, 54, 279–285. [Google Scholar] [CrossRef]
- Sparre, P.; Venema, S.C. Introduction to Tropical Fish Stock Assessment; FAO Fisheries Technical Papers, No. 306/1, Rev. 2 (Part 1); FAO: Rome, Italy, 1998; Available online: https://openknowledge.fao.org/handle/20.500.14283/w5449e (accessed on 20 January 2025).
- Azzurro, E.; Franzitta, G.; Milazzo, M.; Bariche, M.; Fanelli, E. Abundance patterns at the invasion front: The case of Siganus luridus in Linosa (Strait of Sicily, Central Mediterranean Sea). Mar. Freshw. Res. 2016, 68, 697–702. Available online: http://dx.doi.org/10.1071/MF16024 (accessed on 19 October 2024).
- Katsanevakis, S.; Zenetos, A.; Belchior, C.; Cardoso, A.C. Invading European seas: Assessing pathways of introduction of marine aliens. Ocean. Coast. Manag. 2013, 76, 64–74. [Google Scholar] [CrossRef]
- Caddy, J.F.; Mahon, R. Reference Points for Fisheries Management; FAO Fisheries Technical Paper 347; FAO: Rome, Italy, 1995. [Google Scholar]
- Froese, R.; Winker, H.; Gascuel, D.; Sumaila, U.R.; Pauly, D. Minimizing the impact of fishing. Fish Fish. 2016, 17, 785–802. [Google Scholar] [CrossRef]
- Desouky, M.G.; El-Halfawy, M.M.; El-Ganainy, A.A.; El-Regal, M.A.A. Age, Growth and Mortality of the Rabbitfish Siganus rivulatus (Forsskål, 1775) from the Egyptian Mediterranean Coast off Alexandria, Egypt. Egypt. J. Aquat. Biol. Fish. 2022, 26, 557–569. [Google Scholar] [CrossRef]
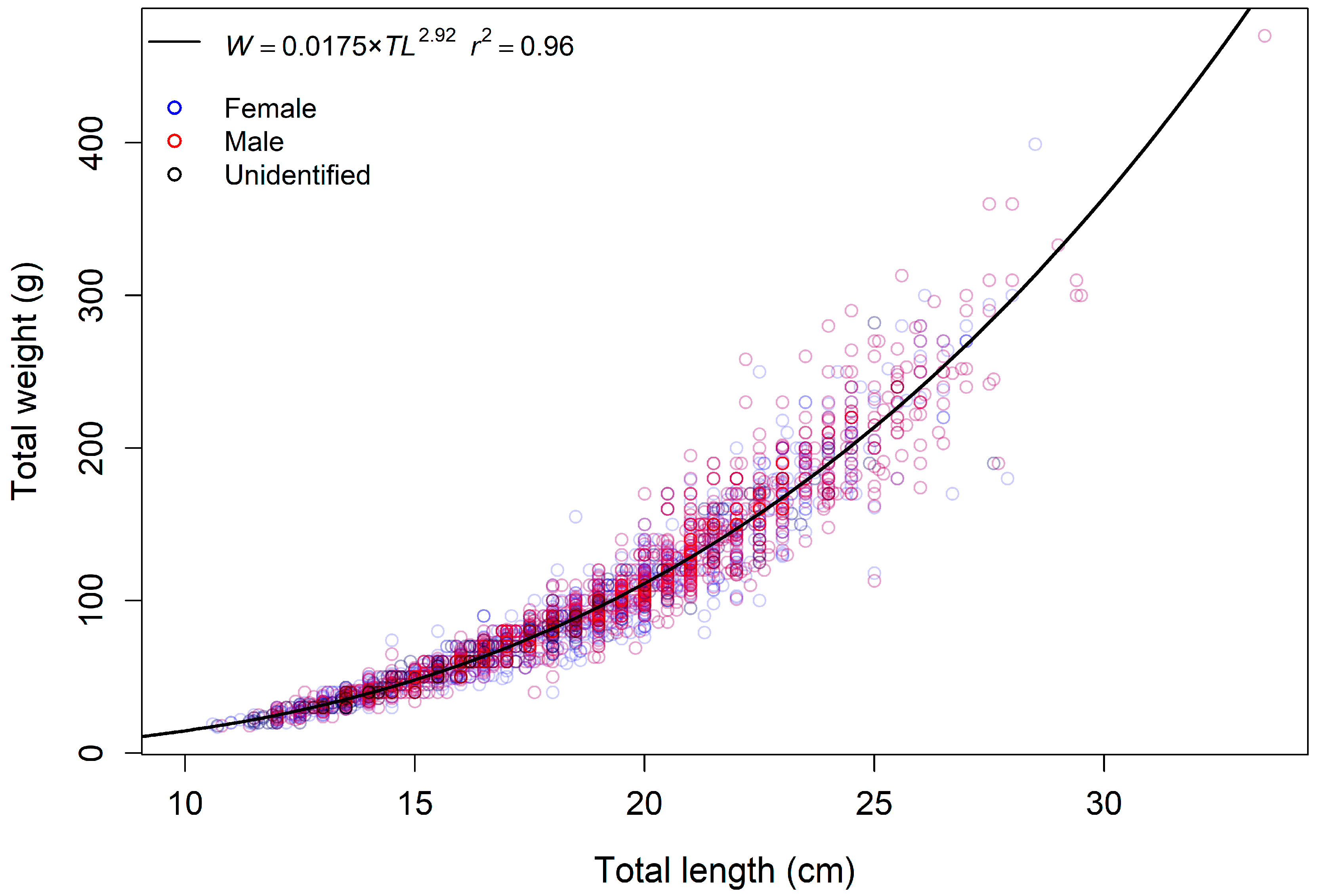
| TL (cm) | W (g) | ||
|---|---|---|---|
| Mean | 18.8 | Mean | 98.8 |
| Max. | 33.5 | Max. | 470.0 |
| Min. | 10.0 | Min. | 17.0 |
| Median | 19.0 | Median | 87.0 |
| Sd | 3.4 | Sd | 64.9 |
| TL-W relationship parameters | 95% CI | ||
| a | 0.0175 | 0.0163–0.0187 | |
| b | 2.92 | 2.90–2.95 | |
| TL Class (cm) | Age (Year) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 12 | 8 | ||||
| 13 | 14 | ||||
| 14 | 7 | ||||
| 15 | 5 | ||||
| 16 | 9 | ||||
| 17 | 9 | ||||
| 18 | 6 | ||||
| 19 | 3 | ||||
| 20 | 13 | ||||
| 21 | 2 | 18 | |||
| 22 | 19 | ||||
| 23 | 11 | ||||
| 24 | 12 | ||||
| 25 | 4 | 1 | |||
| 26 | 11 | ||||
| 27 | 1 | ||||
| 28 | 2 | ||||
| 30 | 1 | ||||
| VPA Outputs | Age Classes | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Survivors (108) | 193.8 | 97.2 | 32.1 | 7.2 | 1.9 | 0 |
| Natural losses (108) | 246.2 | 95.2 | 36.9 | 10.7 | 2.5 | 1.0 |
| Catch × 1000 | 6.0 | 142.5 | 2806.8 | 1411.3 | 279.7 | 99.3 |
| Catch (tonnes) | 0.6 | 14.4 | 283.9 | 142.7 | 28.3 | 10.1 |
| Fishing mortality (F) | 0.0002 | 0.01 | 0.49 | 0.89 | 0.72 | 0.57 |
| Total mortality (Z) | 0.82 | 0.69 | 1.10 | 1.48 | 1.29 | 1.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okba, Z.; Tıraşın, E.M.; Dimech, M. Observations on the Biology and Fishery of the Marbled Spinefoot (Siganus rivulatus Forsskål & Niebuhr, 1775) in the Eastern Red Sea. Fishes 2025, 10, 219. https://doi.org/10.3390/fishes10050219
Okba Z, Tıraşın EM, Dimech M. Observations on the Biology and Fishery of the Marbled Spinefoot (Siganus rivulatus Forsskål & Niebuhr, 1775) in the Eastern Red Sea. Fishes. 2025; 10(5):219. https://doi.org/10.3390/fishes10050219
Chicago/Turabian StyleOkba, Zahra, Eyüp Mümtaz Tıraşın, and Mark Dimech. 2025. "Observations on the Biology and Fishery of the Marbled Spinefoot (Siganus rivulatus Forsskål & Niebuhr, 1775) in the Eastern Red Sea" Fishes 10, no. 5: 219. https://doi.org/10.3390/fishes10050219
APA StyleOkba, Z., Tıraşın, E. M., & Dimech, M. (2025). Observations on the Biology and Fishery of the Marbled Spinefoot (Siganus rivulatus Forsskål & Niebuhr, 1775) in the Eastern Red Sea. Fishes, 10(5), 219. https://doi.org/10.3390/fishes10050219