Integration of Biofloc and Ozone Nanobubbles for Enhanced Pathogen Control in Prenursery of Pacific White Shrimp (Penaeus vannamei)
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Experimental Design
2.3. The Carbohydrate Addition Method in the Biofloc System
2.4. Chemical and Physical Variables of Water
2.5. Real-Time PCR Methods for Vibrio and Muscle Necrosis Virus (MNV)
2.6. Growth Performance and Survival
2.7. Statistical Analysis
3. Results and Discussion
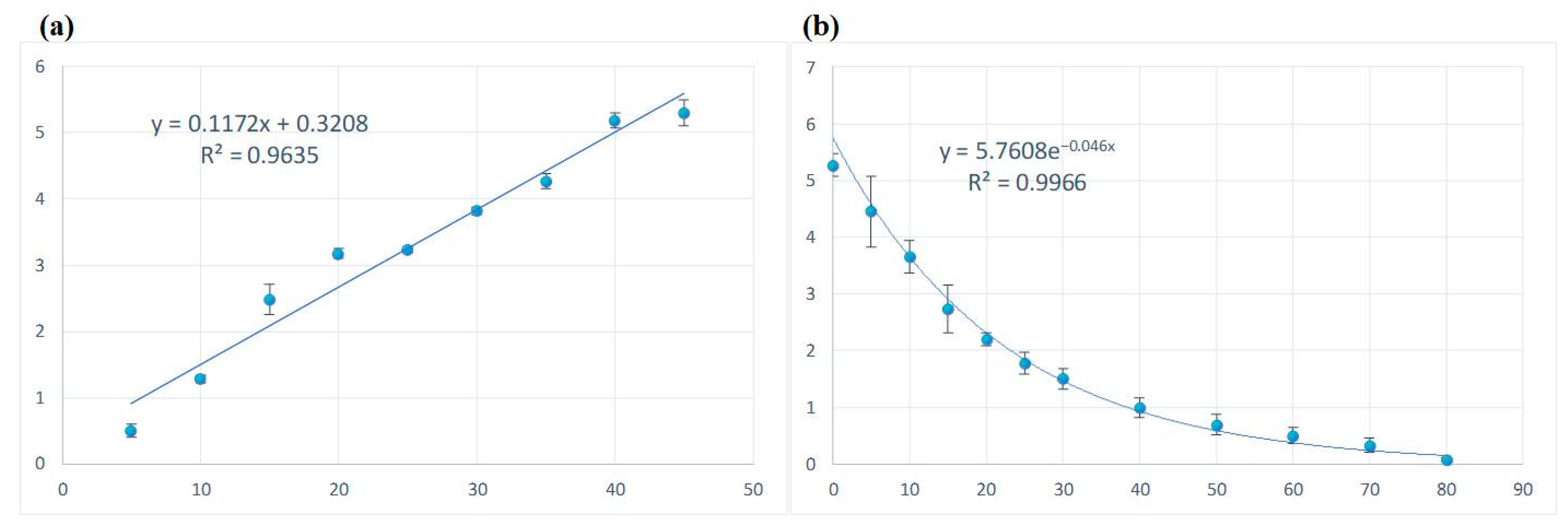
3.1. Temporal Characteristics of Ozone Concentration
3.2. Muscle Necrosis Virus (MNV) and Vibrio Inhibition in Original Water
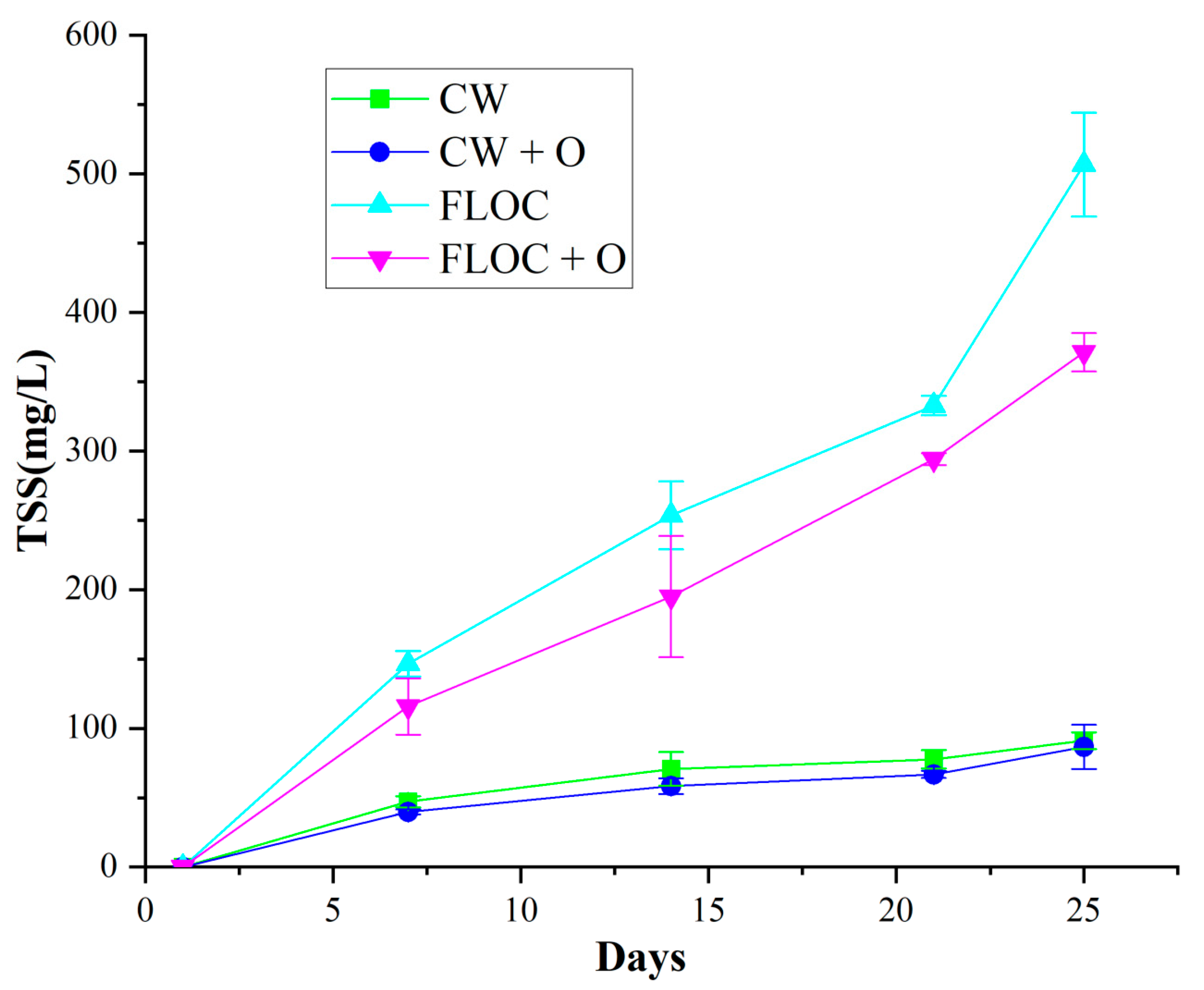
3.3. Water Quality Parameters in Three Treatments
3.4. Protozoan Parasite (Epistylis spp.) Inhibition
3.5. Growth Performance and Survival Rates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wyban, J.; Sweeney, J.N. Intensive Shrimp Production Technology: The Oceanic Institute Shrimp Manual; Oceanic Institute: Hawaii, HI, USA, 1991. [Google Scholar]
- Rego, M.A.S.; Sabbag, O.J.; Soares, R.; Peixoto, S. Risk analysis of the insertion of biofloc technology in a marine shrimp Litopenaeus vannamei production in a farm in Pernambuco, Brazil: A case study. Aquaculture 2017, 469, 67–71. [Google Scholar] [CrossRef]
- Stentiford, G.D. Diseases in aquatic crustaceans: Problems and solutions for global food security. J. Invertebr. Pathol. 2012, 110, 139. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Arthur, J.R.; Ogawa, K.; Chinabut, S.; Adlard, R.; Tan, Z.; Shariff, M. Disease and health management in Asian aquaculture. Vet. Parasitol. 2005, 132, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Rezende, P.C.; Schleder, D.D.; da Silva, H.V.; Henriques, F.M.; de Lorenzo, M.A.; Seiffert, W.Q.; Andreatta, E.R.; Vieira, F.D. Prenursery of the Pacific white shrimp in a biofloc system using different artificial substrates. Aquacult. Eng. 2018, 82, 25–30. [Google Scholar] [CrossRef]
- Kuhn, D.D.; Lawrence, A.L.; Boardman, G.D.; Patnaik, S.; Marsh, L.; Flick, G.J. Evaluation of two types of bioflocs derived from biological treatment of fish effluent as feed ingredients for Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2010, 303, 28–33. [Google Scholar] [CrossRef]
- Ray, A.J.; Seaborn, G.; Leffler, J.W.; Wilde, S.B.; Lawson, A.; Browdy, C.L. Characterization of microbial communities in minimal-exchange, intensive aquaculture systems and the effects of suspended solids management. Aquaculture 2010, 310, 130–138. [Google Scholar] [CrossRef]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Crab, R.; Defoirdt, T.; Bossier, P.; Verstraete, W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture 2012, 356, 351–356. [Google Scholar] [CrossRef]
- Emerenciano, M.; Gaxiola, G.; Cuzon, G. Biofloc technology (BFT): A review for aquaculture application and animal food industry. Biomass Now-Cult. Util. 2013, 12, 301–328. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Hankins, J.A.; Weber, A.L.; Durant, M.D. Ozonation of a recirculating rainbow trout culture system II. Effects on microscreen filtration and water quality. Aquaculture 1997, 158, 57–67. [Google Scholar] [CrossRef]
- Good, C.; Davidson, J.; Welsh, C.; Snekvik, K.; Summerfelt, S. The effects of ozonation on performance, health and welfare of rainbow trout Oncorhynchus mykiss in low-exchange water recirculation aquaculture systems. Aquacult. Eng. 2011, 44, 97–102. [Google Scholar] [CrossRef]
- Good, C.; Redman, N.; Murray, M.; Straus, D.L.; Welch, T.J. Bactericidal activity of peracetic acid to selected fish pathogens in recirculation aquaculture system water. Aquacult. Res. 2022, 53, 4487–4496. [Google Scholar] [CrossRef]
- Pettersson, S.J.; Lindholm-Lehto, P.C.; Pulkkinen, J.T.; Kiuru, T.; Vielma, J. Effect of ozone and hydrogen peroxide on off-flavor compounds and water quality in a recirculating aquaculture system. Aquacult. Eng. 2022, 98, 102277. [Google Scholar] [CrossRef]
- Qi, W.; Skov, P.V.; Gregersen, K.J.d.J.; Mousavi, S.; Pedersen, L.-F.; Mota, V.C. Estimating biofilm activity on biofilter elements in recirculating aquaculture systems (RAS) for rearing Atlantic salmon parr (Salmo salar) during operation with ozone and peracetic acid. Aquaculture 2025, 594, 741381. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; Gagnon, G.A. Ozone application in recirculating aquaculture system: An overview. Ozone Sci. Eng. 2011, 33, 345–367. [Google Scholar] [CrossRef]
- Dinh-Hung, N.; Dong, H.T.; Senapin, S.; Shinn, A.P.; Linh, N.V.; Dien, L.T.; Soontara, C.; Hirono, I.; Chatchaiphan, S.; Rodkhum, C. Using ozone nanobubbles to mitigate the risk of mycobacteriosis in Siamese fighting fish (Betta splendens). Aquaculture 2024, 581, 740390. [Google Scholar] [CrossRef]
- Imaizumi, K.; Tinwongger, S.; Kondo, H.; Hirono, I. Disinfection of an EMS/AHPND strain of Vibrio parahaemolyticus using ozone nanobubbles. J. Fish Dis. 2018, 41, 559–563. [Google Scholar] [CrossRef]
- Ritar, A.J.; Smith, G.G.; Thomas, C.W. Ozonation of seawater improves the survival of larval southern rock lobster, Jasus edwardsii, in culture from egg to juvenile. Aquaculture 2006, 261, 1014–1025. [Google Scholar] [CrossRef]
- Sharrer, M.J.; Summerfelt, S.T. Ozonation followed by ultraviolet irradiation provides effective bacteria inactivation in a freshwater recirculating system. Aquacult. Eng. 2007, 37, 180–191. [Google Scholar] [CrossRef]
- Scolding, J.W.; Powell, A.; Boothroyd, D.P.; Shields, R.J. The effect of ozonation on the survival, growth and microbiology of the European lobster (Homarus gammarus). Aquaculture 2012, 364, 217–223. [Google Scholar] [CrossRef]
- Spiliotopoulou, A.; Rojas-Tirado, P.; Chhetri, R.K.; Kaarsholm, K.M.; Martin, R.; Pedersen, P.B.; Pedersen, L.-F.; Andersen, H.R. Ozonation control and effects of ozone on water quality in recirculating aquaculture systems. Water Res. 2018, 133, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Suurnäkki, S.; Pulkkinen, J.T.; Lindholm-Lehto, P.C.; Tiirola, M.; Aalto, S.L. The effect of peracetic acid on microbial community, water quality, nitrification and rainbow trout (Oncorhynchus mykiss) performance in recirculating aquaculture systems. Aquaculture 2020, 516, 734534. [Google Scholar] [CrossRef]
- Krumins, V.; Ebeling, J.; Wheaton, F. Part-day ozonation for nitrogen and organic carbon control in recirculating aquaculture systems. Aquacult. Eng. 2001, 24, 231–241. [Google Scholar] [CrossRef]
- Suantika, G.; Dhert, P.; Rombaut, G.; Vandenberghe, J.; De Wolf, T.; Sorgeloos, P. The use of ozone in a high density recirculation system for rotifers. Aquaculture 2001, 201, 35–49. [Google Scholar] [CrossRef]
- Davidson, J.; Summerfelt, S.; Espmark, Å.M.O.; Mota, V.C.; Marancik, D.; Earley, R.L.; Snead, A.; Good, C. Effects of ozone on post-smolt Atlantic salmon (Salmo salar) performance, health, and maturation in freshwater recirculation aquaculture systems. Aquaculture 2021, 533, 736208. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, S.; Liu, D.; Guo, X.; Ye, Z. Effects of stocking density of the white shrimp Litopenaeus vannamei (Boone) on immunities, antioxidant status, and resistance against Vibrio harveyi in a biofloc system. Fish Shellfish Immunol. 2017, 67, 19–26. [Google Scholar] [CrossRef]
- Aguilera-Rivera, D.; Prieto-Davó, A.; Escalante, K.; Chávez, C.; Cuzon, G.; Gaxiola, G. Probiotic effect of FLOC on Vibrios in the pacific white shrimp Litopenaeus vannamei. Aquaculture 2014, 424, 215–219. [Google Scholar] [CrossRef]
- Liu, G.; Ye, Z.; Liu, D.; Zhu, S. Inorganic nitrogen control, growth, and immunophysiological response of Litopenaeus vannamei (Boone, 1931) in a biofloc system and in clear water with or without commercial probiotic. Aquacult. Int. 2018, 26, 981–999. [Google Scholar] [CrossRef]
- Rosas, C.; Sanchez, A.; Diaz, E.; Soto, L.A.; Gaxiola, G.; Brito, R. Effect of dietary protein level on apparent heat increment and post-prandial nitrogen excretion of Penaeus setiferus, P. schmitti, P. duorarum, and P. notialis postlarvae. J. World Aquacult. Soc. 1996, 27, 92–102. [Google Scholar] [CrossRef]
- Avnimelech, Y. Carbon nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Summerfelt, S.T. Ozonation and UV irradiation—An introduction and examples of current applications. Aquacult. Eng. 2003, 28, 21–36. [Google Scholar] [CrossRef]
- Toledo, T.M.; Silva, B.C.; Vieira, F.d.N.; Mouriño, J.L.P.; Seiffert, W.Q. Effects of different dietary lipid levels and fatty acids profile in the culture of white shrimp Litopenaeus vannamei (Boone) in biofloc technology: Water quality, biofloc composition, growth and health. Aquacult. Res. 2016, 47, 1841–1851. [Google Scholar] [CrossRef]
- Emerenciano, M.G.; Miranda-Baeza, A.; Martínez-Porchas, M.; Poli, M.A.; Vieira, F.d.N. Biofloc technology (BFT) in shrimp farming: Past and present shaping the future. Front. Mar. Sci. 2021, 8, 813091. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Emerenciano, M.G.C.; Martínez-Córdova, L.R.; Martínez-Porchas, M.; Miranda-Baeza, A. Biofloc technology (BFT): A tool for water quality management in aquaculture. Water Qual. 2017, 5, 92–109. [Google Scholar] [CrossRef]
- Avnimelech, Y. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture 2007, 264, 140–147. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guide Book; The World Aquaculture Society: Baton Rouge, LA, USA, 2012. [Google Scholar]
- Liu, G.; Deng, Y.L.; Verdegem, M.; Ye, Z.Y.; Zhu, S.M. Using poly(beta-hydroxybutyrate-beta-hydroxyvalerate) as carbon source in biofloc-systems: Nitrogen dynamics and shift of Oreochromis niloticus gut microbiota. Sci. Total Environ. 2019, 694, 133664. [Google Scholar] [CrossRef]
- Tango, M.S.; Gagnon, G.A. Impact of ozonation on water quality in marine recirculation systems. Aquacult. Eng. 2003, 29, 125–137. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Rev. Aquacult. 2021, 13, 676–705. [Google Scholar] [CrossRef]
- Nolasco-Alzaga, H.R.; Monreal-Escalante, E.; Gullian-Klanian, M.; de Anda-Montañez, J.A.; Luna-González, A.; Aranceta, F.; Araneda-Padilla, M.E.; Angulo, C. Use of immunostimulants in shrimp farming—A bioeconomic perspective. Animals 2025, 15, 124. [Google Scholar] [CrossRef]
- Bass, D.; Rueckert, S.; Stern, R.; Cleary, A.C.; Taylor, J.D.; Ward, G.M.; Huys, R. Parasites, pathogens, and other symbionts of copepods. Trends Parasitol. 2021, 37, 875–889. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Mozanzadeh, M.T.; Sharifinia, M.; Emerenciano, M.G.C. Biofloc: A sustainable dietary supplement, nutritional value and functional properties. Aquaculture 2023, 562, 738757. [Google Scholar] [CrossRef]
- Glencross, B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquacult. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- Izel-Silva, J.; Dos Santos, R.B.; de Medeiros, P.A.; Suita, S.M.; Wasielesky, W., Jr.; Fugimura, M.M.S.; Affonso, E.G. Brycon amazonicus larviculture cannibalism is reduced in biofloc systems. Aquaculture 2024, 579, 740180. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Cannibalism of decapod crustaceans and implications for their aquaculture: A review of its prevalence, influencing factors, and mitigating methods. Rev. Fish. Sci. Aquacult. 2017, 25, 42–69. [Google Scholar] [CrossRef]
- Mahanand, S.S.; Moulick, S.; Rao, P.S. Optimum formulation of feed for rohu, Labeo rohita (Hamilton), with biofloc as a component. Aquacult. Int. 2013, 21, 347–360. [Google Scholar] [CrossRef]
- Wang, G.; Yu, E.; Xie, J.; Yu, D.; Li, Z.; Luo, W.; Qiu, L.; Zheng, Z. Effect of C/N ratio on water quality in zero-water exchange tanks and the biofloc supplementation in feed on the growth performance of crucian carp, Carassius auratus. Aquaculture 2015, 443, 98–104. [Google Scholar] [CrossRef]
- Santos, S.M.; Wasielesky, W., Jr.; Braga, Í.; Zuñiga, R.; Rosas, V.T.; Christ-Ribeiro, A.; Fóes, G.K. Use of different stocking densities of Litopenaeus vannamei juveniles using “synbiotics”: Effects on water quality, microorganisms, bioflocs composition and zootechnical performance. Aquacult. Int. 2024, 32, 6133–6151. [Google Scholar] [CrossRef]


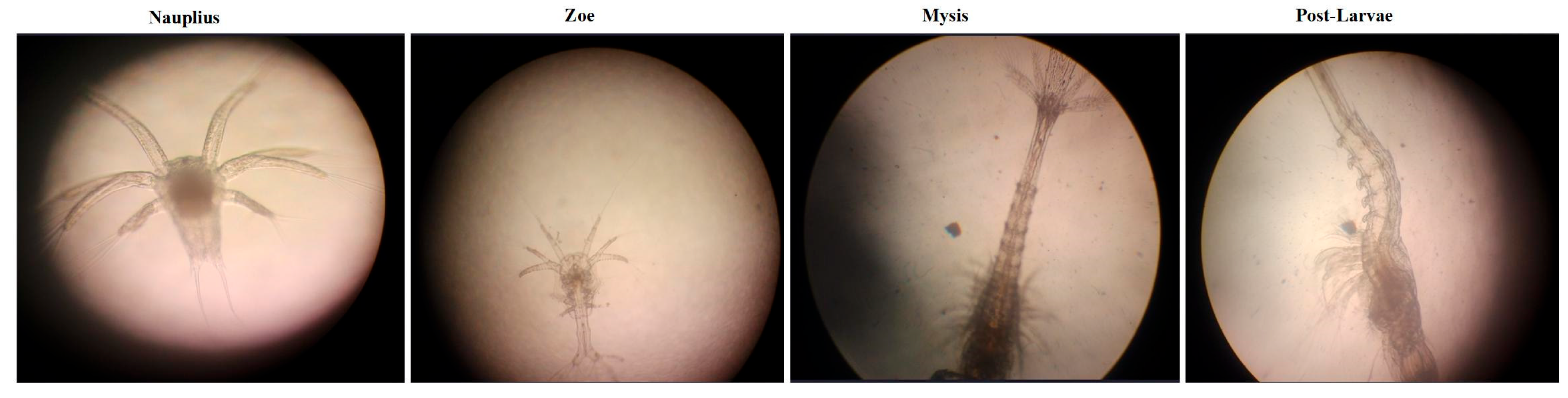
| Growth Stage | Mesh Bag for Feed Filtration | Feed Composition | Feed Weight |
|---|---|---|---|
| Nauplii | NU | Chlorella | 500 mL/tank |
| Zoea stages I and II | 45 µm | Shrimp flakes + prebiotics (gut modifier, spirulina powder, protein products) | 23–65 g/tank |
| Zoea stage III and Mysis stage I | 98 µm | Shrimp flakes + prebiotics (gut modifier, spirulina powder, protein products + Rotifers, Brachionus plicatilis) | 65–90 g/tank, Rotifers 600 mL/tank |
| Mysis stages II and III | 127 µm | Shrimp flakes + prebiotics (spirulina powder, protein products) | 90–120 g/tank, Rotifers 800 mL/tank |
| Post-larvae stages 1–5 | 129 µm | Shrimp flakes + prebiotics (spirulina powder, protein products, Juvenile Brine shrimp, Artemia salina) | 120–180 g/tank, 800 mL/tank |
| Post-larvae stage 5 and beyond | NU | Shrimp flakes | 200–300 g/tank |
| Real-Time Ozone Concentration (mg/L) | Time (min) | Muscle Necrosis Virus (Copies/mL) | Vibrio (CFU/mL) | Total Bacteria (CFU/mL) |
|---|---|---|---|---|
| 2.58 ± 0.12 | 0 | 1064.46 ± 102.33 | 22,884.97 ± 1002.11 | 324,019.01 ± 1287.34 |
| 1.982 ± 0.31 | 2 | 524.91 ± 65.12 | 13,516.23 ± 457.21 | 236,660.19 ± 986.34 |
| 1.463 ± 0.14 | 5 | ND | 12,758.26 ± 321.21 | 132,450.74 ± 765.34 |
| 1.38 ± 0.11 | 10 | ND | 2985.78 ± 102.21 | 26,928.46 ± 453.21 |
| 2.01 ± 0.17 | 20 | ND | 689.59 ± 69.21 | 10,168.36 ± 346.78 |
| 2.25 ± 0.26 | 40 | ND | 637.07 ± 102.23 | 2326.84 ± 154.03 |
| 1.852 ± 0.23 | 60 | ND | 107.18 ± 21.22 | 1511.67 ± 90.11 |
| Real-Time Ozone Concentration (mg/L) | Time (min) | Muscle Necrosis Virus (Copies/mL) | Vibrio (CFU/mL) | Total Bacteria (CFU/mL) |
|---|---|---|---|---|
| 1.25 ± 0.13 | 0 | 1307.32 ± 200.34 | 6881.17 ± 600.36 | 36,146.32 ± 900.32 |
| 1.16 ± 0.22 | 2 | 677.13 ± 43.68 | 1688.08 ± 90.34 | 22,629.91 ± 879.40 |
| 1.068 ± 0.09 | 5 | 222.27 ± 23.49 | 1200.65 ± 100.43 | 12,099.84 ± 766.39 |
| 1.178 ± 0.10 | 10 | 125.47 ± 11.09 | 536.59 ± 67.32 | 2170.48 ± 231.09 |
| 1.348 ± 0.08 | 20 | 53.97 ± 10.24 | 146.17 ± 30.43 | 2730.57 ± 213.90 |
| 1.082 ± 0.21 | 30 | 3.96 ± 0.98 | 211.54 ± 34.87 | 1737.32 ± 215.11 |
| 0.990 ± 0.09 | 40 | 1.26 ± 0.05 | 109.32 ± 10.29 | 1270.36 ± 120.09 |
| 0.972 ± 0.02 | 50 | 0.10 ± 0.01 | 12.30 ± 1.24 | 878.63 ± 79.09 |
| 1.161 ± 0.11 | 60 | 0.06 ± 0.01 | 26.44 ± 2.34 | 1144.49 ± 99.01 |
| Real-Time Ozone Concentration (mg/L) | Time (min) | Muscle Necrosis Virus (Copies/mL) | Vibrio (CFU/mL) | Total Bacteria (CFU/mL) |
|---|---|---|---|---|
| 0.684 ± 0.10 | 0 | 1132.57 ± 109.86 | 7992.12 ± 587.09 | 35,132.13 ± 875.04 |
| 0.746 ± 0.09 | 2 | 429.86 ± 34.49 | 1208.60 ± 107.43 | 6928.46 ± 219.21 |
| 0.802 ± 0.08 | 5 | 129.63 ± 21.08 | 150.08 ± 32.12 | 2788.16 ± 312.98 |
| 0.825 ± 0.12 | 10 | 45.28 ± 11.21 | 189.09 ± 12.09 | 1090.09 ± 109.23 |
| 0.871 ± 0.12 | 15 | 35.42 ± 4.02 | 223.02 ± 20.98 | 1480.45 ± 119.08 |
| 0.706 ± 0.11 | 20 | 9.12 ± 2.14 | 179.36 ± 43.20 | 1127.50 ± 120.32 |
| 0.801 ± 0.03 | 25 | 1.73 ± 0.43 | 61.15 ± 21.22 | 469.79 ± 109.21 |
| 0.761 ± 0.19 | 30 | 0.11 ± 0.02 | 16.12 ± 3.21 | 212.57 ± 34.09 |
| 0.770 ± 0.08 | 40 | ND | 0.82 ± 0.09 | 141.01 ± 21.08 |
| 0.513 ± 0.13 | 50 | ND | 0.96 ± 0.08 | 201.06 ± 11.24 |
| 0.344 ± 0.19 | 60 | ND | 1.15 ± 0.03 | 104.55 ± 31.69 |
| Parameters | Treatments | |||
|---|---|---|---|---|
| CW | CW + O | FLOC | FLOC + O | |
| T (°C) | 29.34 ± 0.53 | 29.34 ± 0.54 | 29.34 ± 0.55 | |
| Salinity (g L−1) | 28.65 ± 0.87 | 28.49 ± 0.88 | 28.96 ± 0.13 | 28.61 ± 0.90 |
| DO (g L−1) | 5.91 ± 0.09 | 6.21 ± 0.10 | 5.80 ± 0.21 | 6.01 ± 0.32 |
| pH | 8.20 ± 0.03 | 8.10 ± 0.02 | 8.21 ± 0.03 | 8.10 ± 0.04 |
| Parameters | Treatments | One-Way ANOVA | Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| CW | CW + O | FLOC | FLOC + O | P | P-O | P-FLOC | P-FLOC + O | |
| Initial | Nauplii | |||||||
| Final weight (mg) | 10.32 ± 1.47 a | 11.68 ± 1.22 b | 11.98 ± 2.13 b | 12.25 ± 1.54 b | 0.020 | 0.323 | 0.012 | 0.125 |
| Final length (mm) | 14.31 ± 1.21 a | 15.01 ± 1.19 b | 15.19 ± 0.41 b | 15.22 ± 1.04 b | 0.018 | 0.214 | 0.015 | 0.319 |
| SGR (%/day) | 7.5 ± 0.45 a | 7.9 ± 0.35 ab | 8.0 ± 0.37 b | 8.1 ± 0.43 b | 0.152 | p < 0.05 | 0.014 | 0.035 |
| FCR | 1.78 ± 0.10 a | 1.45 ± 0.23 b | 1.29 ± 0.22 c | 1.22 ± 0.13 c | 0.031 | p < 0.05 | 0.016 | 0.037 |
| Survival (%) | 40.32 ± 10.23 a | 60.21 ± 18.19 b | 80.55 ± 21.21 c | 82.12 ± 25.12 c | 0.0025 | p < 0.05 | 0.00015 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.; Luan, Y.; Wang, Z.; Niu, J.; Li, Y.; Tang, H.; Li, Z.; Liu, G. Integration of Biofloc and Ozone Nanobubbles for Enhanced Pathogen Control in Prenursery of Pacific White Shrimp (Penaeus vannamei). Fishes 2025, 10, 218. https://doi.org/10.3390/fishes10050218
Liang Q, Luan Y, Wang Z, Niu J, Li Y, Tang H, Li Z, Liu G. Integration of Biofloc and Ozone Nanobubbles for Enhanced Pathogen Control in Prenursery of Pacific White Shrimp (Penaeus vannamei). Fishes. 2025; 10(5):218. https://doi.org/10.3390/fishes10050218
Chicago/Turabian StyleLiang, Qinlang, Yazhi Luan, Zhengwen Wang, Jiangbo Niu, Yasong Li, Hua Tang, Zengting Li, and Gang Liu. 2025. "Integration of Biofloc and Ozone Nanobubbles for Enhanced Pathogen Control in Prenursery of Pacific White Shrimp (Penaeus vannamei)" Fishes 10, no. 5: 218. https://doi.org/10.3390/fishes10050218
APA StyleLiang, Q., Luan, Y., Wang, Z., Niu, J., Li, Y., Tang, H., Li, Z., & Liu, G. (2025). Integration of Biofloc and Ozone Nanobubbles for Enhanced Pathogen Control in Prenursery of Pacific White Shrimp (Penaeus vannamei). Fishes, 10(5), 218. https://doi.org/10.3390/fishes10050218






