Championing Line Breeding and Hybridization in Aquaculture to Safeguard Intellectual Property
Abstract
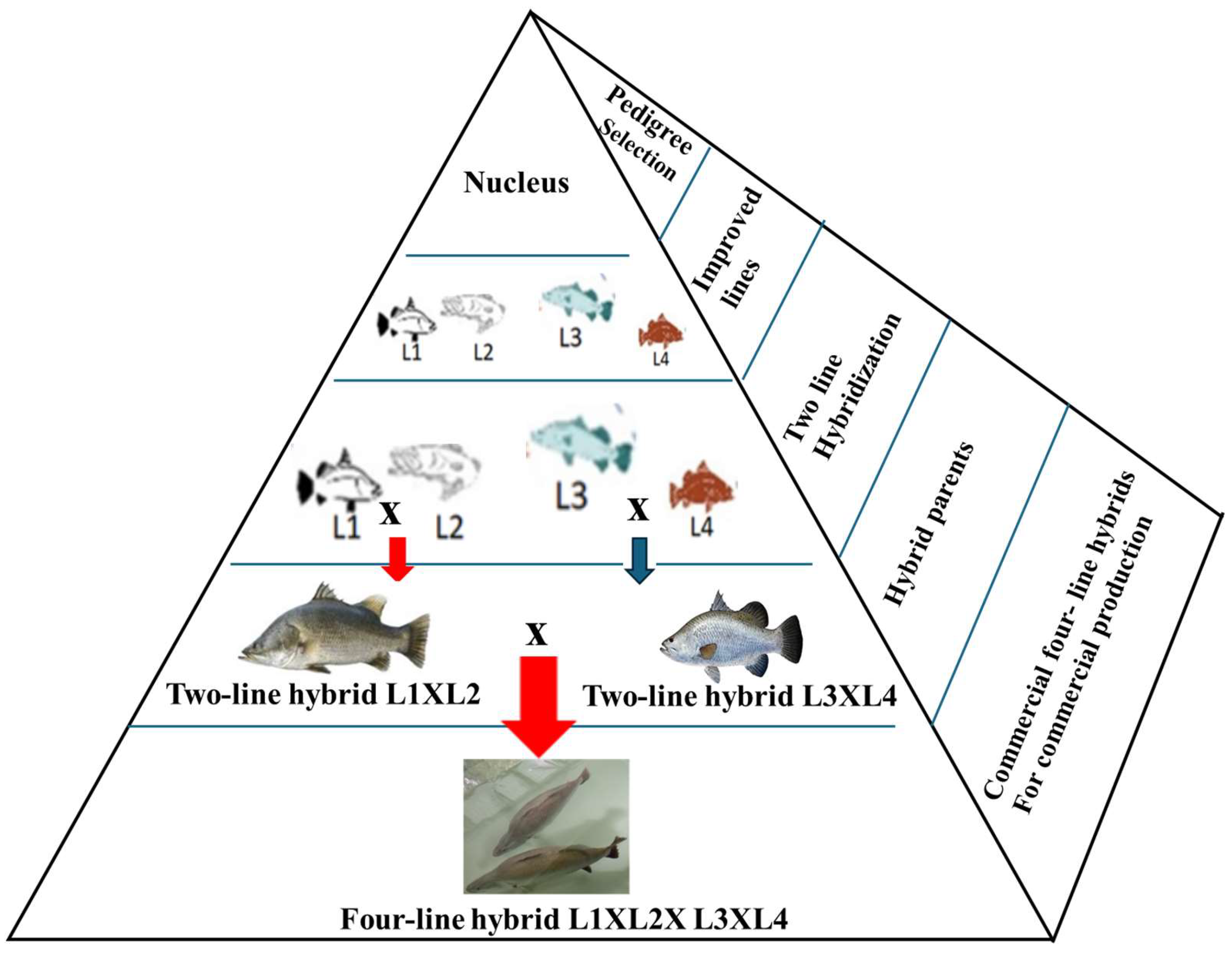
1. Introduction
2. Line Breeding and Line Hybridization: Lessons from Agriculture
| Plant Species | Type of Breeding Used | Key Traits Improved | Examples of Cultivars/Hybrids |
|---|---|---|---|
| Maize (Zea mays) | Line Hybridization | Yield, pest resistance, drought tolerance | Hybrid maize (e.g., Pioneer’s single-cross hybrids) |
| Rice (Oryza sativa) | Line Breeding and Hybridization | Pest resistance, grain yield, flood tolerance | IR8 (Green Revolution), hybrid rice varieties |
| Cotton (Gossypium spp.) | Line Breeding and Hybridization | Fiber quality, pest resistance (Bt cotton) | Bollgard® Bt cotton hybrids |
| Tomato (Solanum lycopersicum) | Line Hybridization | Shelf-life, disease resistance, flavor | Hybrid tomatoes (e.g., Better Boy, Big Beef) |
| Sorghum (Sorghum bicolor) | Line Hybridization | Drought resistance, yield | Hybrid sorghum varieties |
| Canola (Brassica napus) | Line Hybridization | Oil content, herbicide tolerance | Roundup Ready® canola hybrids |
| Sunflower (Helianthus annuus) | Line Hybridization | Oil content, disease resistance | Hybrid sunflower varieties |
| Sugarcane (Saccharum spp.) | Line Hybridization | Sugar content, disease resistance | Co varieties (e.g., Co 86032) |
| Millet (Pennisetum glaucum) | Line Hybridization | Drought tolerance, yield | Hybrid pearl millet varieties |
| Carrot (Daucus carota) | Line Hybridization | Root shape, color, disease resistance | Nantes-type hybrid carrots |
| Cucumber (Cucumis sativus) | Line Hybridization | Disease resistance, fruit quality | Hybrid cucumber varieties |
| Onion (Allium cepa) | Line Hybridization | Bulb size, storage life, disease resistance | Hybrid onions (e.g., Candy, Sweet Spanish) |
| Brinjal/Eggplant (Solanum melongena) | Line Hybridization | Yield, pest resistance | Hybrid eggplant varieties |
| Capsicum/Chili Pepper (Capsicum spp.) | Line Hybridization | Pungency, color, disease resistance | Hybrid pepper varieties |
| Cabbage (Brassica oleracea var. capitata) | Line Hybridization | Head compactness, disease resistance | Hybrid cabbage varieties |
| Species | Type of Breeding Used | Key Traits Improved | Examples of Lines/Hybrids |
|---|---|---|---|
| Chicken (Gallus gallus domesticus) | Line Breeding and Hybridization | Growth rate, egg production, feed efficiency, disease resistance | Broiler hybrids (e.g., Ross 308, Cobb 500), layer hybrids (e.g., Hy-Line, ISA Brown) |
| Turkey (Meleagris gallopavo) | Line Hybridization | Growth rate, meat yield, feed efficiency | Broad Breasted White hybrids |
| Duck (Anas platyrhynchos) | Line Breeding and Hybridization | Growth rate, egg production, disease resistance | Pekin duck hybrids (e.g., Cherry Valley) |
| Cattle (Bos taurus/Bos indicus) | Line Breeding and Hybridization | Milk yield, meat quality, disease resistance | Holstein-Friesian (dairy), Angus–Hereford crosses (beef), Brahman crossbreeds |
| Pig (Sus scrofa domesticus) | Line Breeding and Hybridization | Growth rate, meat quality, litter size | Large White × Landrace hybrids, Duroc terminal sires |
| Sheep (Ovis aries) | Line Breeding and Hybridization | Wool quality, meat production, reproductive efficiency | Merino (wool), Suffolk × Dorset crosses (meat) |
| Asian Seabass (Lates calcarifer) | Line Breeding and Hybridization | Growth rate, omega-3 content, disease resistance | Selected lines from genetic improvement programs |
| Tilapia (Oreochromis spp.) | Line Breeding and Hybridization | Growth rate, salinity tolerance, disease resistance | Genetically Improved Farmed Tilapia (GIFT) |
| Atlantic Salmon (Salmo salar) | Line Breeding and Hybridization | Growth rate, disease resistance, omega-3 content | AquaBounty’s fast-growing salmon, Mowi’s hybrid strains |
| Rainbow Trout (Oncorhynchus mykiss) | Line Breeding and Hybridization | Growth rate, disease resistance | Steelhead hybrids, all-female triploid trout |
| Shrimp (Litopenaeus vannamei) | Line Breeding | Growth rate, disease resistance, salinity tolerance | Specific Pathogen-Free (SPF) lines |
3. Breeding for Genetic Improvement: A Costly but Rewarding Endeavor
4. Line Breeding and Hybridization as IP Protection Strategies
5. Legal Frameworks and International Agreements
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IP | Intellectual property |
| MAS | Marker-assisted selection |
| GS | Genomic selection |
| GE | Genome editing |
References
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization (FAO): Rome, Italy, 2024. [Google Scholar]
- Houston, R.D.; Bean, T.P.; Macqueen, D.J.; Gundappa, M.K.; Jin, Y.H.; Jenkins, T.L.; Selly, S.L.C.; Martin, S.A.; Stevens, J.R.; Santos, E.M. Harnessing genomics to fast-track genetic improvement in aquaculture. Nat. Rev. Genet. 2020, 21, 389–409. [Google Scholar] [CrossRef]
- Yanez, J.M.; Barria, A.; Lopez, M.E.; Moen, T.; Garcia, B.F.; Yoshida, G.M.; Xu, P. Genome-wide association and genomic selection in aquaculture. Rev. Aquac. 2023, 15, 645–675. [Google Scholar] [CrossRef]
- Tixier-Boichard, M.; Leenstra, F.; Flock, D.; Hocking, P.; Weigend, S. A century of poultry genetics. World’s Poult. Sci. J. 2012, 68, 307–321. [Google Scholar] [CrossRef]
- Labroo, M.R.; Studer, A.J.; Rutkoski, J.E. Heterosis and hybrid crop breeding: A multidisciplinary review. Front. Genet. 2021, 12, 643761. [Google Scholar] [CrossRef]
- Yue, G.H. Recent advances of genome mapping and marker-assisted selection in aquaculture. Fish Fish. 2014, 15, 376–396. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Y.; Tay, Y.X.; Yue, G.H. Genome editing and its applications in genetic improvement in aquaculture. Rev. Aquac. 2022, 14, 178–191. [Google Scholar] [CrossRef]
- Yang, Z.T.; Fu, G.H.; Lee, M.; Yeo, S.; Yue, G.H. Genes for editing to improve economic traits in aquaculture fish species. Aquac. Fish. 2025, 10, 1–18. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Gjedrem, T. Selection and Breeding Programs in Aquaculture; Springer: Berlin/Heidelberg, Germany, 2005; Volume 2005. [Google Scholar]
- Castle, D. The Role of Intellectual Property Rights in Biotechnology Innovation; Edward Elgar Publishing: Cheltenham, UK, 2009. [Google Scholar]
- Dutfield, G. Seven Tales of a Patent. In Intellectual Property Rights and the Life Science Industries: A Twentieth Century History; Dutfield, G., Ed.; Routledge: London, UK, 2017; pp. 13–33. [Google Scholar]
- Hallauer, A.R.; Carena, M.J. Maize. In Cereals; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–98. [Google Scholar]
- Glover, K.A.; Otterå, H.; Olsen, R.E.; Slinde, E.; Taranger, G.L.; Skaala, Ø. A comparison of farmed, wild and hybrid Atlantic salmon (Salmo salar L.) reared under farming conditions. Aquaculture 2009, 286, 203–210. [Google Scholar] [CrossRef]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Hamzah, A.; Bakar, K.R.A.; Yee, H.Y. Genetic improvement of Nile tilapia (Oreochromis niloticus) with special reference to the work conducted by the WorldFish Center with the GIFT strain. Rev. Aquac. 2011, 3, 27–41. [Google Scholar] [CrossRef]
- Dunham, R.A.; Masser, M.P. Production of Hybrid Catfish; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2012; Volume 436, pp. 1–7. [Google Scholar]
- Zhang, Z.; Lu, C.K.; Lin, K.B.; You, W.W.; Yang, Z.W. Genetic diversity and genetic structure among four selected strains of whiteleg shrimp (Litopenaeus vannamei) using SSR markers. Fishes 2023, 8, 544. [Google Scholar] [CrossRef]
- Yue, G.H.; Zhu, Z.Y.; Lo, L.C.; Wang, C.M.; Lin, G.; Feng, F.; Pang, H.Y.; Li, J.; Gong, P.; Liu, H.M. Genetic variation and population structure of Asian seabass (Lates calcarifer) in the Asia-Pacific region. Aquaculture 2009, 293, 22–28. [Google Scholar] [CrossRef]
- Yue, G.H.; Guo, C.J. Strategies for managing major diseases in Asian seabass aquaculture. Anim. Dis. 2025, 5, 6. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, F.; Cheng, C.; Qian, Q. A historical review of hybrid rice breeding. J. Integr. Plant Biol. 2024, 66, 532–545. [Google Scholar] [CrossRef]
- Rauf, S.; Shehzad, M.; Al-Khayri, J.M.; Imran, H.M.; Noorka, I.R. Cotton (Gossypium hirsutum L.) breeding strategies. Adv. Plant Breed. Strateg. Ind. Food Crops 2019, 6, 29–59. [Google Scholar]
- Sharma, M.; Adarsh, M.; Kumari, P.; Thakur, M.; Kumar, R.; Sharma, R.; Gautam, N. Hybrid breeding in tomato. Int. J. Farm Sci. 2015, 5, 233–250. [Google Scholar]
- Nguyen, H.T. Sorghum improvement: Past achievements and future prospects. In Crop Improvement: Challenges in the Twenty-First Century; Kang, M.S., Ed.; Food Products Press: New York, NY, USA, 2024; pp. 109–139. [Google Scholar]
- Ton, L.B.; Neik, T.X.; Batley, J. The use of genetic and gene technologies in shaping modern rapeseed cultivars (Brassica napus L.). Genes 2020, 11, 1161. [Google Scholar] [CrossRef]
- Meena, H.; Sujatha, M. Sunflower breeding. In Fundamentals of Field Crop Breeding; Springer: Berlin/Heidelberg, Germany, 2022; pp. 971–1008. [Google Scholar]
- Yeo, S.; Lee, M.; Wang, L.; Endah, S.; Alhuda, N.; Yue, G. Microsatellite analysis of genetic diversity and relationships in 1027 sugarcane accessions. Sugar Tech 2023, 25, 1082–1091. [Google Scholar] [CrossRef]
- Wang, L.; Yeo, S.; Lee, M.; Endah, S.; Alhuda, N.; Yue, G. Combination of GWAS and F ST-based approaches identified loci associated with economic traits in sugarcane. Mol. Genet. Genom. 2023, 298, 1107–1120. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Bollam, S.; Pujarula, V.; Pusuluri, M.; Singh, R.B.; Potupureddi, G.; Gupta, R. Exploitation of heterosis in pearl millet: A review. Plants 2020, 9, 807. [Google Scholar] [CrossRef]
- Kiełkowska, A.; Kiszczak, W. History and current status of haploidization in carrot (Daucus carota L.). Agronomy 2023, 13, 676. [Google Scholar] [CrossRef]
- Jat, G.S.; Behera, T.K.; Lata, S.; Kumar, S. Classical genetics and traditional breeding in cucumber (Cucumis sativus L.). In Cucumber Economic Values and Its Cultivation and Breeding; IntechOpen: London, UK, 2021; pp. 201–215. [Google Scholar]
- Havey, M.J. Onion breeding. Plant Breed. Rev. 2018, 42, 39–85. [Google Scholar]
- Mishra, P.; Tiwari, S.K.; Tiwari, K.N. Genetic improvement of eggplant: Perspectives and challenges. Genet. Eng. Crop Plants Food Health Secur. 2024, 1, 123–149. [Google Scholar]
- Barboza, G.E.; García, C.C.; de Bem Bianchetti, L.; Romero, M.V.; Scaldaferro, M. Monograph of wild and cultivated chili peppers (Capsicum L., Solanaceae). PhytoKeys 2022, 200, 1. [Google Scholar] [CrossRef]
- Parmar, S.S.; Ravindra, I.H.; Kumar, R. Accelerated Approaches for Cabbage Improvement. In Recent Trends in Plant Breeding and Genetic Improvement; IntechOpen: London, UK, 2023. [Google Scholar]
- McCarthy, E.M. Handbook of Avian Hybrids of the World; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Lorenz, F.; Asmundson, V.; Wilson, N. Turkey hybrids: (Meleagris ocellata × Meleagris gallopaVo). J. Hered. 1956, 47, 143–146. [Google Scholar] [CrossRef]
- Liu, M.H.; Churchil, R.R. Duck genetics and breeding. In Duck Production and Management Strategies; Springer: Berlin/Heidelberg, Germany, 2022; pp. 97–156. [Google Scholar]
- Basiel, B.L.; Felix, T.L. Board invited review: Crossbreeding beef× dairy cattle for the modern beef production system. Transl. Anim. Sci. 2022, 6, txac025. [Google Scholar] [CrossRef]
- Mekonnen, T.; Tadesse, Y.; Meseret, S. Genetic improvement strategy of indigenous cattle breeds: Effect of cattle crossbreeding program in production performances. J. Appl. Life Sci. Int. 2020, 23, 23–40. [Google Scholar] [CrossRef]
- Craft, W. Fifty years of progress in swine breeding. J. Anim. Sci. 1958, 17, 960–980. [Google Scholar] [CrossRef]
- Rasali, D.; Shrestha, J.; Crow, G. Development of composite sheep breeds in the world: A review. Can. J. Anim. Sci. 2006, 86, 1–24. [Google Scholar]
- Yue, G.; Wang, L.; Sun, F.; Yang, Z.; Wong, J.; Wen, Y.; Pang, H.; Lee, M.; Yeo, S.; Liang, B. Improving growth, omega-3 contents, and disease resistance of Asian seabass: Status of a 20-year family-based breeding program. Rev. Fish Biol. Fish. 2024, 34, 91–110. [Google Scholar] [CrossRef]
- Moore, M.E.; Goetz, F.A.; Van Doornik, D.M.; Tezak, E.P.; Quinn, T.P.; Reyes-Tomassini, J.J.; Berejikian, B.A. Early marine migration patterns of wild coastal cutthroat trout (Oncorhynchus clarki clarki), steelhead trout (Oncorhynchus mykiss), and their hybrids. PLoS ONE 2010, 5, e12881. [Google Scholar] [CrossRef]
- Alday-Sanz, V.; Brock, J.; Flegel, T.W.; McIntosh, R.; Bondad-Reantaso, M.G.; Salazar, M.; Subasinghe, R. Facts, truths and myths about SPF shrimp in Aquaculture. Rev. Aquac. 2020, 12, 76–84. [Google Scholar] [CrossRef]
- Janssen, K.; Saatkamp, H.; Komen, H. Cost-benefit analysis of aquaculture breeding programs. Genet. Sel. Evol. 2018, 50, 2. [Google Scholar] [CrossRef]
- Wong, J.; Sun, F.; Wang, L.; Yang, Z.; Wen, Y.; Pang, H.; Lee, M.; Yeo, S.; Liang, B.; Chen, K. Changes in genetic diversity of Asian seabass in a 20-year breeding program. Aquaculture 2023, 575, 739738. [Google Scholar] [CrossRef]
- Duvick, D.N. Biotechnology in the 1930s: The development of hybrid maize. Nat. Rev. Genet. 2001, 2, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Saura, M.; Caballero, A.; Santiago, E.; Fernández, A.; Morales-González, E.; Fernández, J.; Cabaleiro, S.; Millán, A.; Martínez, P.; Palaiokostas, C. Estimates of recent and historical effective population size in turbot, seabream, seabass and carp selective breeding programmes. Genet. Sel. Evol. 2021, 53, 85. [Google Scholar] [CrossRef]
- Buck, M.; Hamilton, C. The Nagoya Protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the Convention on Biological Diversity. Rev. Eur. Community Int. Environ. Law 2011, 20, 47–61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, G.H. Championing Line Breeding and Hybridization in Aquaculture to Safeguard Intellectual Property. Fishes 2025, 10, 220. https://doi.org/10.3390/fishes10050220
Yue GH. Championing Line Breeding and Hybridization in Aquaculture to Safeguard Intellectual Property. Fishes. 2025; 10(5):220. https://doi.org/10.3390/fishes10050220
Chicago/Turabian StyleYue, Gen Hua. 2025. "Championing Line Breeding and Hybridization in Aquaculture to Safeguard Intellectual Property" Fishes 10, no. 5: 220. https://doi.org/10.3390/fishes10050220
APA StyleYue, G. H. (2025). Championing Line Breeding and Hybridization in Aquaculture to Safeguard Intellectual Property. Fishes, 10(5), 220. https://doi.org/10.3390/fishes10050220





