Dietary Supplementation of Astragalus Polysaccharides Modulates Growth Physiology, Metabolic Homeostasis, and Innate Immune Responses in Rice Field Eels (Monopterus albus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design and Feeding Management
2.3. Sample Collection and Analysis
2.3.1. Growth Indicators
2.3.2. Hematological Analysis
2.3.3. Lipid Metabolism Analysis
2.3.4. Antioxidant Capacity Assessment and Non-Specific Immunity Evaluation
2.4. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Hematological Parameters
3.3. Lipid Metabolism Parameters
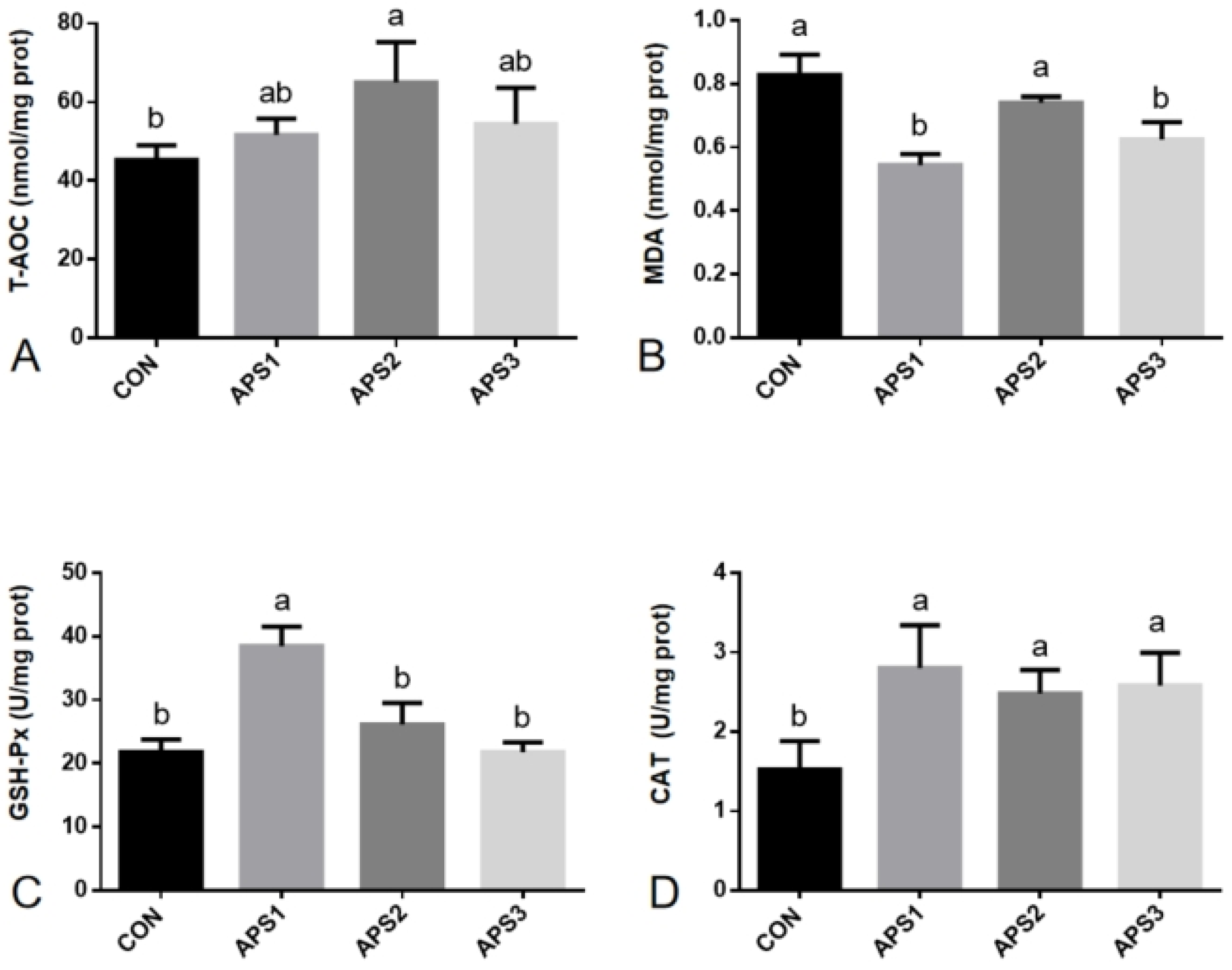
3.4. Antioxidant Capacity Parameters
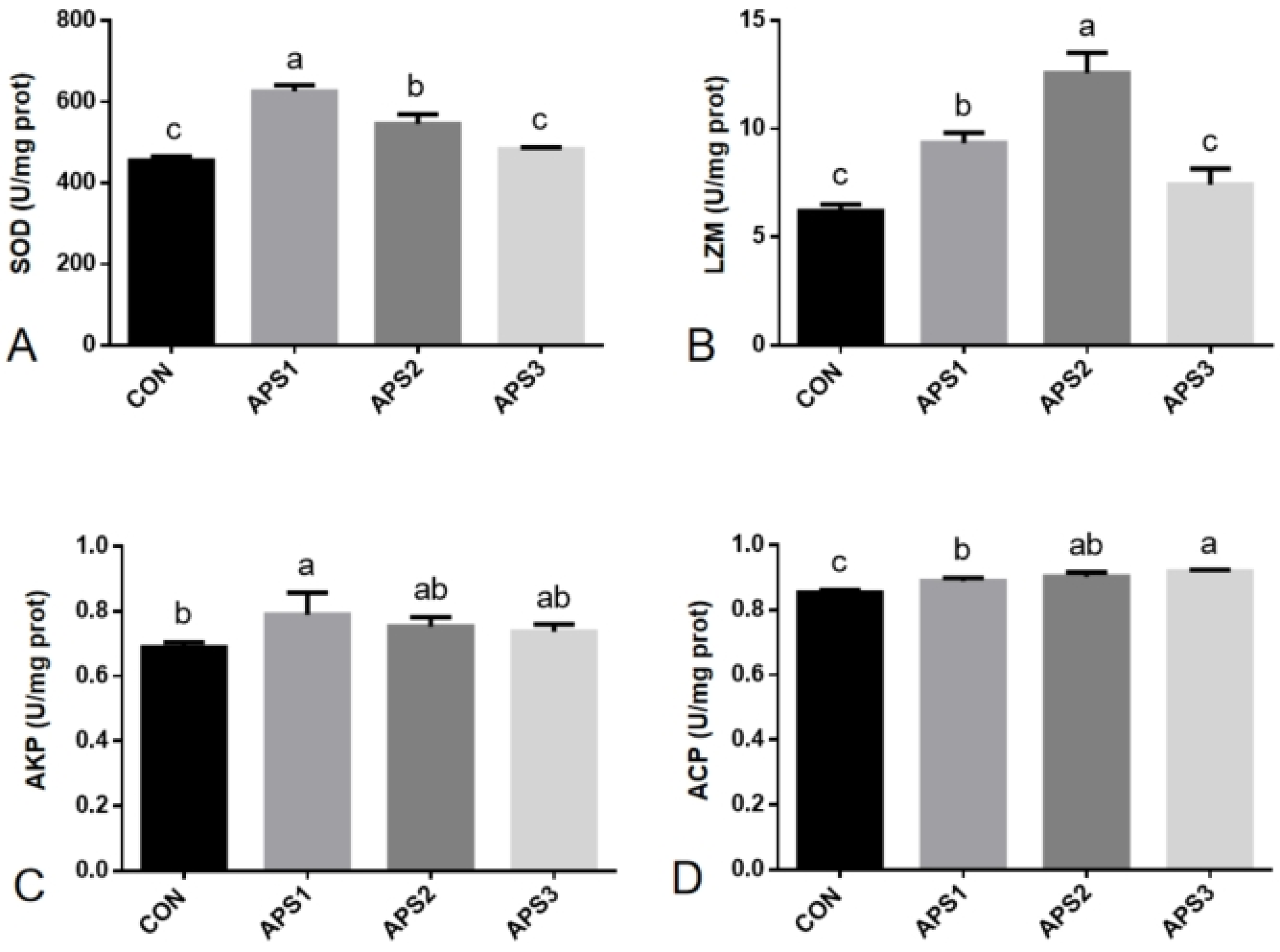
3.5. Non-Specific Immunity Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Agriculture and Rural Affairs. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2024; p. 25.
- Abdel-Latif, H.M.; Abdel-Daim, M.M.; Shukry, M.; Nowosad, J.; Kucharczyk, D. Benefits and applications of Moringa oleifera as a plant protein source in aquafeed: A review. Aquaculture 2022, 547, 737369. [Google Scholar] [CrossRef]
- Shao, B.M.; Xu, W.; Dai, H.; Tu, P.; Li, Z.; Gao, X.M. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem. Biophys. Res. Commun. 2004, 320, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, W.; Zhang, J.; Song, X.; Sun, W.; Fan, Y. The immunoregulatory activities of astragalus polysaccharide liposome on macrophages and dendritic cells. Int. J. Biol. Macromol. 2017, 105, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Yang, W.R.; Yang, H.W.; Wang, Y.; Yang, Z.B.; Jiang, S.Z.; Zhang, G.G. Effects of Astragalus membranaceus on growth performance, carcass characteristics, and antioxidant status of broiler chickens. Acta Agric. Scand. Sect. A 2010, 60, 151–158. [Google Scholar]
- Gu, C.; Zeng, Y.; Tang, Z.; Wang, C.; He, Y.; Feng, X.; Zhou, L. Astragalus polysaccharides affect insulin resistance by regulating the hepatic SIRT1-PGC-1α/PPARα-FGF21 signaling pathway in male Sprague Dawley rats undergoing catch-up growth. Mol. Med. Rep. 2015, 12, 6451–6460. [Google Scholar] [CrossRef]
- Elabd, H.; Wang, H.P.; Shaheen, A.; Yao, H.; Abbass, A. Astragalus membranaceus (AM) enhances growth performance and antioxidant stress profiles in bluegill sunfish (Lepomis macrochirus). Fish Physiol. Biochem. 2016, 42, 955–966. [Google Scholar] [CrossRef]
- Wu, S. Dietary Astragalus membranaceus polysaccharide ameliorates the growth performance and innate immunity of juvenile crucian carp (Carassius auratus). Int. J. Biol. Macromol. 2020, 149, 877–881. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E.; AbdelHamid, F.; Mahgoub, H.A.; Ibrahim, T. Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immun. 2014, 38, 149–157. [Google Scholar] [CrossRef]
- Lin, S.M.; Jiang, Y.; Chen, Y.J.; Luo, L.; Doolgindachbaporn, S.; Yuangsoi, B. Effects of Astragalus polysaccharides (APS) and chitooligosaccharides (COS) on growth, immune response and disease resistance of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immun. 2017, 70, 40–47. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, Y.; Xu, N.; Ding, T.; Cui, K.; Chen, Q.; Zhang, J.; Fang, W.; Mai, K.; Ai, Q. Effects of dietary Astragalus polysaccharides (APS) on survival, growth performance, activities of digestive enzyme, antioxidant responses and intestinal development of large yellow croaker (Larimichthys crocea) larvae. Aquaculture 2020, 517, 734752. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Zhou, H.; Mai, K.; He, G. Dietary Astragalus polysaccharides ameliorates the growth performance, antioxidant capacity and immune responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immun. 2020, 99, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.; Ahmed, H.A.; Shukry, M.; Chaklader, M.R.; Saleh, R.M.; Khallaf, M.A. Astragalus membranaceus extract (AME) enhances growth, digestive enzymes, antioxidant capacity, and immunity of Pangasianodon hypophthalmus juveniles. Fishes 2022, 7, 319. [Google Scholar] [CrossRef]
- Chang, Z.Q.; Ge, Q.Q.; Sun, M.; Wang, Q.; Lv, H.Y.; Li, J. Immune responses by dietary supplement with Astragalus polysaccharides in the Pacific white shrimp, Litopenaeus vannamei. Aquacult. Nutr. 2018, 24, 702–711. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, G.; Pan, J.; Li, Y.; Lu, Q.; Zhou, J.; Li, X. Effects of Astragalus polysaccharides on antioxidant abilities and non-specific immune responses of Chinese mitten crab, Eriocheir sinensis. Aquacult. Int. 2017, 25, 1333–1343. [Google Scholar] [CrossRef]
- Che, C.B.; Shi, Y.; Hu, Y. Effects of two kinds of composite immunopotentiators on growth, immunity and physiological and biochemical indexes of rice field eel (Monopterus albus). Feed Ind. 2020, 41, 43–48. [Google Scholar]
- Jin, M.; Zhao, K.; Huang, Q.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2014, 64, 257–266. [Google Scholar] [CrossRef]
- Liu, H.; Liu, K.; Fu, H.; Ji, R.; Qu, X. Sunlight mediated cadmium release from colored microplastics containing cadmium pigment in aqueous phase. Environ. Pollut. 2020, 263, 114484. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.; Wang, B.; Lu, Y.; Li, L.; Li, Y.; Liu, S. Evaluation of the effects of Astragalus polysaccharides as immunostimulants on the immune response of crucian carp and against SVCV in vitro and in vivo. Comp. Biochem. Phys. C 2022, 253, 109249. [Google Scholar] [CrossRef]
- Ren, F.; Qian, X.H.; Qian, X.L. Astragalus polysaccharide upregulates hepcidin and reduces iron overload in mice via activation of p38 mitogen-activated protein kinase. Biophys. Res. Commun. 2016, 472, 163–168. [Google Scholar] [CrossRef]
- Giardina, B. Hemoglobin: Multiple molecular interactions and multiple functions. An example of energy optimization and global molecular organization. Mol. Asp. Med. 2022, 84, 101040. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Van Doan, H.; Tapingkae, W.; Balasundaram, C.; Arockiaraj, J.; Ringø, E. Changes in immune genes expression, immune response, digestive enzymes-antioxidant status, and growth of catla (Catla catla) fed with Astragalus polysaccharides against edwardsiellosis disease. Fish Shellfish Immun. 2022, 121, 418–436. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Tao, Y.F.; Bao, J.W.; Chen, D.J.; Li, H.X.; He, J.; Xu, P. High fat diet-induced miR-122 regulates lipid metabolism and fat deposition in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) liver. Front. Physiol. 2018, 9, 1422. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, C.; Wen, H.; Zhang, C.; Jiang, M.; Liu, W.; Tian, J.; Yu, L.; Lu, X. Improving low-temperature stress tolerance of tilapia, Oreochromis niloticus: A functional analysis of Astragalus membranaceus. J. World Aquacult. Soc. 2019, 50, 749–762. [Google Scholar] [CrossRef]
- Li, B.; Hong, Y.; Gu, Y.; Ye, S.; Hu, K.; Yao, J.; Ding, K.; Zhao, A.; Jia, W.; Li, H. Functional metabolomics reveals that Astragalus polysaccharides improve lipids metabolism through microbial metabolite 2-hydroxybutyric acid in obese mice. Engineering 2022, 9, 111–122. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Khalil, S.R.; Moustafa, A.A.; Mahmoud, H.K.; Abdel-Latif, H.M. Astragalus membranaceus polysaccharides modulate growth, hemato-biochemical indices, hepatic antioxidants, and expression of HSP70 and apoptosis-related genes in Oreochromis niloticus exposed to sub-lethal thallium toxicity. Fish Shellfish Immun. 2021, 118, 251–260. [Google Scholar] [CrossRef]
- Li, M.; Qiang, J.; Zhu, X.; Bao, J.; Tao, Y.; Zhu, H. Effect of Siberian ginseng water extract as a dietary additive on growth performance, blood biochemical indexes, lipid metabolism, and expression of PPARs pathway-related genes in genetically improved farmed tilapia (Oreochromis niloticus). Fishes 2022, 7, 149. [Google Scholar] [CrossRef]
- Xu, D.; Han, P.; Xia, L.; Gan, J.; Xu, Q. A comparative transcriptome analysis focusing on immune responses of Asian swamp eel following infection with Aeromonas hydrophila. Aquaculture 2021, 539, 736655. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.L.; Yan, Y.Y.; Zhang, C.X.; Ye, J.D.; Lu, K.L.; Hu, L.H.; Zhang, J.J.; Ruan, L.; Sun, Y.Z. Effects of fructooligosaccharide on growth, immunity and intestinal microbiota of shrimp (Litopenaeus vannamei) fed diets with fish meal partially replaced by soybean meal. Aquacult. Nutr. 2019, 25, 194–204. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Cheng, A.; Hao, E.; Huang, X.; Chen, X. Immunomodulatory and antioxidant effects of Astragalus polysaccharide liposome in large yellow croaker (Larimichthys crocea). Fish Shellfish Immun. 2020, 100, 126–136. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Khalil, S.R.; El-Hady, E.W.; Elhady, W.M.; Ismail, T.A.; Marini, C.; Cerbo, A.; Abdel-Latif, H.M. Immunosuppressive effects of thallium toxicity in Nile Tilapia fingerlings: Elucidating the rescue role of Astragalus membranaceus polysaccharides. Front. Vet. Sci. 2022, 9, 843031. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Wang, Z.; Yu, S.; Long, T.; Zhou, X.; Bao, Y. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci. Rep. 2017, 7, 44822. [Google Scholar]
| Item | CON | APS1 | APS2 | APS3 |
|---|---|---|---|---|
| Initial weight (g) | 25.28 ± 0.09 a | 25.44 ± 0.10 a | 25.28 ± 0.10 a | 25.06 ± 0.10 b |
| Final weight (g) | 34.07 ± 0.42 b | 39.61 ± 0.42 a | 40.11 ± 0.26 a | 34.28 ± 0.81 b |
| WG (%) | 34.80 ± 1.24 b | 55.68 ± 1.12 a | 58.70 ± 0.52 a | 36.82 ± 3.62 b |
| FCR | 2.80 ± 0.20 a | 2.01 ± 0.08 b | 2.01 ± 0.04 b | 2.92 ± 0.17 a |
| SR (%) | 95.56 ± 1.93 | 97.78 ± 1.92 | 97.78 ± 0.96 | 95.00 ± 1.67 |
| Item | CON | APS1 | APS2 | APS3 |
|---|---|---|---|---|
| WBC (109/L) | 165.33 ± 16.19 | 168.07 ± 5.63 | 162.90 ± 7.11 | 166.77 ± 6.21 |
| RBC (1012/L) | 1.74 ± 0.09 | 1.76 ± 0.11 | 1.71 ± 0.04 | 1.76 ± 0.34 |
| HGB (g/L) | 140.67 ± 2.08 b | 168.67 ± 10.26 a | 173.00 ± 8.72 a | 146.33 ± 4.93 b |
| HCT (%) | 21.70 ± 1.35 | 21.53 ± 2.07 | 22.70 ± 0.66 | 22.43 ± 0.61 |
| PLT (109/L) | 48.33 ± 1.53 | 51.67 ± 3.22 | 53.00 ± 4.36 | 50.33 ± 7.37 |
| MPV (fL) | 6.10 ± 0.26 | 6.23 ± 0.68 | 6.40 ± 0.36 | 6.13 ± 0.68 |
| PDW (%) | 19.33 ± 0.50 | 19.20 ± 0.46 | 19.23 ± 0.35 | 19.40 ± 0.36 |
| Item | CON | APS1 | APS2 | APS3 |
|---|---|---|---|---|
| TG (mmol/L) | 3.53 ± 1.25 a | 2.75 ± 0.08 ab | 2.00 ± 0.34 bc | 1.20 ± 0.05 c |
| CHO (mmol/L) | 3.69 ± 0.26 a | 3.41 ± 0.21 a | 3.47 ± 0.22 a | 2.53 ± 0.26 b |
| HDL (mmol/L) | 0.58 ± 0.06 c | 1.47 ± 0.11 a | 1.62 ± 0.16 a | 0.93 ± 0.15 b |
| LDL (mmol/L) | 1.56 ± 0.38 a | 1.49 ± 0.18 a | 1.13 ± 0.04 ab | 0.73 ± 0.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Yang, H.; Yang, Y.; Yuan, Q.; Lv, W.; Ayana, G.U.; Li, M.; Su, D.; Zhou, W.; Zhang, Q. Dietary Supplementation of Astragalus Polysaccharides Modulates Growth Physiology, Metabolic Homeostasis, and Innate Immune Responses in Rice Field Eels (Monopterus albus). Fishes 2025, 10, 213. https://doi.org/10.3390/fishes10050213
Wu C, Yang H, Yang Y, Yuan Q, Lv W, Ayana GU, Li M, Su D, Zhou W, Zhang Q. Dietary Supplementation of Astragalus Polysaccharides Modulates Growth Physiology, Metabolic Homeostasis, and Innate Immune Responses in Rice Field Eels (Monopterus albus). Fishes. 2025; 10(5):213. https://doi.org/10.3390/fishes10050213
Chicago/Turabian StyleWu, Chengcheng, Hang Yang, Yutong Yang, Quan Yuan, Weiwei Lv, Gelana Urgesa Ayana, Mingyou Li, Di Su, Wenzong Zhou, and Qinghua Zhang. 2025. "Dietary Supplementation of Astragalus Polysaccharides Modulates Growth Physiology, Metabolic Homeostasis, and Innate Immune Responses in Rice Field Eels (Monopterus albus)" Fishes 10, no. 5: 213. https://doi.org/10.3390/fishes10050213
APA StyleWu, C., Yang, H., Yang, Y., Yuan, Q., Lv, W., Ayana, G. U., Li, M., Su, D., Zhou, W., & Zhang, Q. (2025). Dietary Supplementation of Astragalus Polysaccharides Modulates Growth Physiology, Metabolic Homeostasis, and Innate Immune Responses in Rice Field Eels (Monopterus albus). Fishes, 10(5), 213. https://doi.org/10.3390/fishes10050213









