Effects of Astragalus–Ginseng Dietary Supplementation on the Growth and Stress Resistance of Yellow Catfish (Pelteobagrus fulvidraco)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Experimental Animals
2.3. Growth Performance Assay
- Weight gain (WG) = (FBW − IBW) × 100%
- Weight gain rate (WGR) = (FBW − IBW)/IBW × 100%
- Specific growth rate (SGR) = (lnFBW − lnIBW)/Days of test × 100%
- Viscera-somatic index (VSI) = (Visceral Weight/FBW − Visceral Weight) × 100%
- Hepato-somatic index (HSI) = (Liver Weight/FBW − Liver Weight) × 100%
2.4. Sample Collection
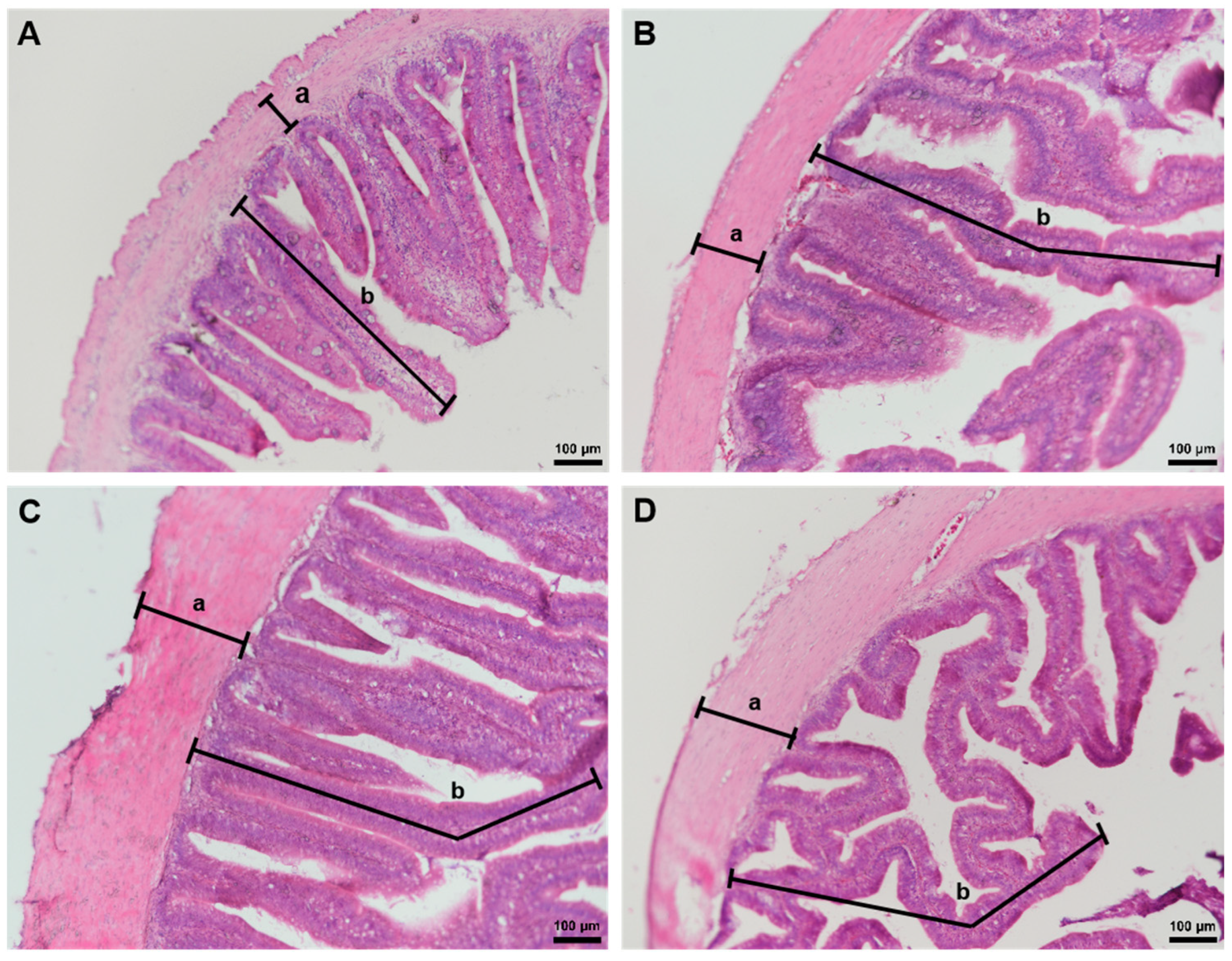
2.5. Intestinal Microvillus Morphology
2.6. Enzyme Activity Assay
2.7. Quantitative Real-Time PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Intestinal Morphology
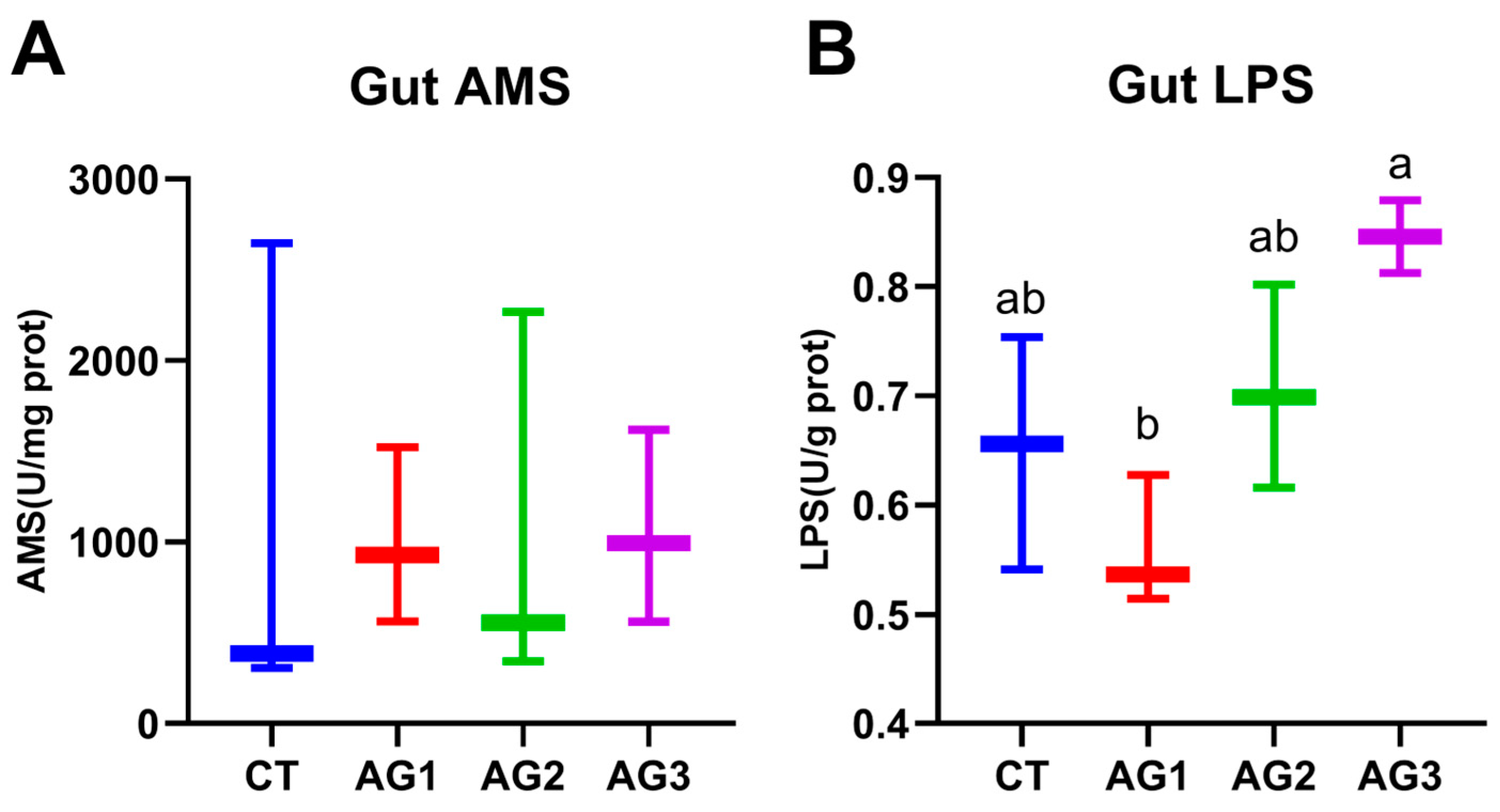
3.3. Digestive Enzyme Activity
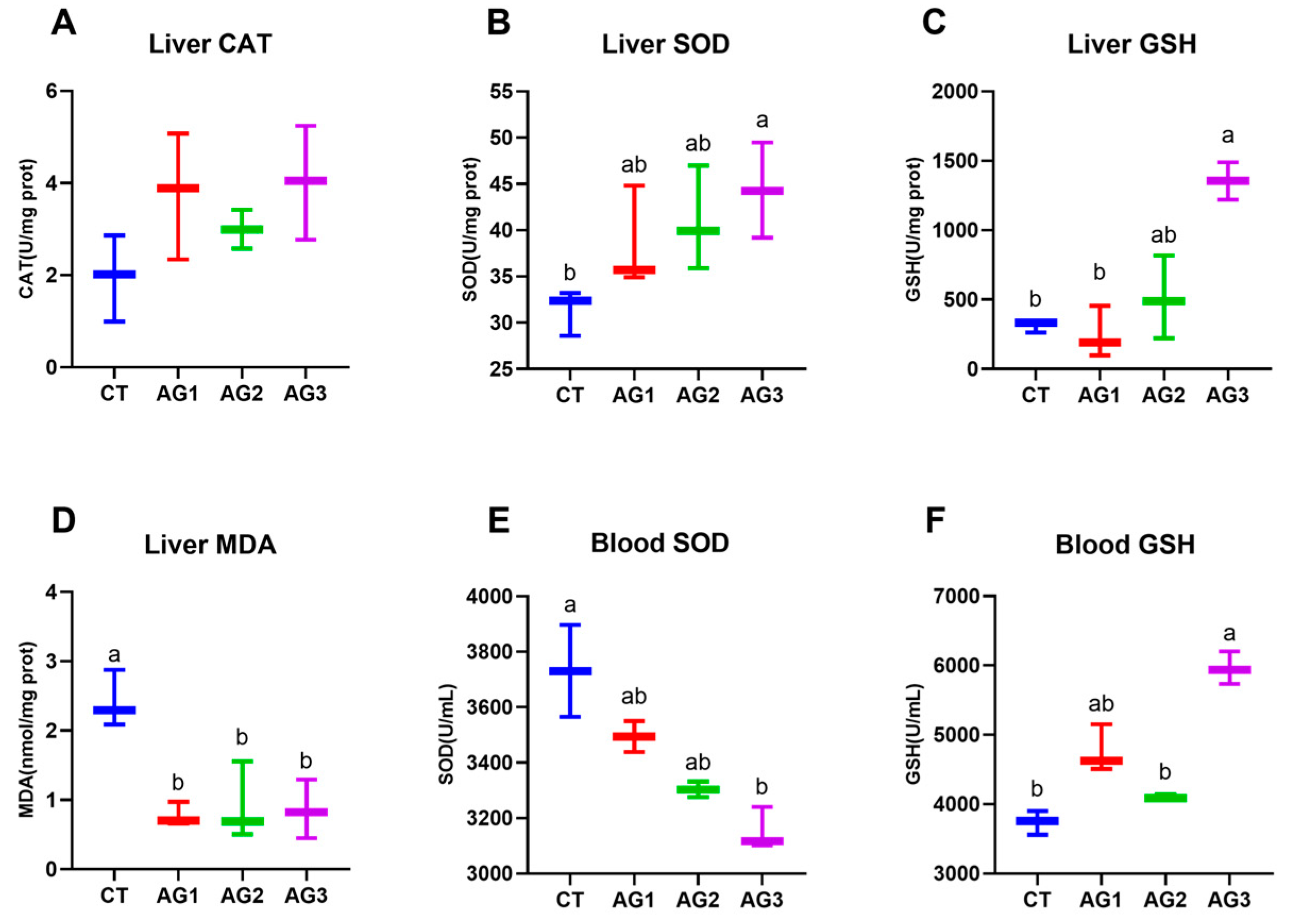
3.4. Antioxidant Enzyme Activity
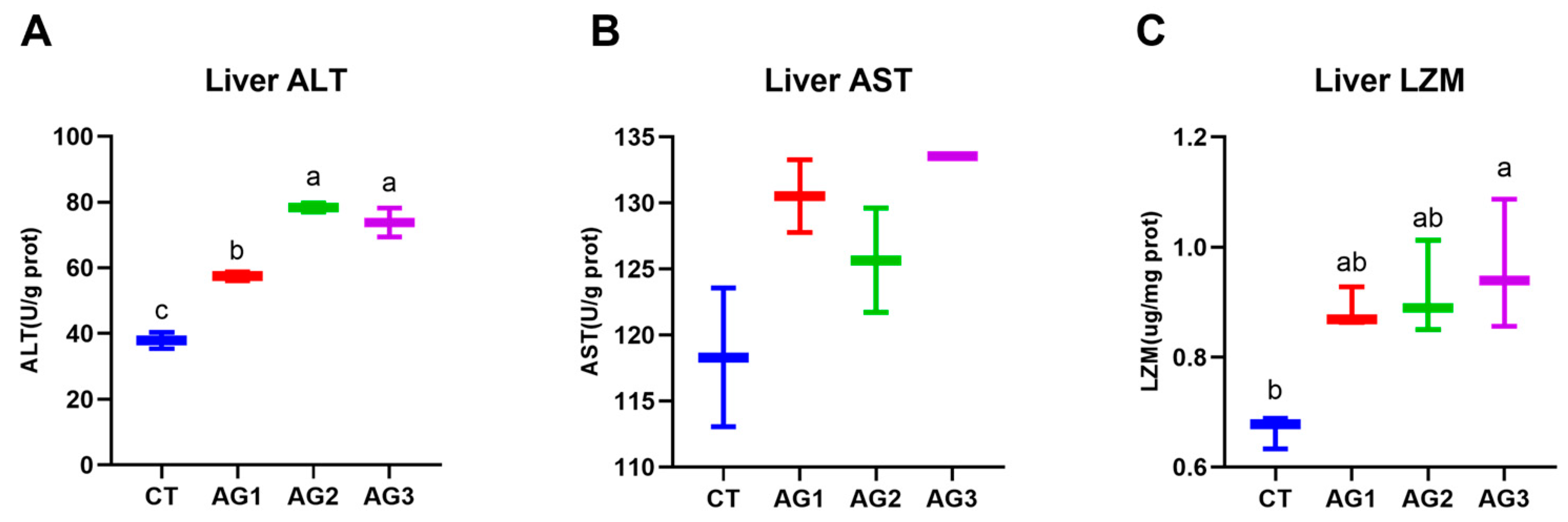
3.5. Immune-Related Enzyme Activity
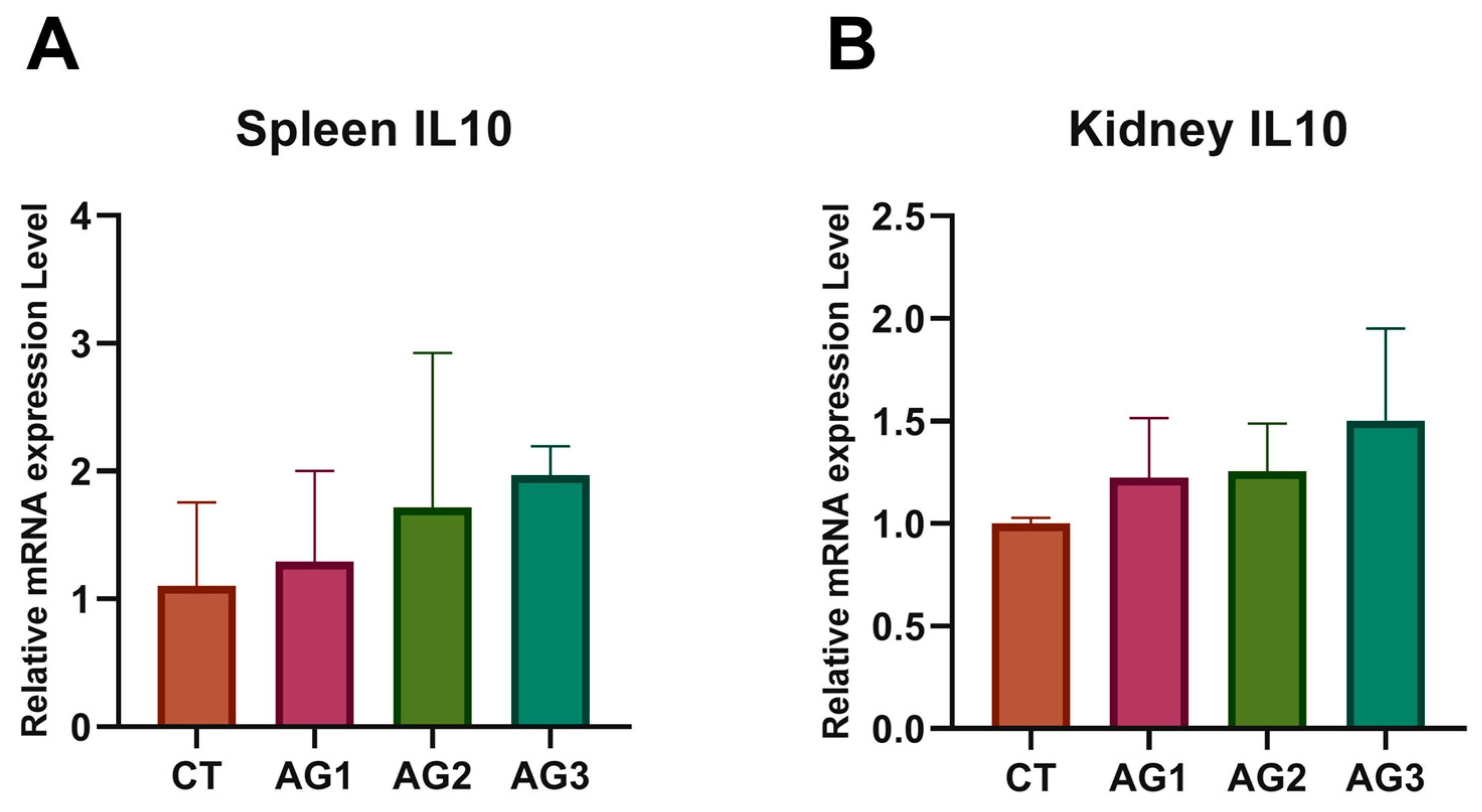
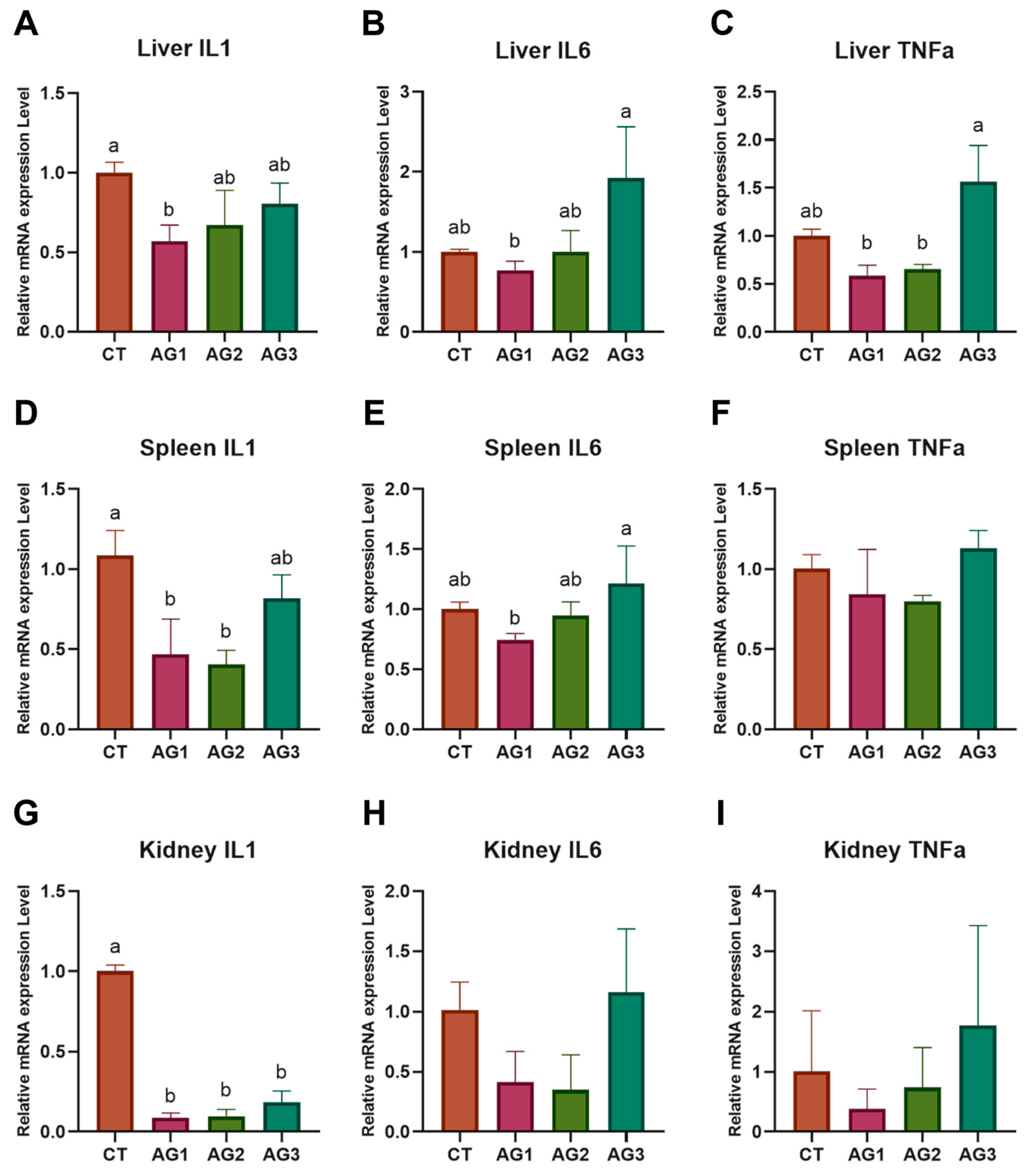
3.6. Immune-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Genes | Forward Primer | Reverse Primer | Accession No. |
|---|---|---|---|
| IL-1β | 5-CCTGTGTGTTTGGGGATTG-3 | 5-CCTTGATGGTCTTTAGGCTCT-3 | MF770571.1 |
| IL-6 | 5-CCATCGGAGGAACACAGA-3 | 5-GTAGATAAGGCGCAGACATT-3 | XM_027176013.2 |
| IL-10 | 5-TCTGTAGGTTCCTCCTGCTT-3 | 5-AGGTCATCCTTGGATTCGT-3 | XM_027144360.2 |
| TNF-α | 5-CAGGCAAACACACAAAGGC-3 | 5-GAGAAAGCTCCGAAAAACG-3 | XM_027133763.2 |
| β-actin | 5-CCTAAAGCCAACAGGGAAAAG-3 | 5-GTCACGGCCAGCCAAATC-3 | XM_027148463.2 |
Appendix B
References
- Li, X.P.; Li, J.R.; Wang, Y.B.; Fu, L.L.; Fu, Y.Y.; Li, B.Q.; Jiao, B.H. Aquaculture Industry in China: Current State, Challenges, and Outlook. Rev. Fish. Sci. 2011, 19, 187–200. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Arthur, J.R.; Ogawa, K.; Chinabut, S.; Adlard, R.; Tan, Z.; Shariff, M. Disease and health management in Asian aquaculture. Vet. Parasitol. 2005, 132, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, K.D.; Harvell, C.D.; Conrad, J.M.; Friedman, C.S.; Kent, M.L.; Kuris, A.M.; Powell, E.N.; Rondeau, D.; Saksida, S.M. Infectious Diseases Affect Marine Fisheries and Aquaculture Economics. Annu. Rev. Mar. Sci. 2015, 7, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dolz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Tan, B.K.H.; Vanitha, J. Immunomodulatory and antimicrobial effects of some traditional Chinese medicinal herbs: A review. Curr. Med. Chem. 2004, 11, 1423–1430. [Google Scholar] [CrossRef]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S60–S84. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef]
- Liu, D.; Tang, W.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Monosaccharide composition analysis of polysaccharides from natural sources: Hydrolysis condition and detection method development. Food Hydrocoll. 2021, 116, 106641. [Google Scholar] [CrossRef]
- Xu, D.-J.; Xia, Q.; Wang, J.-J.; Wang, P.-P. Molecular Weight and Monosaccharide Composition of Astragalus Polysaccharides. Molecules 2008, 13, 2408–2415. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-G.; Chen, Y.; Zhang, Y.-Q. Effects of Astragalus polysaccharide on nephritis induced by cationic bovine serum albumin in rats. J. Chin. Med. Mater. 2010, 33, 1913–1916. [Google Scholar]
- Shao, B.M.; Xu, W.; Dai, H.; Tu, P.; Li, Z.; Gao, X.M. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem. Biophys. Res. Commun. 2004, 320, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Zhao, X.J.; Wang, J.Y. Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poult. Sci. 2009, 88, 519–525. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E.; AbdelHamid, F.; Mahgoub, H.A.; Ibrahim, T. Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2014, 38, 149–157. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology—Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Attele, A.S.; Zhou, Y.P.; Xie, J.T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef]
- Mochizuki, M.; Yoo, Y.C.; Matsuzawa, K.; Sato, K.; Saiki, I.; Tonooka, S.; Samukawa, K.; Azuma, I. Inhibitory Effect of Tumor-Metastasis in Mice by Saponins, Ginsenoside-Rb2, 20(R)-Ginsenoside-Rg3 and 20(S)-Ginsenoside-Rg3, of Red-ginseng. Biol. Pharm. Bull. 1995, 18, 1197–1202. [Google Scholar] [CrossRef]
- Shibata, S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J. Korean Med. Sci. 2001, 16, S28–S37. [Google Scholar] [CrossRef]
- Fu, Y.; Ji, L.L. Chronic ginseng consumption attenuates age-associated oxidative stress in rats. J. Nutr. 2003, 133, 3603–3609. [Google Scholar] [CrossRef]
- Jung, S.; Lee, M.-S.; Shin, Y.; Kim, C.-T.; Kim, I.-H.; Kim, Y. High Hydrostatic Pressure Extract of Red Ginseng Attenuates Inflammation in Rats with High-fat Diet Induced Obesity. Prev. Nutr. Food Sci. 2015, 20, 253–259. [Google Scholar] [CrossRef]
- Uluisik, D.; Keskin, E. The Effects of Ginseng and Echinacea on Some Plasma Cytokine Levels in Rats. Kafkas Univ. Vet. Fak. Derg. 2012, 18, 65–68. [Google Scholar]
- Ovodov, Y.S.; Solov’eva, T.F. Polysaccharides ofPanax ginseng. Chem. Nat. Compd. 1966, 2, 243–245. [Google Scholar] [CrossRef]
- Sun, L.; Wu, D.; Ning, X.; Yang, G.; Lin, Z.; Tian, M.; Zhou, Y. α-Amylase-assisted extraction of polysaccharides from Panax ginseng. Int. J. Biol. Macromol. 2015, 75, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, L.; Bi, H.; Li, X.; Ni, W.; Han, H.; Li, N.; Wang, B.; Zhou, Y.; Tai, G. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. Carbohydr. Polym. 2009, 77, 544–552. [Google Scholar] [CrossRef]
- Jiang, X.L.; Ma, G.F.; Zhao, B.B.; Meng, Y.; Chen, L.L. Structural characterization and immunomodulatory activity of a novel polysaccharide from Panax notoginseng. Front. Pharmacol. 2023, 14, 1190233. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, M.; Shimada, K.; Konno, C.; Sugiyama, K.; Hikino, H.J.P.M. Partial structure of panaxan A, a hypoglycaemic glycan of Panax ginseng roots. Planta Medica 1984, 50, 436–438. [Google Scholar] [CrossRef]
- Cheng, H.; Li, S.; Fan, Y.; Gao, X.; Hao, M.; Wang, J.; Zhang, X.; Tai, G.; Zhou, Y. Comparative studies of the antiproliferative effects of ginseng polysaccharides on HT-29 human colon cancer cells. Med. Oncol. 2011, 28, 175–181. [Google Scholar] [CrossRef]
- Na, H.S.; Lim, Y.-J.; Yun, Y.S.; Choi, Y.H.; Oh, J.S.; Rhee, J.H.; Lee, H.C. Protective Effect of Ginsan Against Vibrio vulnificus Infection. J. Bacteriol. Virol. 2009, 39, 113–118. [Google Scholar] [CrossRef][Green Version]
- He, Z.; Wang, X.; Li, G.; Zhao, Y.; Zhang, J.; Niu, C.; Zhang, L.; Zhang, X.; Ying, D.; Li, S. Antioxidant activity of prebiotic ginseng polysaccharides combined with potential probiotic Lactobacillus plantarum C88. Int. J. Food Sci. Technol. 2015, 50, 1673–1682. [Google Scholar] [CrossRef]
- Li, Y.G.; Dong, X.H.; Zhang, Y.L.; Xiao, T.Y.; Zhao, Y.R.; Wang, H.Q. Astragalus polysaccharide improves the growth, meat quality, antioxidant capacity and bacterial resistance of Furong crucian carp (Furong carp♀ × red crucian carp♂). Int. J. Biol. Macromol. 2023, 244, 124999. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Liu, X.Y.; Xia, C.G.; Li, M.Y.; Niu, X.T.; Wang, G.Q.; Zhang, D.M. Effects of dietary Astragalus propinquus Schischkin polysaccharides on growth performance, immunological parameters, antioxidants responses and inflammation-related gene expression in Channa argus. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2021, 249, 109121. [Google Scholar] [CrossRef]
- Sun, Y.K.; Wang, X.; Zhou, H.H.; Mai, K.S.; He, G. Dietary Astragalus polysaccharides ameliorates the growth performance, antioxidant capacity and immune responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2020, 99, 603–608. [Google Scholar] [CrossRef]
- Pan, Y.C.; Liu, Z.; Quan, J.Q.; Gu, W.; Wang, J.W.; Zhao, G.Y.; Lu, J.H.; Wang, J.F. Purified Astragalus Polysaccharide Combined with Inactivated Vaccine Markedly Prevents Infectious Haematopoietic Necrosis Virus Infection in Rainbow Trout (Oncorhynchus mykiss). ACS Biomater. Sci. Eng. 2024, 10, 6938–6953. [Google Scholar] [CrossRef]
- Lin, G.X.; Da, F.; Wan, X.J.; Huang, Y.C.; Yang, S.P.; Jian, J.C.; Cai, S.H. Immune-enhancing effects of Astragalus polysaccharides and Ganoderma lucidum polysaccharides on Vibrio harveyi flgJ DNA vaccine in grouper. J. Fish Dis. 2023, 46, 147–156. [Google Scholar] [CrossRef]
- Bulfon, C.; Bongiorno, T.; Messina, M.; Volpatti, D.; Tibaldi, E.; Tulli, F. Effects of Panax ginseng extract in practical diets for rainbow trout (Oncorhynchus mykiss) on growth performance, immune response and resistance to Yersinia ruckeri. Aquac. Res. 2017, 48, 2369–2379. [Google Scholar] [CrossRef]
- In-Chul, B.; Kwon, M.G.; Cho, S.H. Effects of Dietary Inclusion of Red Ginseng Byproduct on Growth, Body Composition, Serum Chemistry, and Lysozyme Activity in Juvenile Olive Flounder (Paralichthys olivaceus). Fish. Aquat. Sci. 2010, 13, 300–307. [Google Scholar]
- Shao, J.C.; Wang, X.X.; Liu, Q.Q.; Lv, H.Y.; Qi, Q.; Li, C.H.; Zhang, J.N.; Chen, X.J.; Chen, X.H. Eucommia ulmoides leaf extracts combined with Astragalus polysaccharides: Effects on growth, antioxidant capacity, and intestinal inflammation in juvenile large yellow croaker (Larimichthys crocea). Fish Shellfish. Immunol. 2025, 161, 110229. [Google Scholar] [CrossRef]
- Jin, R.M.; Huang, H.Z.; Zhou, Y.; Wang, Y.Y.; Fu, H.C.; Li, Z.; Fu, X.Z.; Li, N.Q. Characterization of mandarin fish (Siniperca chuatsi) IL-6 and IL-6 signal transducer and the association between their SNPs and resistance to ISKNV disease. Fish Shellfish Immunol. 2021, 113, 139–147. [Google Scholar] [CrossRef]
- Xiong, Y.; Zheng, X.; Ke, W.; Gong, G.; Wang, Y.; Dan, C.; Huang, P.; Wu, J.; Guo, W.; Mei, J. Function and association analysis of Cyclophilin A gene with resistance to Edwardsiella ictaluri in yellow catfish. Dev. Comp. Immunol. 2020, 113, 103783. [Google Scholar] [CrossRef]
- Ye, S.; Li, H.; Qiao, G.; Li, Z. First case of Edwardsiella ictaluri infection in China farmed yellow catfish Pelteobagrus fulvidraco. Aquaculture 2009, 292, 6–10. [Google Scholar] [CrossRef]
- Bai, D.Q.; Xu, H.L.; Wu, X.; Zhai, S.L.; Yang, G.; Qiao, X.T.; Guo, Y.J. Effect of Dietary Ganoderma lucidum Polysaccharides (GLP) on Cellular Immune Responses and Disease Resistance of Yellow Catfish (Pelteobagrus fulvidraco). Isr. J. Aquac.-Bamidgeh 2015, 67, 9. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Wu, S. Dietary chitosan modulates the growth performance, body composition and nonspecific immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Int. J. Biol. Macromol. 2022, 217, 188–192. [Google Scholar] [CrossRef]
- Song, Z.X.; Jiao, C.R.; Chen, B.Y.; Xu, W.Y.; Wang, M.Y.; Zou, J.H.; Xu, W.Q.; Xu, Z.; Wang, Q.C. Dietary Acanthopanax senticosus extracts modulated the inflammatory and apoptotic responses of yellow catfish to protect against Edwardsiella ictaluriinfection. Aquac. Res. 2021, 52, 5078–5092. [Google Scholar] [CrossRef]
- Wei, F.; Abulahaiti, D.; Tian, C.C.; Chen, Y.; Jiang, S.S.; Lu, J.X.; Zhang, G.H. Effects of dietary Astragalus mongholicus, Astragalus polysaccharides and Lactobacillus on growth performance, immunity and antioxidant status in Qingjiaoma finishing broilers. Czech J. Anim. Sci. 2022, 67, 275–285. [Google Scholar] [CrossRef]
- Huang, Z.F.; Ye, Y.L.; Xu, A.L.; Li, Z.B. Effects of Astragalus membranaceus Polysaccharides on Growth Performance, Physiological and Biochemical Parameters, and Expression of Genes Related to Lipid Metabolism of Spotted Sea Bass, Lateolabrax maculatus. Aquac. Nutr. 2023, 2023, 6191330. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Liu, Z.; Sha, J.; Li, S.; Dong, L.; Sun, Y. Effects of ginseng soluble dietary fiber on serum antioxidant status, immune factor levels and cecal health in healthy rats. Food Chem. 2021, 365, 130641. [Google Scholar] [CrossRef]
- Heidarieh, M.; Mirvaghefi, A.R.; Akbari, M.; Farahmand, H.; Sheikhzadeh, N.; Shahbazfar, A.A.; Behgar, M. Effect of dietary Ergosan on growth performance, digestive enzymes, intestinal histology, hematological parameters and body composition of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2012, 38, 1169–1174. [Google Scholar] [CrossRef]
- Li, Y.; Ran, C.; Wei, K.; Xie, Y.; Xie, M.; Zhou, W.; Yang, Y.; Zhang, Z.; Lv, H.; Ma, X.; et al. The effect of Astragalus polysaccharide on growth, gut and liver health, and anti-viral immunity of zebrafish. Aquaculture 2021, 540, 736677. [Google Scholar] [CrossRef]
- Liu, L.; Ling, H.Y.; Zhang, W.; Zhou, Y.; Li, Y.G.; Peng, N.; Zhao, S.M. Functional Comparison of Clostridium butyricum and Sodium Butyrate Supplementation on Growth, Intestinal Health, and the Anti-inflammatory Response of Broilers. Front. Microbiol. 2022, 13, 914212. [Google Scholar] [CrossRef]
- Liang, H.; Tao, S.M.; Wang, Y.Y.; Zhao, J.; Yan, C.; Wu, Y.J.; Liu, N.; Qin, Y.H. Astragalus polysaccharide: Implication for intestinal barrier, anti-inflammation, and animal production. Front. Nutr. 2024, 11, 1364739. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Zhang, H.R.; Han, Q.J.; Lan, J.H.; Chen, G.Y.; Cao, G.T.; Yang, C.M. Effects of astragalus and ginseng polysaccharides on growth performance, immune function and intestinal barrier in weaned piglets challenged with lipopolysaccharide. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1096–1105. [Google Scholar] [CrossRef]
- Liu, Y.T.; Miao, Y.Q.; Xu, N.; Ding, T.; Cui, K.; Chen, Q.C.; Zhang, J.Z.; Fang, W.; Mai, K.S.; Ai, Q.H. Effects of dietary Astragalus polysaccharides (APS) on survival, growth performance, activities of digestive enzyme, antioxidant responses and intestinal development of large yellow croaker (Larimichthys crocea) larvae. Aquaculture 2020, 517, 734752. [Google Scholar] [CrossRef]
- Qiao, Y.Y.; Liu, C.Z.; Guo, Y.P.; Zhang, W.; Guo, W.B.; Oleksandr, K.; Wang, Z.X. Polysaccharides derived from Astragalus membranaceus and Glycyrrhiza uralensis improve growth performance of broilers by enhancing intestinal health and modulating gut microbiota. Poult. Sci. 2022, 101, 101905. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Zheng, W.; Zhao, Y.; Xu, T. METTL3-Mediated m6A Modification of TRIF and MyD88 mRNAs Suppresses Innate Immunity in Teleost Fish, Miichthys miiuy. J. Immunol. 2023, 211, 130–139. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Zhang, X.; Wang, X.-Y.; Jia, W. Effect of long-term intake of ginseng extracts on gut microbiota in rats. China J. Chin. Mater. Medica 2018, 43, 3927–3932. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.B.; Liu, T.Y.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Chang, Z.Q.; Ge, Q.Q.; Sun, M.; Wang, Q.; Lv, H.Y.; Li, J. Immune responses by dietary supplement with Astragalus polysaccharides in the Pacific white shrimp, Litopenaeus vannamei. Aquac. Nutr. 2018, 24, 702–711. [Google Scholar] [CrossRef]
- Weini, Z.; Yongyang, W.; Anyi, C.; Ruoyu, L.; Fuyu, K.; Jinpeng, Z.; Jianchun, S.; Xiaohong, H.; Xinhua, C. Protective effects of dietary Astragalus polysaccharides on large yellow croaker (Larimichthys crocea) against Vibrio alginolyticus infection. Aquaculture 2024, 581, 740398. [Google Scholar] [CrossRef]
- Elabd, H.; Wang, H.P.; Shaheen, A.; Yao, H.; Abbass, A. Feeding Glycyrrhiza glabra (liquorice) and Astragalus membranaceus (AM) alters innate immune and physiological responses in yellow perch (Perca flavescens). Fish Shellfish Immunol. 2016, 54, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Devi, G.; Doan, H.V.; Tapingkae, W.; Balasundaram, C.; Arockiaraj, J.; Ringo, E. Changes in immune genes expression, immune response, digestive enzymes-antioxidant status, and growth of catla (Catla catla) fed with Astragalus polysaccharides against edwardsiellosis disease. Fish Shellfish Immunol. 2022, 121, 418–436. [Google Scholar] [CrossRef] [PubMed]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Song, Q.; Xiao, Y.; Xiao, Z.H.; Liu, T.; Li, J.C.; Li, P.; Han, F. Lysozymes in Fish. J. Agric. Food Chem. 2021, 69, 15039–15051. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.J.; Wang, B.; Lu, Y.T.; Li, L.; Li, Y.H.; Liu, S.J. Evaluation of the effects of Astragalus polysaccharides as immunostimulants on the immune response of crucian carp and against SVCV in vitro and in vivo. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2022, 253, 109249. [Google Scholar] [CrossRef]
- Anderson, F.H.; Zeng, L.C.; Rock, N.R.; Yoshida, E.M. An assessment of the clinical utility of serum ALT and AST in chronic hepatitis C. Hepatol. Res. 2000, 18, 63–71. [Google Scholar] [CrossRef]
- Huynh, T.; Zhang, J.; Hu, K.Q. Hepatitis C Virus Clearance by Direct-acting Antiviral Results in Rapid Resolution of Hepatocytic Injury as Indicated by Both Alanine Aminotransferase and Aspartate Aminotransferase Normalization. J. Clin. Transl. Hepatol. 2018, 6, 258–263. [Google Scholar] [CrossRef]
- Hoffmann, J.A.; Kafatos, F.C.; Janeway, C.A.; Ezekowitz, R.A.B. Phylogenetic perspectives in innate immunity. Science 1999, 284, 1313–1318. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Nie, L.; Zhu, G.; Xiang, L.X.; Shao, J.Z. Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts. Dev. Comp. Immunol. 2013, 39, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. IL-6: From its discovery to clinical applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef]
- Tracey, K.J.; Cerami, A. Tumor necrosis factor: A pleiotropic cytokine and therapeutic target. Annu. Rev. Med. 1994, 45, 491–503. [Google Scholar] [CrossRef]
- Moore, K.W.; Malefyt, R.D.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Yin, G.J.; Jeney, G.; Racz, T.; Xu, P.; Jun, M.; Jeney, Z. Effect of two Chinese herbs (Astragalus radix and Scutellaria radix) on non-specific immune response of tilapia, Oreochromis niloticus. Aquaculture 2006, 253, 39–47. [Google Scholar] [CrossRef]
- Shi, L.S.; Xue, M.Y.; Xing, Y.Y.; Xu, C.; Jiang, N.; Fan, Y.D.; Chen, J.W.; Liu, W.; Wu, Y.Y.; Wu, M.L.; et al. Dietary supplementation of Astragalus fermentation products improves the growth performance, immunological characteristics, and disease resistance of crucian carp (Carassius auratus). Isr. J. Aquac.-Bamidgeh 2024, 76, 190–199. [Google Scholar] [CrossRef]
- Yin, G.J.; Ardó, L.; Thompson, K.D.; Adams, A.; Jeney, Z.; Jeney, G. Chinese herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp, Cyprinus carpio, and protection against Aeromonas hydrophila. Fish Shellfish Immunol. 2009, 26, 140–145. [Google Scholar] [CrossRef]
| Ingredients | Experimental Feeds (Dry Matter %) | |||
|---|---|---|---|---|
| CT | AG1 | AG2 | AG3 | |
| Fish meal | 44 | 44 | 44 | 44 |
| Flour | 19.55 | 19.55 | 19.55 | 19.55 |
| Cottonseed meal | 4 | 4 | 4 | 4 |
| Soybean meal | 15 | 15 | 15 | 15 |
| Corn gluten meal | 5 | 5 | 5 | 5 |
| Fish oil | 4.5 | 4.5 | 4.5 | 4.5 |
| Brewer’s yeast | 6 | 6 | 6 | 6 |
| Minerals | 1 | 1 | 1 | 1 |
| Choline chloride | 0.6 | 0.6 | 0.6 | 0.6 |
| Multivitamin | 0.2 | 0.2 | 0.2 | 0.2 |
| Mold inhibitor | 0.15 | 0.15 | 0.15 | 0.15 |
| Total | 100 | 100 | 100 | 100 |
| Astragalus–ginseng mixture (mg/kg) | 0 | 500 | 1000 | 2000 |
| Parameters | 0 mg/kg | 500 mg/kg | 1000 mg/kg | 2000 mg/kg |
|---|---|---|---|---|
| IBW (g) | 5.13 ± 0.12 | 5.04 ± 0.23 | 5.05 ± 0.22 | 5.06 ± 0.26 |
| FBW (g) | 9.75 ± 0.55 b | 10.81 ± 0.12 ab | 10.97 ± 0.27 ab | 11.61 ± 0.76 a |
| WG (%) | 46.35 ± 3.88 b | 56.9 ± 1.41 ab | 59.16 ± 0.58 a | 65.53 ± 6.33 a |
| WGR (%) | 90.54 ± 4.72 b | 111.23 ± 8.43 a | 117.20 ± 4.08 a | 129.60 ± 12.36 a |
| SGR (%) | 1.43 ± 0.05 b | 1.66 ± 0.08 ab | 1.72 ± 0.04 a | 1.84 ± 0.12 a |
| VSI (%) | 12.06 ± 1.80 | 13.18 ± 1.11 | 12.76 ± 1.15 | 13.24 ± 0.91 |
| HSI (%) | 1.01 ± 0.01 c | 1.29 ± 0.06 b | 1.63 ± 0.03 a | 1.53 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Xu, H.; Ma, X.; Yin, Z.; Wang, A.; Qiu, J.; Li, M. Effects of Astragalus–Ginseng Dietary Supplementation on the Growth and Stress Resistance of Yellow Catfish (Pelteobagrus fulvidraco). Fishes 2025, 10, 208. https://doi.org/10.3390/fishes10050208
Lin W, Xu H, Ma X, Yin Z, Wang A, Qiu J, Li M. Effects of Astragalus–Ginseng Dietary Supplementation on the Growth and Stress Resistance of Yellow Catfish (Pelteobagrus fulvidraco). Fishes. 2025; 10(5):208. https://doi.org/10.3390/fishes10050208
Chicago/Turabian StyleLin, Wenkai, Haijing Xu, Xinlan Ma, Zifeng Yin, Aimin Wang, Junqiang Qiu, and Mingyou Li. 2025. "Effects of Astragalus–Ginseng Dietary Supplementation on the Growth and Stress Resistance of Yellow Catfish (Pelteobagrus fulvidraco)" Fishes 10, no. 5: 208. https://doi.org/10.3390/fishes10050208
APA StyleLin, W., Xu, H., Ma, X., Yin, Z., Wang, A., Qiu, J., & Li, M. (2025). Effects of Astragalus–Ginseng Dietary Supplementation on the Growth and Stress Resistance of Yellow Catfish (Pelteobagrus fulvidraco). Fishes, 10(5), 208. https://doi.org/10.3390/fishes10050208







