eDNA Metabarcoding Reveals the Depth-Structured Variation of Coral Reef Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. DNA Extraction and PCR Amplification
2.3. Sequence Analysis
2.4. Functional Group
2.5. Functional Diversity
2.6. Data Analysis
3. Results
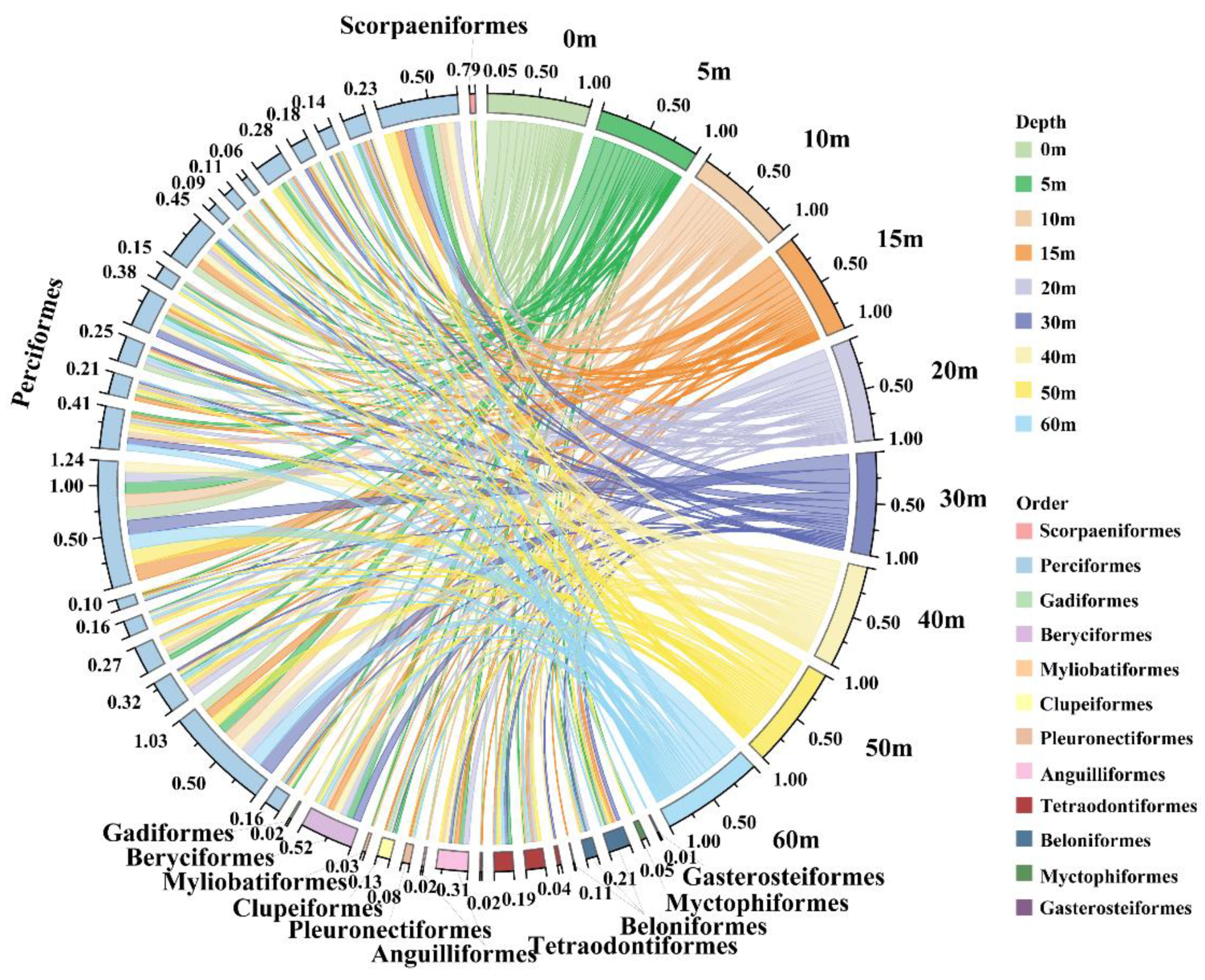
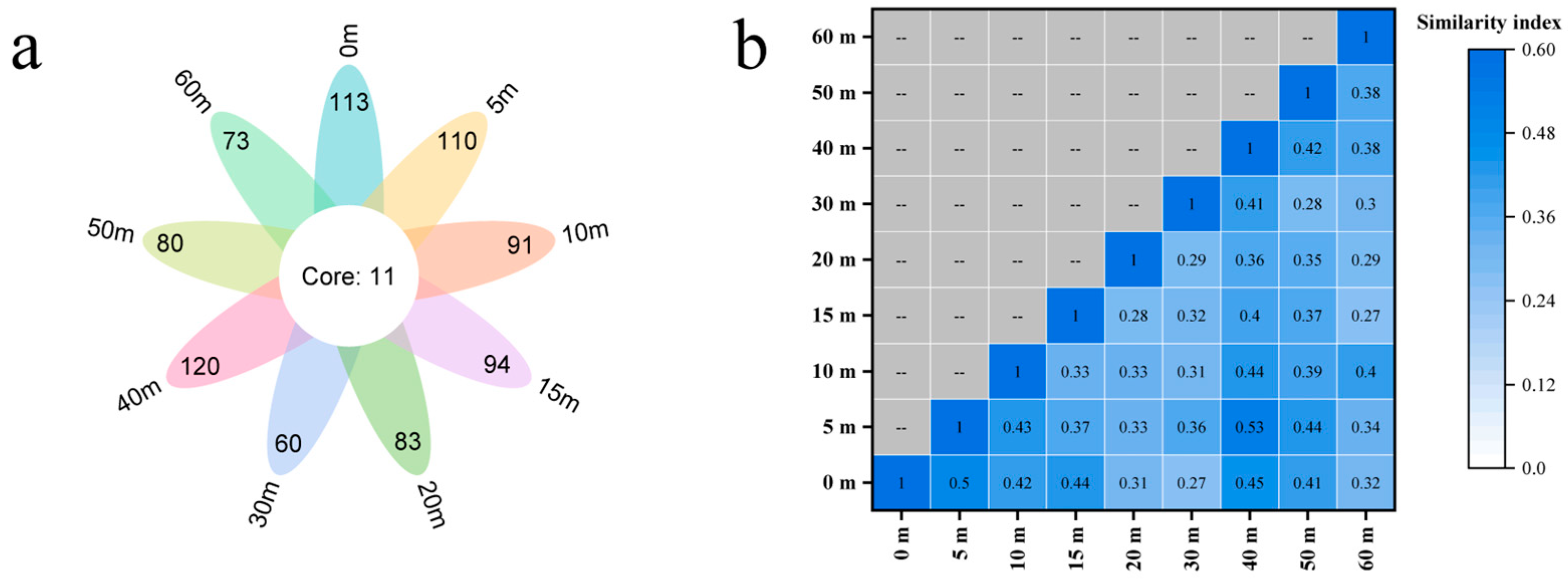
3.1. Fish Composition and Community Assembly
3.2. Distinguish Fish Communities by Depth
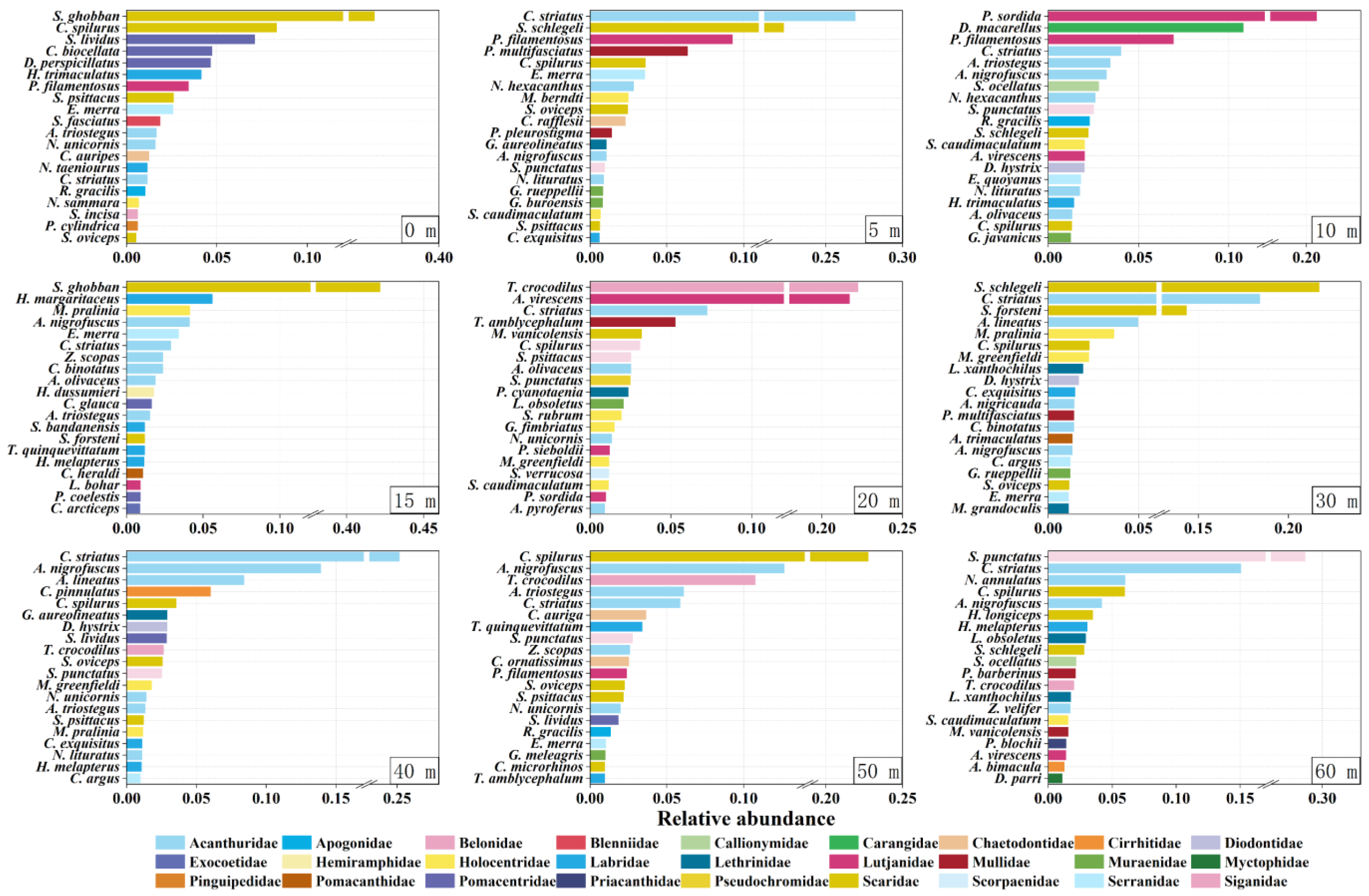
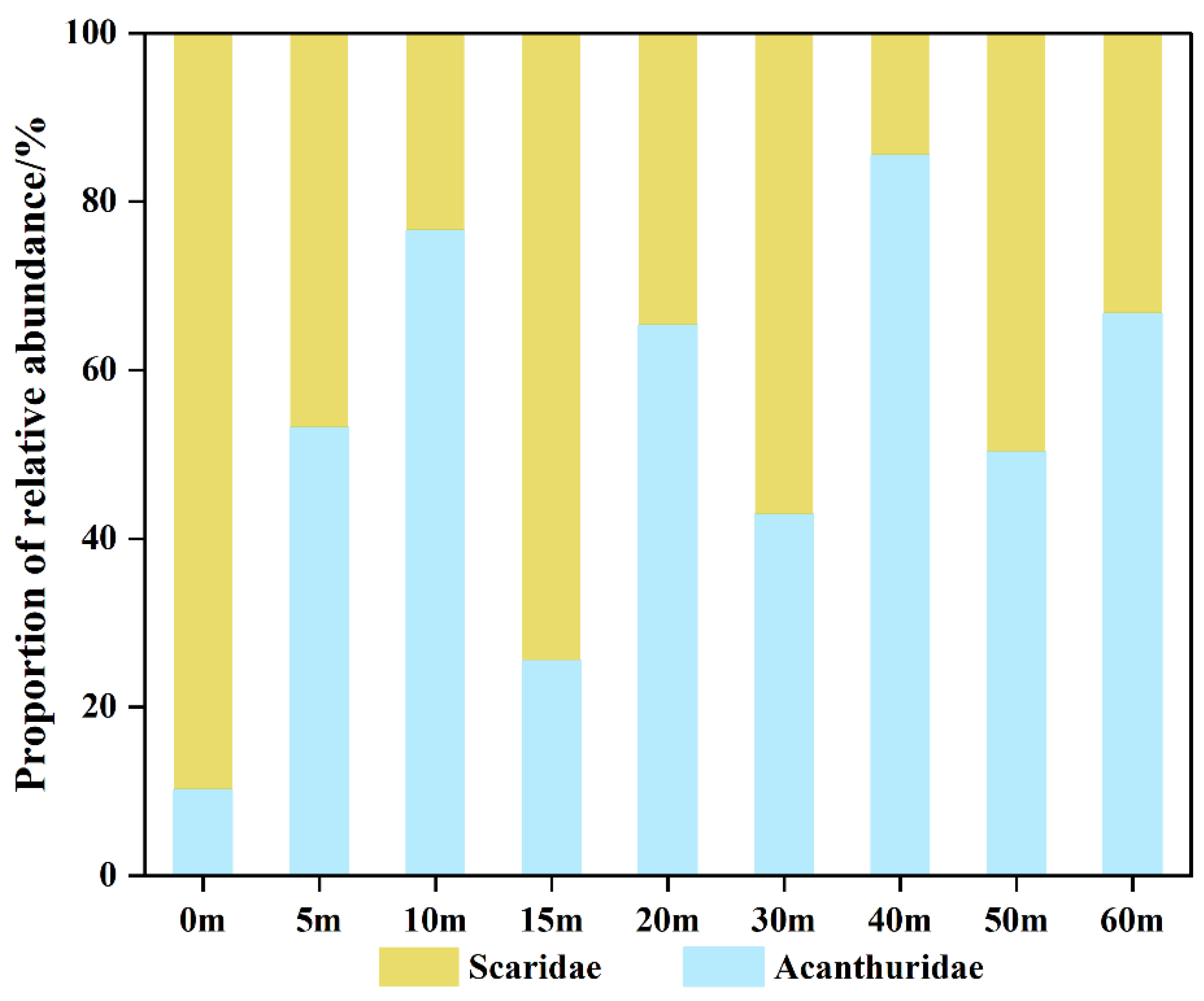
3.3. Dominant and Special Species at Different Depths
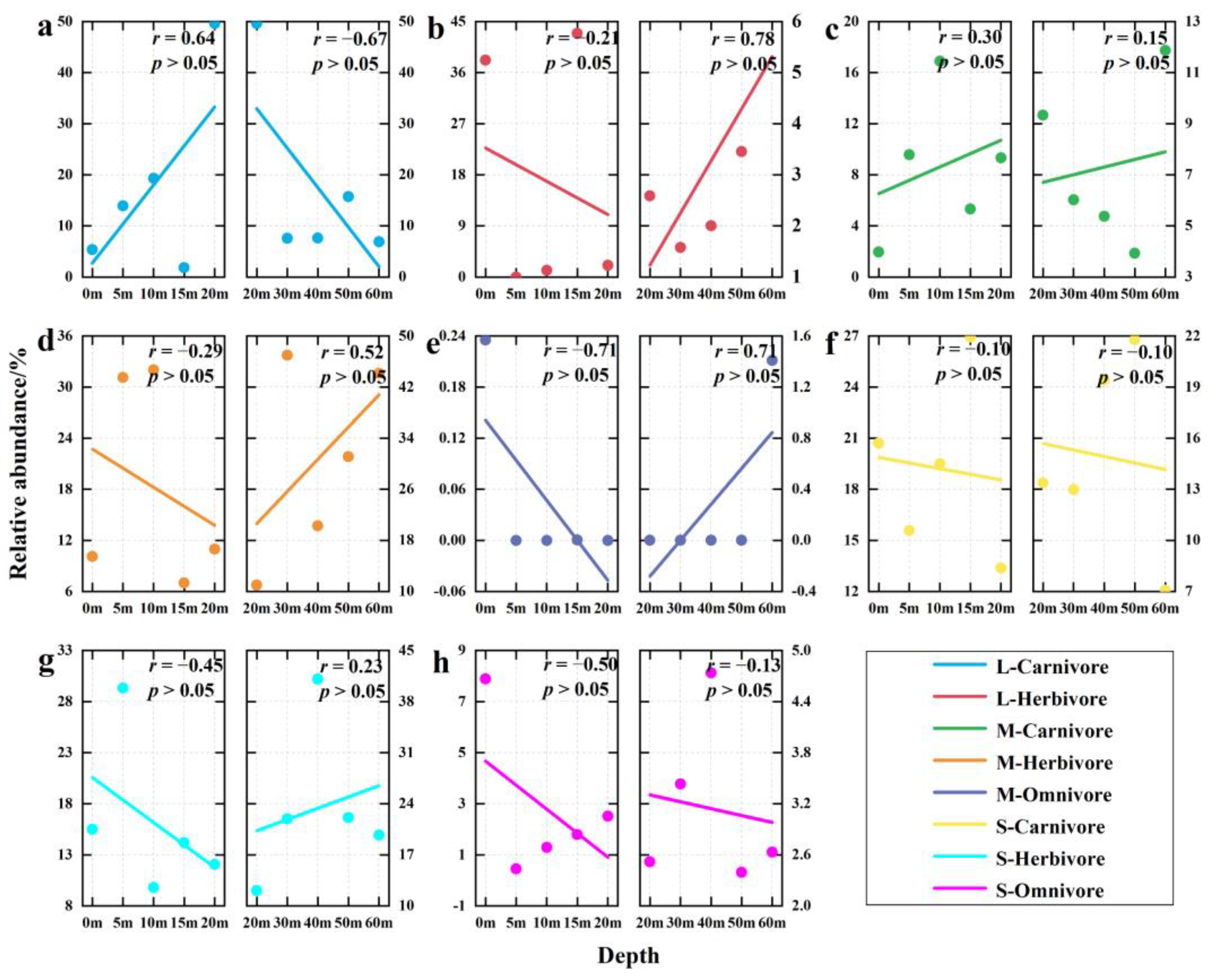
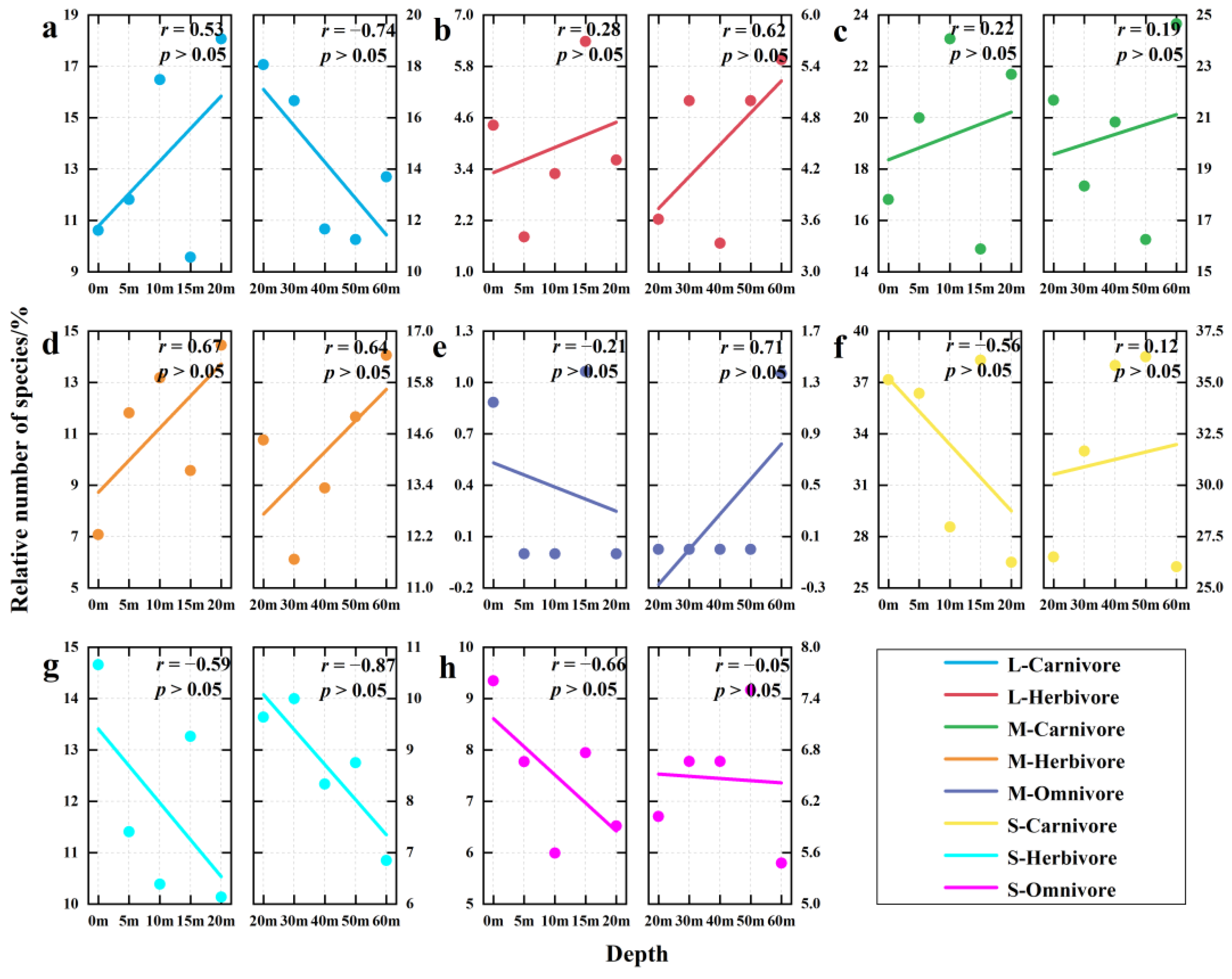
3.4. Depth Gradient Variation of Functional Group Structure
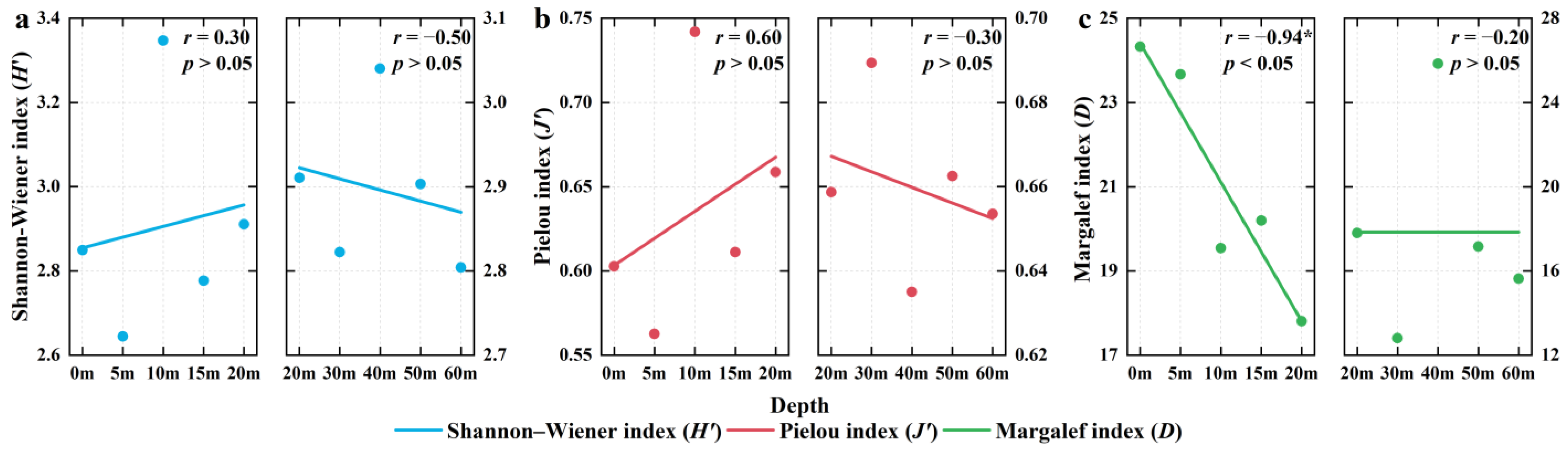
3.5. Depth Gradient Variation of Biological Diversity
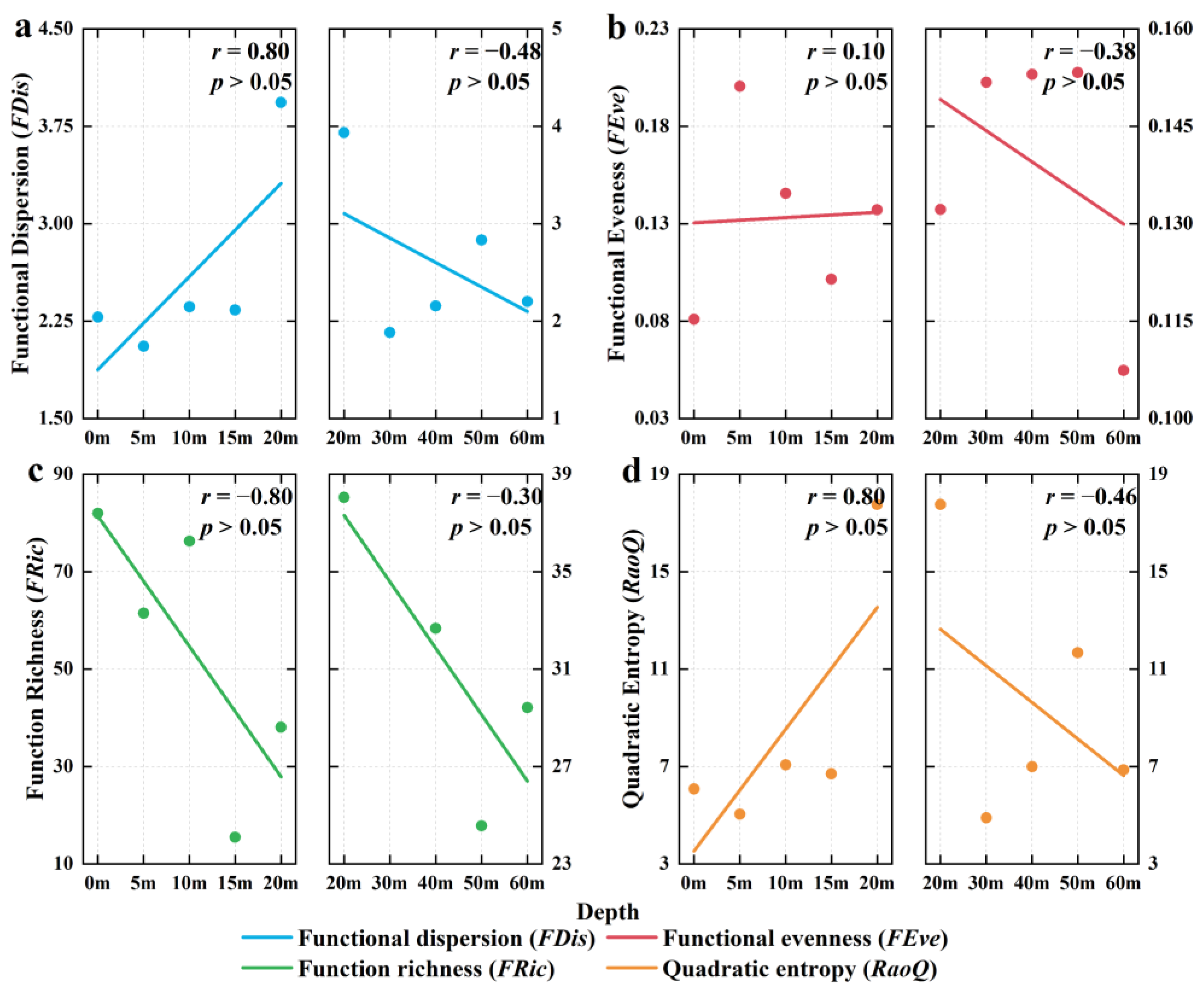
3.6. Depth Gradient Variation of Functional Diversity
4. Discussion
4.1. eDNA Metabarcoding Plays a Positive Role in Understanding Biodiversity
4.2. Fish Community Structure Reflects Habitat Status
4.3. eDNA Metabarcoding Reveals the Depth-Structured Variation of Fish
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, R.; O’Leary, R.A.; Low-Choy, S.; Mengersen, K.; Knowlton, N.; Brainard, R.E.; Caley, M.J. Species Richness on Coral Reefs and the Pursuit of Convergent Global Estimates. Curr. Biol. 2015, 25, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.C.; Kleypas, J.; van de Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Coker, D.J.; Wilson, S.K.; Pratchett, M.S. Importance of live coral habitat for reef fishes. Rev. Fish Biol. Fish. 2014, 24, 89–126. [Google Scholar] [CrossRef]
- Robinson, J.P.W.; Wilson, S.K.; Robinson, J.; Gerry, C.; Lucas, J.; Assan, C.; Govinden, R.; Jennings, S.; Graham, N.A.J. Productive instability of coral reef fisheries after climate-driven regime shifts. Nat. Ecol. Evol. 2019, 3, 183–190. [Google Scholar] [CrossRef]
- Shi, J.; Li, C.H.; Wang, T.; Zhao, J.F.; Liu, Y.; Xiao, Y.Y. Distribution Pattern of Coral Reef Fishes in China. Sustainability 2022, 14, 15107. [Google Scholar] [CrossRef]
- Cheal, A.J.; Macneil, M.A.; Emslie, M.J.; Sweatman, H. The threat to coral reefs from more intense cyclones under climate change. Glob. Change Biol. 2017, 23, 1511–1524. [Google Scholar] [CrossRef]
- Perry, C.T.; Alvarez-Filip, L.; Graham, N.A.; Mumby, P.J.; Wilson, S.K.; Kench, P.S.; Manzello, D.P.; Morgan, K.M.; Slangen, A.; Thomson, D.P. Loss of coral reef growth capacity to track future increases in sea level. Nature 2018, 558, 396–400. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Dulvy, N.K.; Gill, J.A.; Côté, I.M.; Watkinson, A.R. Flattening of Caribbean coral reefs: Region-wide declines in architectural complexity. Proc. R. Soc. B Biol. Sci. 2009, 276, 3019–3025. [Google Scholar] [CrossRef]
- Des Roches, S.; Post, D.M.; Turley, N.E.; Bailey, J.K.; Hendry, A.P.; Kinnison, M.T.; Schweitzer, J.A.; Palkovacs, E.P. The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2018, 2, 57–64. [Google Scholar] [CrossRef]
- Brandl, S.J.; Rasher, D.B.; Côté, I.M.; Casey, J.M.; Darling, E.S.; Lefcheck, J.S.; Duffy, J.E. Coral reef ecosystem functioning: Eight core processes and the role of biodiversity. Front. Ecol. Environ. 2019, 17, 445–454. [Google Scholar] [CrossRef]
- Pilly, S.S.; Richardson, L.E.; Turner, J.R.; Roche, R.C. Atoll-dependent variation in depth zonation of benthic communities on remote reefs. Mar. Environ. Res. 2022, 173, 105520. [Google Scholar] [CrossRef] [PubMed]
- Karisa, J.F.; Obura, D.O.; Chen, C.A. Spatial heterogeneity of coral reef benthic communities in Kenya. PLoS ONE 2020, 15, e0237397. [Google Scholar] [CrossRef] [PubMed]
- Pilly, S.S.; Roche, R.C.; Richardson, L.E.; Turner, J.R. Depth variation in benthic community response to repeated marine heatwaves on remote Central Indian Ocean reefs. R. Soc. Open Sci. 2024, 11, 231246. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.F.; Uthicke, S.; Humphrey, C.; Fabricius, K.E. Gradients in water column nutrients, sediment parameters, irradiance and coral reef development in the Whitsunday Region, central Great Barrier Reef. Estuar. Coast. Shelf Sci. 2007, 74, 458–470. [Google Scholar] [CrossRef]
- Nash, K.L.; Graham, N.A.; Wilson, S.K.; Bellwood, D.R. Cross-scale habitat structure drives fish body size distributions on coral reefs. Ecosystems 2013, 16, 478–490. [Google Scholar] [CrossRef]
- Cyronak, T.; Takeshita, Y.; Courtney, T.A.; DeCarlo, E.H.; Eyre, B.D.; Kline, D.I.; Martz, T.; Page, H.; Price, N.N.; Smith, J.; et al. Diel temperature and pH variability scale with depth across diverse coral reef habitats. Limnol. Oceanogr. Lett. 2020, 5, 193–203. [Google Scholar] [CrossRef]
- Liaño-Carrera, F.; Camarena-Luhrs, T.; Gómez-Barrero, A.; Martos-Fernández, F.J.; Ramírez-Macias, J.I.; Salas-Monreal, D. New coral reef structures in a tropical coral reef system. Lat. Am. J. Aquat. Res. 2019, 47, 270–281. [Google Scholar] [CrossRef]
- Radice, V.Z.; Hoegh-Guldberg, O.; Fry, B.; Fox, M.D.; Dove, S.G. Upwelling as the major source of nitrogen for shallow and deep reef-building corals across an oceanic atoll system. Funct. Ecol. 2019, 33, 1120–1134. [Google Scholar] [CrossRef]
- Schlaefer, J.A.; Tebbett, S.B.; Bowden, C.L.; Collins, W.P.; Duce, S.; Hemingson, C.R.; Huertas, V.; Mihalitsis, M.; Morais, J.; Morais, R.A.; et al. A snapshot of sediment dynamics on an inshore coral reef. Mar. Environ. Res. 2022, 181, 105763. [Google Scholar] [CrossRef]
- Titlyanov, E.; Titlyanova, T. Reef-building corals—Symbiotic autotrophic organisms: 2. Pathways and mechanisms of adaptation to light. Russ. J. Mar. Biol. 2002, 28, S16–S31. [Google Scholar] [CrossRef]
- Duckworth, A.; Giofre, N.; Jones, R. Coral morphology and sedimentation. Mar. Pollut. Bull. 2017, 125, 289–300. [Google Scholar] [CrossRef]
- Darling, E.S.; Côté, I.M. Seeking resilience in marine ecosystems. Science 2018, 359, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, H.T.; MacDonald, C.; Quimbayo, J.P.; Shepherd, B.; Phelps, T.A.; Loss, A.C.; Teixeira, J.B.; Rocha, L.A. Assembly rules of coral reef fish communities along the depth gradient. Curr. Biol. 2023, 33, 1421–1430. [Google Scholar] [CrossRef]
- Robinson, J.P.W.; Benkwitt, C.E.; Maire, E.; Morais, R.; Schiettekatte, N.M.D.; Skinner, C.; Brandl, S.J. Quantifying energy and nutrient fl uxes in coral reef food webs. Trends Ecol. Evol. 2024, 39, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Osuka, K.E.; Stewart, B.D.; Samoilys, M.; McClean, C.J.; Musembi, P.; Yahya, S.; Hamad, A.R.; Mbugua, J. Depth and habitat are important drivers of abundance for predatory reef fish off Pemba Island, Tanzania. Mar. Environ. Res. 2022, 175, 105587. [Google Scholar] [CrossRef] [PubMed]
- Brokovich, E.; Einbinder, S.; Shashar, N.; Kiflawi, M.; Kark, S. Descending to the twilight-zone: Changes in coral reef fish assemblages along a depth gradient down to 65 m. Mar. Ecol. Prog. Ser. 2008, 371, 253–262. [Google Scholar] [CrossRef]
- Cooper, A.M.; MacDonald, C.; Roberts, T.E.; Bridge, T.C.L. Variability in the functional composition of coral reef fish communities on submerged and emergent reefs in the central Great Barrier Reef, Australia. PLoS ONE 2019, 14, e0216785. [Google Scholar] [CrossRef]
- Richardson, L.E.; Williams, G.J. Depth zonation of reef fish is predictable but disrupted on contemporary coral reefs. Nat. Ecol. Evol. 2023, 7, 1759–1760. [Google Scholar] [CrossRef]
- DiBattista, J.D.; Reimer, J.D.; Stat, M.; Masucci, G.D.; Biondi, P.; De Brauwer, M.; Bunce, M. Digging for DNA at depth: Rapid universal metabarcoding surveys (RUMS) as a tool to detect coral reef biodiversity across a depth gradient. Peer J 2019, 7, e6379. [Google Scholar] [CrossRef]
- Feary, D.A.; McCormick, M.I.; Jones, G.P. Growth of reef fishes in response to live coral cover. J. Exp. Mar. Biol. Ecol. 2009, 373, 45–49. [Google Scholar] [CrossRef]
- Sheppard, C.E.; Williams, G.J.; Exton, D.A.; Keith, S.A. Co-occurrence of herbivorous fish functional groups correlates with enhanced coral reef benthic state. Glob. Ecol. Biogeogr. 2023, 32, 435–449. [Google Scholar] [CrossRef]
- Valentini, A.; Taberlet, P.; Miaud, C.; Civade, R.; Herder, J.; Thomsen, P.F.; Bellemain, E.; Besnard, A.; Coissac, E.; Boyer, F. Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol. Ecol. 2016, 25, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.K.; Bekkevold, D.; Clausen, L.W.; Nielsen, E.E. The sceptical optimist: Challenges and perspectives for the application of environmental DNA in marine fisheries. Fish Fish. 2018, 19, 751–768. [Google Scholar] [CrossRef]
- Halperin, A.A.; Lichowski, F.; Morioka, J.; O’Brien, K.; Suka, R.; Huntington, B. Coral cover remains suppressed three years after derelict net removal in a remote shallow water coral reef ecosystem. Mar. Pollut. Bull. 2023, 188, 114703. [Google Scholar] [CrossRef]
- Gilbey, J.; Carvalho, G.; Castilho, R.; Coscia, I.; Coulson, M.W.; Dahle, G.; Derycke, S.; Francisco, S.M.; Helyar, S.J.; Johansen, T. Life in a drop: Sampling environmental DNA for marine fishery management and ecosystem monitoring. Mar. Policy 2021, 124, 104331. [Google Scholar] [CrossRef]
- Veilleux, H.D.; Misutka, M.D.; Glover, C.N. Environmental DNA and environmental RNA: Current and prospective applications for biological monitoring. Sci. Total Environ. 2021, 782, 146891. [Google Scholar] [CrossRef]
- Stat, M.; Huggett, M.J.; Bernasconi, R.; DiBattista, J.D.; Berry, T.E.; Newman, S.J.; Harvey, E.S.; Bunce, M. Ecosystem biomonitoring with eDNA: Metabarcoding across the tree of life in a tropical marine environment. Sci. Rep. 2017, 7, 12240. [Google Scholar] [CrossRef]
- Tsuji, S.; Shibata, N.; Inui, R.; Nakao, R.; Akamatsu, Y.; Watanabe, K. Environmental DNA phylogeography: Successful reconstruction of phylogeographic patterns of multiple fish species from cups of water. Mol. Ecol. Resour. 2023, 23, 1050–1065. [Google Scholar] [CrossRef]
- Bohmann, K.; Evans, A.; Gilbert, M.T.P.; Carvalho, G.R.; Creer, S.; Knapp, M.; Douglas, W.Y.; De Bruyn, M. Environmental DNA for wildlife biology and biodiversity monitoring. Trends Ecol. Evol. 2014, 29, 358–367. [Google Scholar] [CrossRef]
- Oladi, M.; Leontidou, K.; Stoeck, T.; Shokri, M.R. Environmental DNA-based profiling of benthic bacterial and eukaryote communities along a crude oil spill gradient in a coral reef in the Persian Gulf. Mar. Pollut. Bull. 2022, 184, 114143. [Google Scholar] [CrossRef]
- DiBattista, J.D.; Coker, D.J.; Sinclair-Taylor, T.H.; Stat, M.; Berumen, M.L.; Bunce, M. Assessing the utility of eDNA as a tool to survey reef-fish communities in the Red Sea. Coral Reefs 2017, 36, 1245–1252. [Google Scholar] [CrossRef]
- Gelis, E.R.E.; Kamal, M.M.; Subhan, B.; Bachtiar, I.; Sani, L.M.I.; Madduppa, H. Environmental biomonitoring of reef fish community structure with eDNA metabarcoding in the Coral Triangle. Environ. Biol. Fishes 2021, 104, 887–903. [Google Scholar] [CrossRef]
- Port, J.A.; O’Donnell, J.L.; Romero-Maraccini, O.C.; Leary, P.R.; Litvin, S.Y.; Nickols, K.J.; Yamahara, K.M.; Kelly, R.P. Assessing vertebrate biodiversity in a kelp forest ecosystem using environmental DNA. Mol. Ecol. 2016, 25, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Canals, O.; Mendibil, I.; Santos, M.; Irigoien, X.; Rodríguez-Ezpeleta, N. Vertical stratification of environmental DNA in the open ocean captures ecological patterns and behavior of deep-sea fishes. Limnol. Oceanogr. Lett. 2021, 6, 339–347. [Google Scholar] [CrossRef]
- Jeunen, G.J.; Lamare, M.D.; Knapp, M.; Spencer, H.G.; Taylor, H.R.; Stat, M.; Bunce, M.; Gemmell, N.J. Water stratification in the marine biome restricts vertical environmental DNA (eDNA) signal dispersal. Environ. DNA 2020, 2, 99–111. [Google Scholar] [CrossRef]
- Mathon, L.; Baletaud, F.; Lebourges-Dhaussy, A.; Lecellier, G.; Menkes, C.; Bachelier, C.; Bonneville, C.; Dejean, T.; Dumas, M.; Fiat, S. Three-dimensional conservation planning of fish biodiversity metrics to achieve the deep-sea 30 × 30 conservation target. Conserv. Biol. 2024, 39, e14368. [Google Scholar] [CrossRef]
- Doxa, A.; Almpanidou, V.; Katsanevakis, S.; Queirós, A.M.; Kaschner, K.; Garilao, C.; Kesner-Reyes, K.; Mazaris, A.D. 4D marine conservation networks: Combining 3D prioritization of present and future biodiversity with climatic refugia. Glob. Change Biol. 2022, 28, 4577–4588. [Google Scholar] [CrossRef]
- Dali, M.Z.M.; Nasir, M.; Khaleel, A.G.; Chun, L.M.; Gan, H.M.; Wan, N.; Umar, R.; Kamarudin, A.S. Predicting Cherax quadricarinatus Habitat Distribution Patterns Through the Usage of GIS and eDNA Analysis in Terengganu, Malaysia. Sains Malays. 2023, 52, 343–354. [Google Scholar] [CrossRef]
- Dali, M.Z.M.; Umar, R.; Ismail, N.; Juahir, H.; Nasir, M.; Khaleel, A.G.; Madiran, N.A.; Kari, Z.A.; Wei, L.S.; Tahiluddin, A.B.; et al. Detection and Management of Freshwater Invasive Alien Species through Environmental DNA Analysis and Geographic Information Systems: A Review. Sustainability 2023, 15, 9497. [Google Scholar] [CrossRef]
- Wiggins, J.J.; Tobias, V.D.; Holcombe, E.F.; Karpenko, K.; Huber, E.R.; Goodman, A.C. Leveraging environmental DNA (eDNA) to optimize targeted removal of invasive fishes. J. Freshw. Ecol. 2024, 39, 2378841. [Google Scholar] [CrossRef]
- Kasai, A.; Yamazaki, A.; Ahn, H.; Yamanaka, H.; Kameyama, S.; Masuda, R.; Azuma, N.; Kimura, S.; Karaki, T.; Kurokawa, Y.; et al. Distribution of Japanese Eel Anguilla japonica Revealed by Environmental DNA. Front. Ecol. Evol. 2021, 9, 621461. [Google Scholar] [CrossRef]
- Bessey, C.; Depczynski, M.; Goetze, J.S.; Moore, G.; Fulton, C.J.; Snell, M.; Parsons, S.K.; Berry, O.; Wilson, S. Cryptic biodiversity: A portfolio-approach to coral reef fish surveys. Limnol. Oceanogr. Methods 2023, 21, 594–605. [Google Scholar] [CrossRef]
- Hoban, M.L.; Bunce, M.; Bowen, B.W. Plumbing the depths with environmental DNA (eDNA): Metabarcoding reveals biodiversity zonation at 45–60 m on mesophotic coral reefs. Mol. Ecol. 2023, 32, 5590–5608. [Google Scholar] [CrossRef] [PubMed]
- Mathon, L.; Marques, V.; Mouillot, D.; Albouy, C.; Andrello, M.; Baletaud, F.; Borrero-Perez, G.H.; Dejean, T.; Edgar, G.J.; Grondin, J.; et al. Cross-ocean patterns and processes in fish biodiversity on coral reefs through the lens of eDNA metabarcoding. Proc. R. Soc. B—Biol. Sci. 2022, 289, 20220162. [Google Scholar] [CrossRef]
- Li, Y.Z.; Jia, X.P.; Chen, G.B. Coral Reef Fish Stocks in the South China Sea; Ocean Press: Beijing, China, 2007; pp. 1–446. [Google Scholar]
- Huang, H.; Dong, Z.J.; Lian, J.S. Establishment of nature reserve of coral reef ecosystem on the Xisha Islands. Trop. Geogr. 2008, 28, 540–544. [Google Scholar]
- Gu, S.S.; Deng, Y.; Wang, P.Y.; Li, C.H.; Shi, D.J.; Wang, S.P. Assessing riverine fish community diversity and stability by eDNA metabarcoding. Ecol. Indic. 2023, 157, 111222. [Google Scholar] [CrossRef]
- Miya, M.; Sato, Y.; Fukunaga, T.; Sado, T.; Poulsen, J.Y.; Sato, K.; Minamoto, T.; Yamamoto, S.; Yamanaka, H.; Araki, H.; et al. MiFish, a set of universal PCR primers for metabarcoding environmental DNA from fishes: Detection of more than 230 subtropical marine species. R. Soc. Open Sci. 2015, 2, 150088. [Google Scholar] [CrossRef]
- Iwasaki, W.; Fukunaga, T.; Isagozawa, R.; Yamada, K.; Maeda, Y.; Satoh, T.P.; Sado, T.; Mabuchi, K.; Takeshima, H.; Miya, M.; et al. MitoFish and MitoAnnotator: A Mitochondrial Genome Database of Fish with an Accurate and Automatic Annotation Pipeline. Mol. Biol. Evol. 2013, 30, 2531–2540. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, T.; Li, C.; Shi, J.; Xie, H.; Luo, L.; Xiao, Y.; Liu, Y. Seven decades of transformation: Evaluating the dynamics of coral reef fish communities in the Xisha Islands, South China Sea. Rev. Fish Biol. Fish. 2024, 34, 1261–1281. [Google Scholar] [CrossRef]
- Peter, F.S. The Ecology of Fishes on Coral Reefs; Academic Press: New York, NY, USA, 1991; pp. 47–51. [Google Scholar]
- Wang, T.; Liu, Y.; Li, C.H.; Xiao, Y.Y.; Lin, L.; Li, C.R.; Xie, Y.F.; Wu, P. Characteristics of fish community structure in coral reefs adjacent to yongxing island of xisha islands. Acta Hydrobiol. Sin. 2023, 47, 674–683. [Google Scholar]
- Toussaint, A.; Charpin, N.; Brosse, S.; Villéger, S. Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Sci. Rep. 2016, 6, 22125. [Google Scholar] [CrossRef] [PubMed]
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Zintzen, V.; Anderson, M.J.; Roberts, C.D.; Diebel, C.E. Increasing variation in taxonomic distinctness reveals clusters of specialists in the deep sea. Ecography 2011, 34, 306–317. [Google Scholar] [CrossRef]
- Margalef, R. Perspectives in Ecological Theory; University of Chicago Press: Chicago, IL, USA, 1968; pp. 121–127. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Pielou, E. The use of information theory in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Villéger, S.; Mason, N.W.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C.; Cheng, Q.; Zhang, Y.; Wang, C.; Tian, M.; Yang, W.; Sun, B.; Zheng, W.; Zheng, B. Fishes of the South China Sea; Science Press: Beijing, China, 1962; pp. 1–1184. [Google Scholar]
- Donovan, M.K.; Friedlander, A.M.; Lecky, J.; Jouffray, J.B.; Williams, G.J.; Wedding, L.M.; Crowder, L.B.; Erickson, A.L.; Graham, N.A.J.; Gove, J.M.; et al. Combining fish and benthic communities into multiple regimes reveals complex reef dynamics. Sci. Rep. 2018, 8, 16943. [Google Scholar] [CrossRef]
- Hemingson, C.R.; Mihalitsis, M.; Bellwood, D.R. Are fish communities on coral reefs becoming less colourful? Glob. Change Biol. 2022, 28, 3321–3332. [Google Scholar] [CrossRef]
- Kelly, R.P.; Port, J.A.; Yamahara, K.M.; Martone, R.G.; Lowell, N.; Thomsen, P.F.; Mach, M.E.; Bennett, M.; Prahler, E.; Caldwell, M.R. Harnessing DNA to improve environmental management. Science 2014, 344, 1455–1456. [Google Scholar] [CrossRef]
- Evans, N.T.; Shirey, P.D.; Wieringa, J.G.; Mahon, A.R.; Lamberti, G.A. Comparative Cost and Effort of Fish Distribution Detection via Environmental DNA Analysis and Electrofishing. Fisheries 2017, 42, 90–99. [Google Scholar] [CrossRef]
- Ji, Y.; Ashton, L.; Pedley, S.M.; Edwards, D.P.; Tang, Y.; Nakamura, A.; Kitching, R.; Dolman, P.M.; Woodcock, P.; Edwards, F.A. Reliable, verifiable and efficient monitoring of biodiversity via metabarcoding. Ecol. Lett. 2013, 16, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Buckley, M.C.; Gallego, R.; Arranz, V.; Halafihi, T.; Stone, K.; Erdmann, M.; Tornabene, L.M. Comparing anesthetic stations and environmental DNA sampling to determine community composition of cryptobenthic coral reef fishes of Vava’u, Kingdom of Tonga. Coral Reefs 2023, 42, 785–797. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, S.; Zhao, J.D.; Yao, M. Passive eDNA sampling facilitates biodiversity monitoring and rare species detection. Environ. Int. 2024, 187, 108706. [Google Scholar] [CrossRef]
- West, K.M.; Stat, M.; Harvey, E.S.; Skepper, C.L.; DiBattista, J.D.; Richards, Z.T.; Travers, M.J.; Newman, S.J.; Bunce, M. eDNA metabarcoding survey reveals fine-scale coral reef community variation across a remote, tropical island ecosystem. Mol. Ecol. 2020, 29, 1069–1086. [Google Scholar] [CrossRef]
- Blanchette, A.; Ely, T.; Zeko, A.; Sura, S.A.; Turba, R.; Fong, P. Damselfish Stegastes nigricans increase algal growth within their territories on shallow coral reefs via enhanced nutrient supplies. J. Exp. Mar. Biol. Ecol. 2019, 513, 21–26. [Google Scholar] [CrossRef]
- MacDonald, C.; Tauati, M.I.; Jones, G.P. Depth patterns in microhabitat versatility and selectivity in coral reef damselfishes. Mar. Biol. 2018, 165, 138. [Google Scholar] [CrossRef]
- Ross, S.W.; Quattrini, A.M.; Roa-Varón, A.Y.; McClain, J.P. Species composition and distributions of mesopelagic fishes over the slope of the north-central Gulf of Mexico. Deep-Sea Res. Part II—Top. Stud. Oceanogr. 2010, 57, 1926–1956. [Google Scholar] [CrossRef]
- Sassa, C.; Kawaguchi, K.; Hirota, Y.; Ishida, M. Distribution depth of the transforming stage larvae of myctophid fishes in the subtropical-tropical waters of the western North Pacific. Deep-Sea Res. Part I—Oceanogr. Res. Pap. 2007, 54, 2181–2193. [Google Scholar] [CrossRef]
- Rissik, D.; Suthers, I.M. Enhanced feeding by pelagic juvenile myctophid fishes within a region of island-induced flow disturbance in the Coral Sea. Mar. Ecol. Prog. Ser. 2000, 203, 263–273. [Google Scholar] [CrossRef]
- Flynn, A.J.; Paxton, J.R. Spawning aggregation of the lanternfish Diaphus danae (family Myctophidae) in the north-western Coral Sea and associations with tuna aggregations. Mar. Freshw. Res. 2012, 63, 1255–1271. [Google Scholar] [CrossRef]
- Srinivasan, M. Depth distributions of coral reef fishes: The influence of microhabitat structure, settlement, and post-settlement processes. Oecologia 2003, 137, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Monuki, K.; Barber, P.H.; Gold, Z. eDNA captures depth partitioning in a kelp forest ecosystem. PLoS ONE 2021, 16, e0253104. [Google Scholar] [CrossRef]
- Beng, K.C.; Corlett, R.T. Applications of environmental DNA (eDNA) in ecology and conservation: Opportunities, challenges and prospects. Biodivers. Conserv. 2020, 29, 2089–2121. [Google Scholar] [CrossRef]
- Antinero, A.T.; Balaba, M.P.; Leopardas, V.E.; Aspe, N.M.; Kajita, T. Is it really there? Addressing Inadequate Sampling and False Detection in Environmental DNA Metabarcoding. J. Environ. Sci. Manag. 2024, 27, 61–76. [Google Scholar] [CrossRef]
- Norström, A.V.; Nyström, M.; Lokrantz, J.; Folke, C. Alternative states on coral reefs: Beyond coral–macroalgal phase shifts. Mar. Ecol. Prog. Ser. 2009, 376, 295–306. [Google Scholar] [CrossRef]
- Bell, J.J.; Davy, S.K.; Jones, T.; Taylor, M.W.; Webster, N.S. Could some coral reefs become sponge reefs as our climate changes? Glob. Change Biol. 2013, 19, 2613–2624. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef]
- Noble, M.M.; van Laake, G.; Berumen, M.L.; Fulton, C.J. Community Change within a Caribbean Coral Reef Marine Protected Area following Two Decades of Local Management. PLoS ONE 2013, 8, e54069. [Google Scholar] [CrossRef]
- Li, Y.C.; Wu, Z.J.; Liang, J.L.; Chen, S.Q.; Zhao, J.M. Analysis on the outbreak period and cause of Acanthaster planci in Xisha Islands in recent 15 years. Chin. Sci. Bull. 2019, 64, 3478–3484. [Google Scholar]
- Wang, T.; Shi, J.; Yu, Y.F.; Zhao, J.F.; Xiao, Y.Y.; Liu, Y.; Li, C.H. Research progress and conservation suggestions of coral reef fishes in the Xisha Islands. Chin. J. Ecol. 2023, 42, 1755–1763. [Google Scholar] [CrossRef]
- Mumby, P.J.; Steneck, R.S.; Adjeroud, M.; Arnold, S.N. High resilience masks underlying sensitivity to algal phase shifts of Pacific coral reefs. Oikos 2016, 125, 644–655. [Google Scholar] [CrossRef]
- Kapur, M.R.; Franklin, E.C. Simulating future climate impacts on tropical fisheries: Are contemporary spatial fishery management strategies sufficient? Can. J. Fish. Aquat. Sci. 2017, 74, 1974–1989. [Google Scholar] [CrossRef]
- Lindahl, U.; Öhman, M.C.; Schelten, C.K. The 1997/1998 mass mortality of corals:: Effects on fish communities on a Tanzanian coral reef. Mar. Pollut. Bull. 2001, 42, 127–131. [Google Scholar] [CrossRef]
- Cheal, A.J.; Wilson, S.K.; Emslie, M.J.; Dolman, A.M.; Sweatman, H. Responses of reef fish communities to coral declines on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2008, 372, 211–223. [Google Scholar] [CrossRef]
- Bonaldo, R.M.; Hoey, A.S.; Bellwood, D.R. The ecosystem roles of parrotfishes on tropical reefs. Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 81–132. [Google Scholar]
- Cramer, K.L.; O’Dea, A.; Clark, T.R.; Zhao, J.-x.; Norris, R.D. Prehistorical and historical declines in Caribbean coral reef accretion rates driven by loss of parrotfish. Nat. Commun. 2017, 8, 14160. [Google Scholar] [CrossRef]
- Bonaldo, R.; Krajewski, J.; Bellwood, D. Relative impact of parrotfish grazing scars on massive Porites corals at Lizard Island, Great Barrier Reef. Mar. Ecol. Prog. Ser. 2011, 423, 223–233. [Google Scholar] [CrossRef][Green Version]
- Ferreira, C.E.L.; Gonçalves, J.E.A. Community structure and diet of roving herbivorous reef fishes in the Abrolhos Archipelago, south-western Atlantic. J. Fish Biol. 2006, 69, 1533–1551. [Google Scholar] [CrossRef]
- Streit, R.; Hoey, A.; Bellwood, D. Feeding characteristics reveal functional distinctions among browsing herbivorous fishes on coral reefs. Coral Reefs 2015, 34, 1037–1047. [Google Scholar] [CrossRef]
- Nugues, M.M.; Bak, R.P.M. Long-term dynamics of the brown macroalga Lobophora variegata on deep reefs in Curacao. Coral Reefs 2008, 27, 389–393. [Google Scholar] [CrossRef]
- Brokovich, E.; Ayalon, I.; Einbinder, S.; Segev, N.; Shaked, Y.; Genin, A.; Kark, S.; Kiflawi, M. Grazing pressure on coral reefs decreases across a wide depth gradient in the Gulf of Aqaba, Red Sea. Mar. Ecol. Prog. Ser. 2010, 399, 69–80. [Google Scholar] [CrossRef]
- Sheppard, C.; Harris, A.; Sheppard, A. Archipelago-wide coral recovery patterns since 1998 in the Chagos Archipelago, central Indian Ocean. Mar. Ecol. Prog. Ser. 2008, 362, 109–117. [Google Scholar] [CrossRef]
- Marcelino, L.A.; Westneat, M.W.; Stoyneva, V.; Henss, J.; Rogers, J.D.; Radosevich, A.; Turzhitsky, V.; Siple, M.; Fang, A.; Swain, T.D. Modulation of light-enhancement to symbiotic algae by light-scattering in corals and evolutionary trends in bleaching. PLoS ONE 2013, 8, e61492. [Google Scholar] [CrossRef] [PubMed]
- Marlow, J.; Haris, A.; Jompa, J.; Werorilangi, S.; Bates, T.; Bennett, H.; Bell, J.J. Spatial variation in the benthic community composition of coral reefs in the Wakatobi Marine National Park, Indonesia: Updated baselines and limited benthic community shifts. J. Mar. Biol. Assoc. United Kingd. 2020, 100, 37–44. [Google Scholar] [CrossRef]
- Loiseau, N.; Villéger, S.; Le Bozec, C.; Gimenez, M.; Kawahara, S.L.; Claverie, T. Mesophotic reefs are not refugia for neither taxonomic nor functional diversity of reef fishes. Coral Reefs 2023, 42, 63–75. [Google Scholar] [CrossRef]
- Li, S.; Yu, K.; Shi, Q.; Chen, T.; Zhao, M.; Zhao, J. Interspecies and spatial diversity in the symbiotic zooxanthellae density in corals from northern South China Sea and its relationship to coral reef bleaching. Chin. Sci. Bull. 2008, 53, 295–303. [Google Scholar] [CrossRef]
- Houlbrèque, F.; Ferrier-Pagès, C. Heterotrophy in tropical scleractinian corals. Biol. Rev. 2009, 84, 1–17. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Brown, E.K.; Jokiel, P.L.; Smith, W.R.; Rodgers, K.S. Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs 2003, 22, 291–305. [Google Scholar] [CrossRef]
- Quimpo, T.J.R.; Cabaitan, P.C.; Olavides, R.D.D.; Dumalagan, E.E.; Munar, J.; Siringan, F.P. Spatial variability in reef-fish assemblages in shallow and upper mesophotic coral ecosystems in the Philippines. J. Fish Biol. 2019, 94, 17–28. [Google Scholar] [CrossRef]
- MacDonald, C.; Bridge, T.C.L.; Jones, G.P. Depth, bay position and habitat structure as determinants of coral reef fish distributions: Are deep reefs a potential refuge? Mar. Ecol. Prog. Ser. 2016, 561, 217–231. [Google Scholar] [CrossRef]
- Cure, K.; Currey-Randall, L.; Galaiduk, R.; Radford, B.; Wakeford, M.; Heyward, A. Depth gradients in abundance and functional roles suggest limited depth refuges for herbivorous fishes. Coral Reefs 2021, 40, 365–379. [Google Scholar] [CrossRef]
- Asher, J.; Williams, I.D.; Harvey, E.S. Mesophotic Depth Gradients Impact Reef Fish Assemblage Composition and Functional Group Partitioning in the Main Hawaiian Islands. Front. Mar. Sci. 2017, 4, 98. [Google Scholar] [CrossRef]
- Levy, N.; Simon-Blecher, N.; Ben-Ezra, S.; Yuval, M.; Doniger, T.; Leray, M.; Karako-Lampert, S.; Tarazi, E.; Levy, O. Evaluating biodiversity for coral reef reformation and monitoring on complex 3D structures using environmental DNA (eDNA) metabarcoding. Sci. Total Environ. 2023, 856, 159051. [Google Scholar] [CrossRef]
- Alexander, J.B.; Bunce, M.; White, N.; Wilkinson, S.P.; Adam, A.A.S.; Berry, T.; Stat, M.; Thomas, L.; Newman, S.J.; Dugal, L.; et al. Development of a multi-assay approach for monitoring coral diversity using eDNA metabarcoding. Coral Reefs 2020, 39, 159–171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Lin, L.; Liu, Y.; Wang, T.; Liu, Y.; Xiao, Y.; Shen, J.; Xie, H.; Huang, H.; Han, Q. eDNA Metabarcoding Reveals the Depth-Structured Variation of Coral Reef Fish. Fishes 2025, 10, 209. https://doi.org/10.3390/fishes10050209
Zhao J, Lin L, Liu Y, Wang T, Liu Y, Xiao Y, Shen J, Xie H, Huang H, Han Q. eDNA Metabarcoding Reveals the Depth-Structured Variation of Coral Reef Fish. Fishes. 2025; 10(5):209. https://doi.org/10.3390/fishes10050209
Chicago/Turabian StyleZhao, Jinfa, Lin Lin, Yong Liu, Teng Wang, Yu Liu, Yayuan Xiao, Jianzhong Shen, Hongyu Xie, Hai Huang, and Qiuying Han. 2025. "eDNA Metabarcoding Reveals the Depth-Structured Variation of Coral Reef Fish" Fishes 10, no. 5: 209. https://doi.org/10.3390/fishes10050209
APA StyleZhao, J., Lin, L., Liu, Y., Wang, T., Liu, Y., Xiao, Y., Shen, J., Xie, H., Huang, H., & Han, Q. (2025). eDNA Metabarcoding Reveals the Depth-Structured Variation of Coral Reef Fish. Fishes, 10(5), 209. https://doi.org/10.3390/fishes10050209





