1. Introduction
Global climate change has profoundly affected marine ecosystems and fisheries production. Since the 1970s, rising anthropogenic greenhouse gas (GHG) emissions have led to a continuous increase in global sea surface temperature (SST) and upper ocean (0–700 m) temperature [
1], while ocean acidification due to decreasing pH levels has also been intensifying. Notably, the frequency of marine heatwaves has nearly doubled since the 1980s, and the occurrence of hot extremes and heatwaves on land has become more frequent. Concurrently, rising sea levels and changes in ocean circulation have negatively affected fisheries and shellfish aquaculture production [
1].
The World Meteorological Organization [
2] reports that global glaciers have shrunk by an average of 1 m per year over the past decade, while sea levels have risen at an average rate of 4.5 mm per year. Additionally, ocean acidification is progressing more rapidly in the mid-latitude regions of the North Pacific than in other regions, accelerating coral reef degradation and altering fish habitats [
2]. These transformations are expected to significantly impact not only global marine ecosystems but also Korea’s coastal and offshore fisheries.
According to the National Institute of Fisheries Science [
3], the annual mean SST in Korean waters has increased by approximately 1.44 °C over the past 56 years (1968–2023), with an average warming rate of 0.026 °C per year. In contrast, the global mean SST has risen by 0.70 °C over the same period, with a warming rate of 0.0125 °C per year, demonstrating that the SST increase in Korea’s coastal waters has been more than twice as fast as the global average.
This rapid warming of Korea’s coastal and offshore waters is expected to have profound implications for fisheries production, potentially influencing species distribution, recruitment success, and trophic interactions. While multiple factors contribute to fluctuations in fisheries production, climate variability is increasingly acknowledged as a significant influence. Korea’s coastal and offshore fisheries production, which peaked at approximately 1,725,820 tons in 1986, has since declined to 1,011,536 tons in 2018 and further dropped below the symbolic threshold of 1 million tons in 2023, reaching 951,722 tons.
Notably, major pelagic fish species such as chub mackerel (
Scomber japonicus) and Japanese anchovy (
Engraulis japonicus), which showed an increasing trend until the early 2000s, have exhibited a recent declining trend, while Japanese common squid (
Todarodes pacificus) has experienced a significant decline in catch since the 2010s [
3,
4,
5,
6,
7]. Likewise, once-dominant species in the 1980s–1990s, such as filefish (
Stephanolepis cirrhifer), walleye pollock (
Gadus chalcogrammus), and Japanese sardine (
Sardinops melanostictus), have undergone severe stock depletion since the 2000s [
3,
4,
8,
9].
In contrast, warm-water species, including Japanese amberjack (
Seriola quinqueradiata), Japanese horse mackerel (
Trachurus japonicus), and Japanese Spanish mackerel (
Scomberomorus niphonius), have shown a steady increase in catch over the past four decades, reflecting shifts in species composition driven by ocean warming [
3,
10,
11,
12]. These shifts in species composition highlight the need for further empirical research to quantify the impact of climate variability on fisheries production in Korean waters.
While previous studies in Korea have predominantly focused on the impacts of climate change on agricultural production [
13,
14,
15,
16,
17], empirical research specifically addressing the effects of climate variability on coastal and offshore fisheries remains limited. Although climate-driven shifts in marine ecosystems have raised concerns, comprehensive quantitative analyses on the combined effects of SST rise, carbon dioxide (CO
2)-induced ocean acidification, and precipitation variability on fisheries production in Korean waters remain scarce.
To address these research gaps, this study investigates the dynamic relationships between total CO
2 emissions, annual mean SST, annual mean precipitation, and coastal and offshore fisheries production in Korea using the autoregressive distributed lag (ARDL) model. While numerous studies have examined the impacts of climate change on fisheries production [
18,
19,
20,
21,
22] using correlation-based and regression-based models, this study offers a more thorough analysis by distinguishing between short-run and long-run effects through the ARDL bounds testing approach for cointegration. Furthermore, to ensure the robustness of the ARDL estimates, two widely used cointegration techniques—Fully Modified Ordinary Least Squares (FMOLS) and Canonical Cointegrating Regression (CCR)—are employed as complementary validation methods.
By integrating multiple climatic stressors into a unified empirical framework, this study quantitatively assesses the combined effects of ocean warming, acidification, and hydrological variability on fisheries production. Using the ARDL bounds testing approach, this research estimates the elasticities of fisheries production in response to changes in SST, CO2 emissions, and precipitation, offering quantitative insights into climate-induced pressures on Korea’s fisheries sector.
The findings of this study contribute to empirical research on climate-resilient fisheries management. Identifying key climatic drivers of declining fisheries production facilitates the development of evidence-based adaptive management strategies, enhances stock assessment models, and supports international cooperation for the sustainable management of transboundary fish stocks. Moreover, this study emphasizes the importance of climate-resilient fisheries policies, including flexible harvest regulations, ecosystem-based management strategies, and increased investment in sustainable aquaculture.
The structure of this paper is as follows.
Section 2 reviews the existing literature on the effects of climate variability on fisheries production, with a particular focus on SST, CO
2 emissions, and precipitation variability.
Section 3 describes the data sources, variable definitions, and econometric methodology employed in this study.
Section 4 presents the empirical findings, including stationarity diagnostics, cointegration analysis, and ARDL model estimation results. Finally,
Section 5 interprets the key findings, discusses their policy implications, and outlines study limitations, concluding with recommendations for future research and climate-resilient fisheries management.
4. Empirical Results and Model Validation
4.1. Stationarity Tests and Optimal Lag Selection
This study applies unit root tests to determine the integration order of the variables and ensure that none exhibit I (2) behavior, which would invalidate the ARDL model. In time-series econometrics, stationarity implies that a series maintains stable statistical properties, including variance, covariance, and mean, over time. The ARDL model is valid only when the variables are integrated at order I (0) or I (1), as the presence of I (2) or higher-order integration can lead to estimation issues, such as spurious regression and model misspecification.
The results presented in
Table 3 confirm that all variables are either I (0) or I (1), satisfying the stationarity requirements for ARDL estimation. Since the ARDL model accommodates regressors with different integration orders, all variables were analyzed in their log-transformed levels to improve consistency and maintain economic interpretability.
Given the mixed integration orders—LSST and LPREC being stationary at level [I (0)], and LCOFP and LCO2 being integrated of order one [I (1)]—the ARDL bounds testing approach is particularly well-suited for this study. The ARDL framework is specifically designed to handle such combinations, provided none of the variables is integrated of order two [I (2)]. This flexibility, along with its applicability in small-sample contexts, ensures both the reliability and efficiency of the estimation procedure.
For robustness, unit root tests were conducted using both the Augmented Dickey-Fuller (ADF) and Phillips–Perron (PP) tests, yielding consistent results.
After confirming the integration order, the optimal lag length was determined based on multiple information criteria to enhance the robustness of model specification. Choosing an appropriate lag structure is essential for adequately capturing both short-run dynamics and long-run equilibrium relationships in the model.
Table 4 presents the lag order selection criteria for the vector autoregression (VAR) model. The AIC criterion indicates that a lag length of 4 minimizes information loss, making it the most suitable lag specification. Among the various information criteria, AIC was preferred as it selects more flexible lag structures, thereby improving the model’s ability to capture complex dynamic relationships among variables.
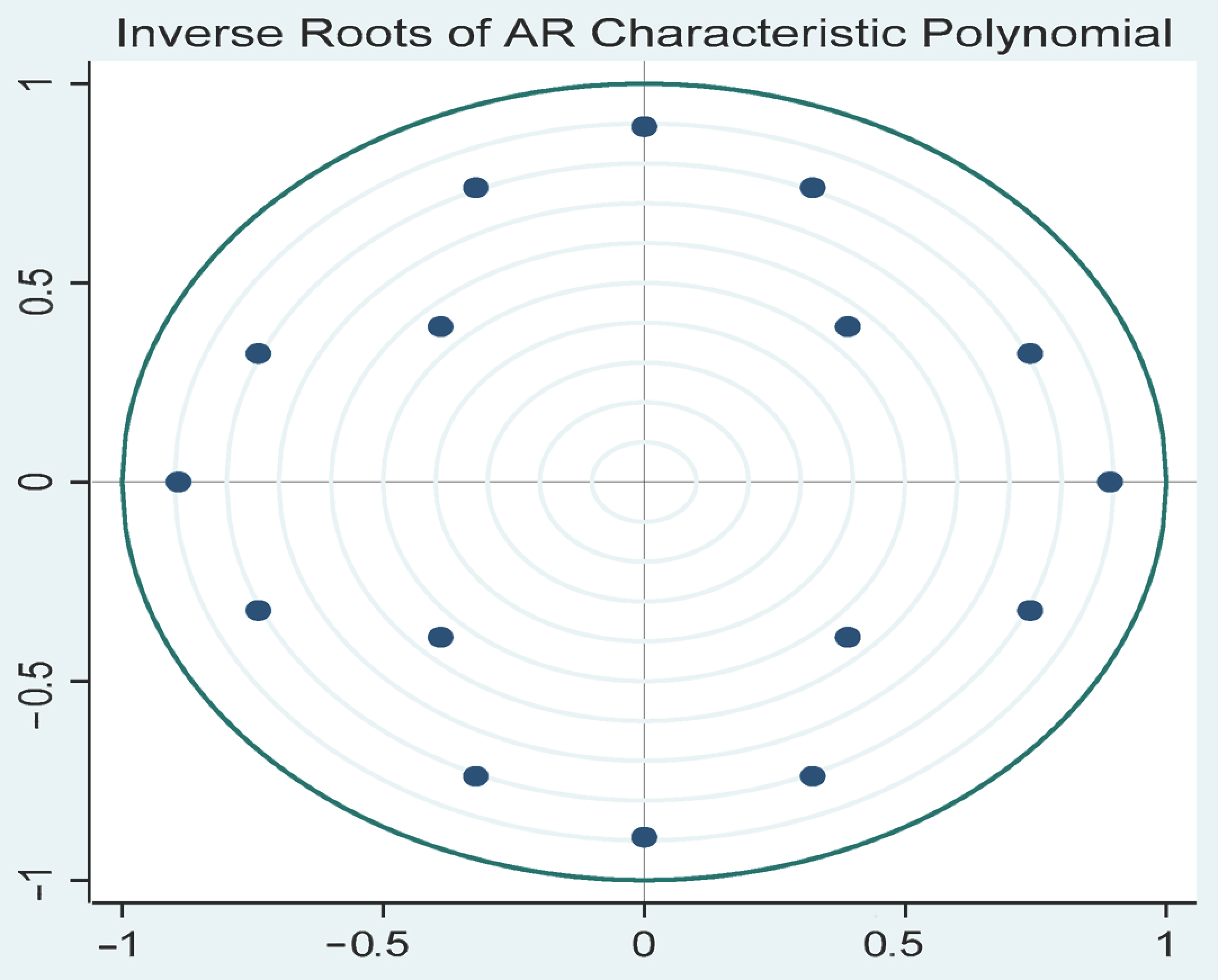
To further evaluate the stability of the estimated VAR model, the inverse roots of the characteristic polynomial were examined (
Figure 2). The results confirm that all roots lie within the unit circle, indicating that the model satisfies the necessary stability conditions for valid statistical inference. Ensuring model stability is crucial, as an unstable VAR process can lead to explosive behavior, potentially compromising the reliability of impulse response functions and variance decomposition.
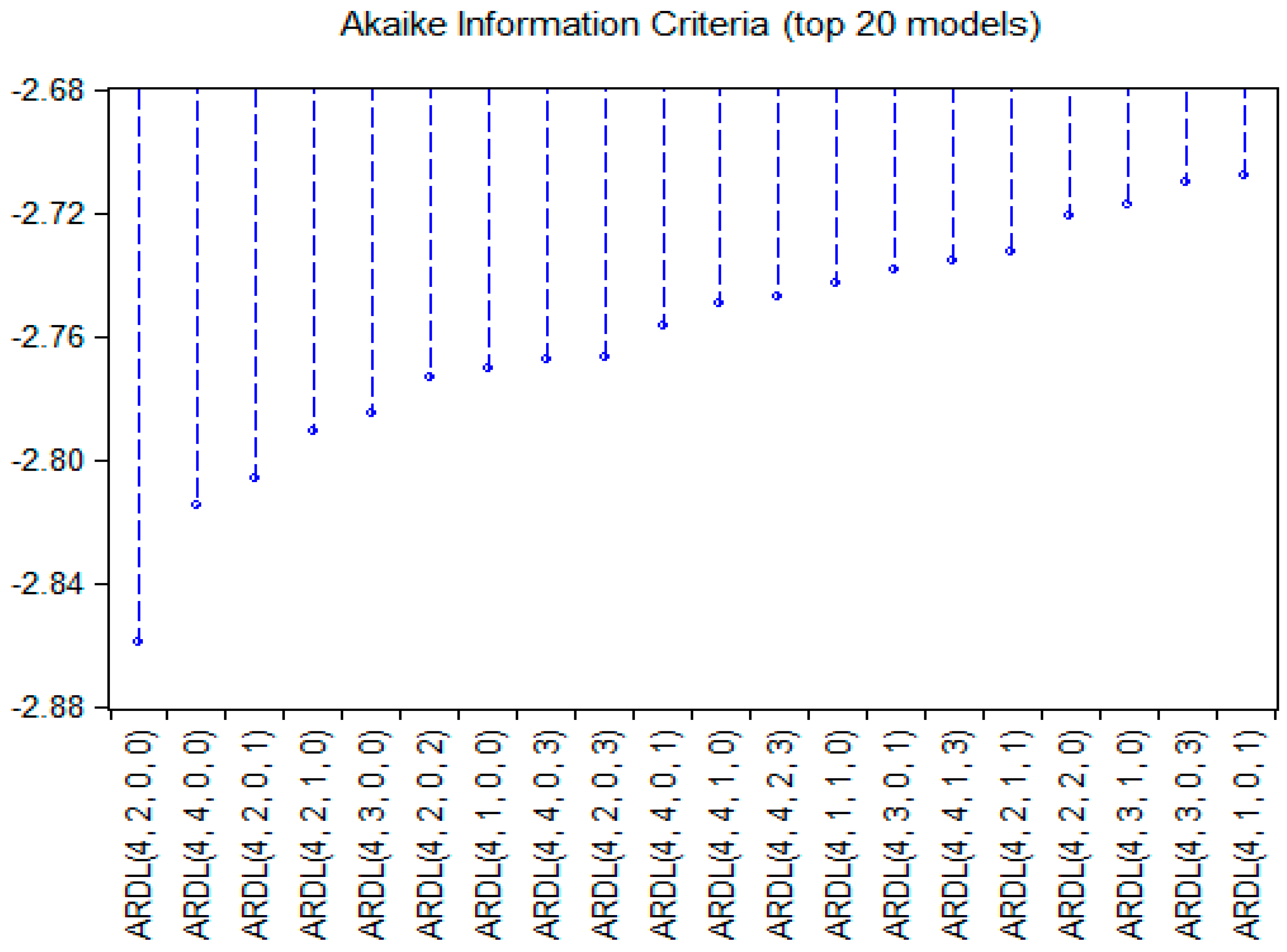
Subsequently, we identified the optimal lag structure for the ARDL model by evaluating various specifications using the AIC. To enhance methodological transparency and robustness, the top 20 models ranked by AIC values are illustrated in
Figure 3. Among these, the ARDL (4, 2, 0, 0) model exhibited the lowest AIC, reflecting the most favorable balance between goodness-of-fit and model parsimony.
This specification also satisfies the lag-length sufficiency condition for capturing both immediate and delayed dynamics in the dependent and key explanatory variables, especially given the limited sample size.
Therefore, this specification was adopted for the main empirical analysis.
4.2. The ARDL Bounds Test for Cointegration
The ARDL bounds test was conducted to assess the cointegration relationship between Korea’s LCOFP and key climatic variables, namely LSST, LCO2, and LPREC. This test determines whether a stable long-run equilibrium relationship exists between the dependent and independent variables, confirming the presence of cointegration.
Since cointegration was confirmed, the ARDL model was extended to estimate the corresponding ECM, which captures short-run dynamics and quantifies the speed of adjustment toward long-run equilibrium.
As shown in
Table 5, the computed F-statistic (5.8280) exceeds the upper bound critical value at the 5% significance level (5.4260), leading to the rejection of the null hypothesis of no cointegration.
Thus, for the period 1993–2023, the results provide strong empirical evidence of a long-run equilibrium relationship among Korea’s LCOFP, LSST, LCO2, and LPREC.
Accordingly, the ARDL model was employed to estimate both the long-run relationships and the ECM, enabling the quantification of short-run adjustments toward equilibrium.
4.3. Long and Short-Run Estimations
The ARDL bounds test confirms the presence of a long-run cointegration relationship, allowing the estimation of both long-run and short-run elasticities.
Table 6 summarizes these estimation results, highlighting the key dynamics among the variables.
In the long run, LSST, LCO2, and LPREC are statistically significantly associated with COFP at the 1% level. Specifically, a 1% increase in LSST is associated with a 3.52% decrease in COFP, suggesting that rising sea surface temperatures may negatively influence fisheries production. Similarly, a 1% increase in LCO2 is linked to a 0.82% decline in COFP, reflecting the potential environmental stress imposed by elevated carbon emissions. Additionally, a 1% increase in LPREC corresponds to a 0.34% reduction in COFP, indicating a possible association between precipitation variability and long-term changes in fisheries output. These findings provide robust statistical evidence of a significant long-run equilibrium relationship between climatic factors and fisheries production.
Given the established long-run cointegration, short-run elasticities were estimated. The lagged values of COFP exhibit varying effects on short-term production. A 1% increase in COFP from two periods ago (LCOFP (−2)) is associated with an approximately 0.49% increase in the current level of COFP at the 10% significance level, while a 1% increase in COFP from three periods ago (LCOFP (−3)) is linked to a larger 0.78% increase at the 1% level. This suggests that past fluctuations in fisheries production may have meaningful associations with current output, with a stronger relationship observed for longer lags.
The immediate effect of LSST on COFP (LSST) is statistically insignificant, indicating that short-term fluctuations in sea surface temperature do not exert an immediate impact on fisheries production. However, the lagged effect (LSST (−1)) remains negative, though statistically insignificant, suggesting that while short-term climatic variations may not exhibit an immediate influence, their cumulative impact over multiple periods remains economically relevant.
The error correction term (CointEq (−1) = −0.75, p < 0.01) is negative and highly significant, confirming that short-term deviations from the long-run equilibrium are adjusted toward equilibrium at an annual rate of approximately 75.33%. This implies that the system exhibits a strong tendency to revert to its equilibrium path within roughly one year and four months (1.33 years) following a shock. The relatively rapid adjustment speed suggests that short-run deviations from the long-run equilibrium are corrected efficiently, indicating a stable dynamic response of Korea’s COFP to external climatic variability.
4.4. Model Diagnostics and Structural Stability Tests
The reliability and stability of the ARDL model were evaluated through a series of diagnostic tests. The results, presented in
Table 7, indicate that the model does not exhibit issues related to serial correlation, heteroskedasticity, or non-normality. Additionally, the Ramsey RESET test does not suggest significant misspecification, confirming that the chosen functional form is appropriate.
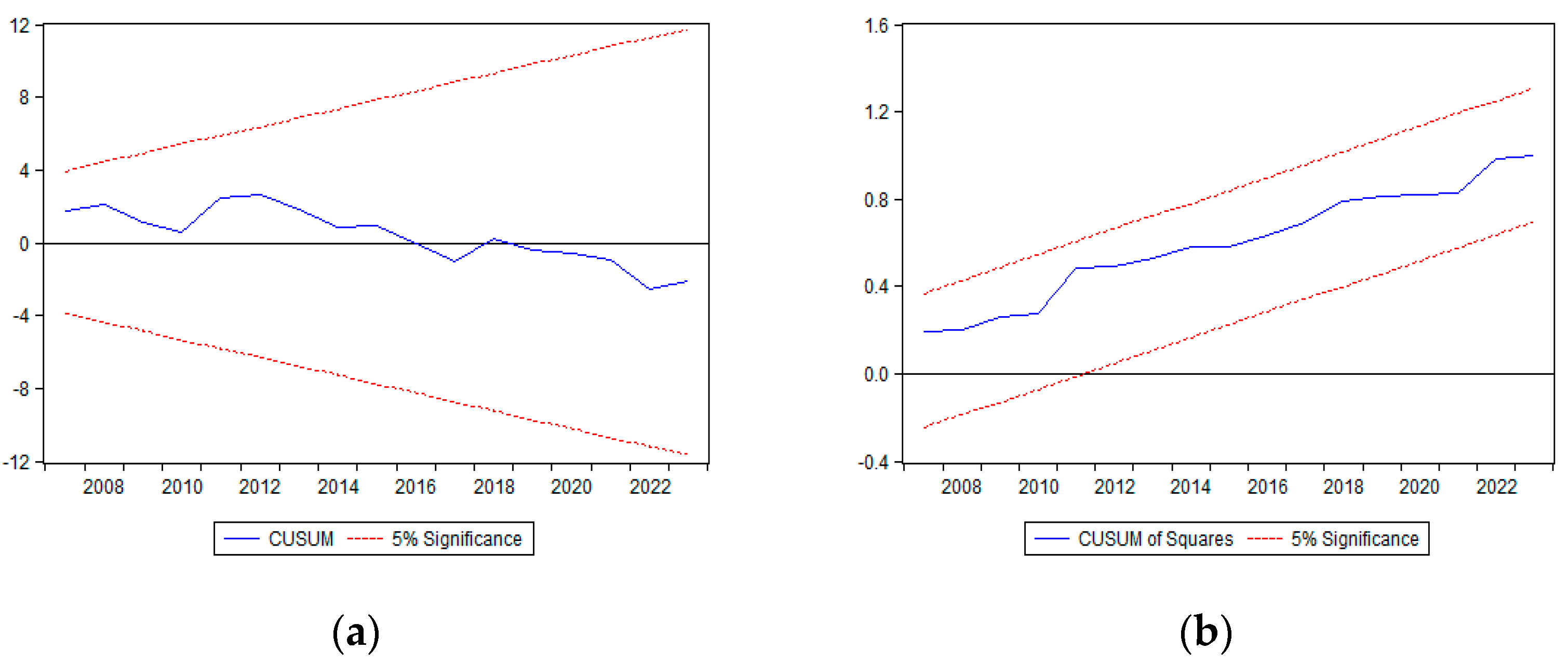
The structural stability of the estimated parameters was assessed using the CUSUM and CUSUM of squares tests. As shown in
Figure 4, both test statistics remain well within the 5% significance bounds, suggesting that the estimated coefficients do not exhibit substantial structural breaks or instability.
All diagnostic tests confirm that the ARDL model satisfies key econometric assumptions, supporting its robustness for empirical analysis. The Breusch–Godfrey LM test (p = 0.15) suggests the absence of serial correlation, while the Breusch–Pagan–Godfrey test (p = 0.35) confirms that residuals are homoskedastic. The Jarque–Bera test (p = 0.68) indicates that the residuals follow a normal distribution, suggesting that the estimated parameters are unbiased and consistent. Furthermore, the Ramsey RESET test (p = 0.49) does not reveal any major misspecification, implying that the functional form of the model is correctly specified.
To further assess parameter stability, the CUSUM and CUSUM of squares tests were performed. As illustrated in
Figure 4a, the CUSUM test statistic remains within the 5% significance bounds, indicating no significant structural breaks in the estimated coefficients. Similarly,
Figure 4b presents the results of the CUSUM of squares test, which shows a gradual increase but remains within the critical bounds. This suggests that while minor parameter variability may exist, the overall stability of the model is maintained throughout the sample period.
These results confirm that the estimated ARDL model is structurally stable, reinforcing its suitability for long-term fisheries production analysis under changing climatic conditions.
4.5. Robustness Assessment Using Alternative Estimation Methods
The robustness of the ARDL estimation results was evaluated using FMOLS and CCR as alternative estimation techniques (
Table 8). These methods assume the presence of a single cointegrating relationship and apply semi-parametric corrections to mitigate potential biases arising from long-run correlations between the cointegrating equation and stochastic disturbances. Comparing these estimates with the ARDL results provides a comprehensive assessment of the stability and reliability of the estimated long-run coefficients.
The FMOLS and CCR estimations were performed using the Bartlett kernel and the Newey–West automatic bandwidth selection. The Bartlett kernel is widely employed in long-run variance estimation, offering an optimal balance between bias and efficiency. Meanwhile, the Newey–West automatic bandwidth selection ensures that standard errors remain robust to heteroskedasticity and autocorrelation, thereby enhancing the reliability of statistical inference.
The estimation results confirm that all key variables remain statistically significant in the long run across all estimation methods, reinforcing the validity of the ARDL model. The coefficients for LCO2 and LPREC in FMOLS and CCR closely align with the ARDL estimates, particularly in terms of sign direction and statistical significance, with only minor variations in magnitude. However, the SST coefficient exhibits a more noticeable discrepancy across estimation methods—for example, −3.52 in the ARDL model compared to approximately −1.85 in both FMOLS and CCR. This difference likely stems from methodological and structural distinctions among the estimators. While FMOLS and CCR isolate long-run equilibrium relationships under static assumptions, the ARDL framework incorporates dynamic lag structures that can interact with underlying data properties—such as autocorrelation and lagged feedback effects—to influence long-run coefficient estimation. Moreover, although ARDL formally distinguishes between short-run and long-run components, the presence of higher-order lags and finite sample effects can induce sensitivity in parameter estimates, particularly when the dependent variable exhibits strong inertia. Therefore, the larger magnitude of the SST elasticity in the ARDL specification may reflect its greater responsiveness to dynamic adjustment mechanisms and short-horizon volatility, which are abstracted away in FMOLS and CCR formulations.
Despite these differences, the overall consistency in statistical significance across estimation methods confirms the robustness of the ARDL long-run model. These findings suggest that the relationships identified within the ARDL framework remain structurally stable across different econometric approaches, further validating the model’s reliability for empirical analysis and policy implications.
5. Discussion and Conclusions
5.1. Summary of Empirical Findings: Effects of SST, CO2, and PREC on COFP
This study presents empirical evidence that increases in LSST and LCO2, as well as variability in LPREC, are statistically associated with long-term reductions in Korea’s COFP. The ARDL analysis confirms that these climatic variables exhibit statistically significant negative elasticities, suggesting potential long-term associations with marine ecosystem stress.
Specifically, a 1% increase in LSST is associated with a 3.52% decline in COFP, suggesting that ocean warming may negatively influence fisheries production. Similarly, a 1% increase in LCO2 is linked to a 0.82% reduction in COFP, consistent with the hypothesis that rising carbon emissions could exert environmental stress through ocean acidification. Additionally, a 1% increase in LPREC corresponds to a 0.34% decline in COFP, indicating a potential association between hydrological variability and changes in coastal oceanographic conditions and nutrient dynamics. Robustness checks using FMOLS and CCR estimations corroborate the statistical reliability of these long-run associations.
Over the study period (1993–2023), Korea’s annual average COFP was 1,153,201 tons. Climate variability appears to be associated with the decline of key commercial species, potentially contributing to disruptions in fisheries production. The observed negative elasticity of LSST (−3.52%) may reflect ecological stressors related to rising temperatures, such as altered species distributions, recruitment challenges, and changes in trophic dynamics. Likewise, the negative associations with LCO2 and LPREC variability underscore the potential compounded pressures of ocean acidification and hydrological fluctuations on marine resource production.
5.2. Effects of Rising SST on COFP
Rising SST appears to be statistically associated with long-term declines in Korea’s COFP, potentially through ecological mechanisms such as shifts in species distributions, reduced recruitment success, and disruptions in trophic interactions. The persistent downturn in COFP aligns closely with the observed collapse of key commercial species, including Japanese common squid, walleye pollock, and Japanese sardine. The pronounced reduction in landings of these species—occurring alongside sustained SST increases—suggests that warming waters may have exerted substantial pressure on fisheries production. These patterns are consistent with prior studies indicating that temperature-sensitive species are particularly vulnerable to recruitment failure, habitat compression, and range shifts under rising thermal stress.
Among the affected species, Japanese common squid—one of the most commercially important cephalopods in Korea and Japan—has experienced a dramatic population decline. Korea’s annual landings fell from 252,618 tons in 1996 to 23,375 tons in 2023 (−90.7%), while Japan’s landings decreased from 253,840 tons in 2003 to 29,700 tons in 2022 (−88.3%). The negative elasticity of SST (−3.52%) observed in this study may reflect the ecological stressors associated with these population declines.
Empirical research suggests that rising SST alters the spatial distribution of Japanese common squid, shifting their migration patterns northward and reducing their presence in traditional fishing grounds [
7,
32]. Furthermore, warming waters disrupt optimal spawning conditions. Japanese common squid eggs and larvae exhibit the highest survival rates at SSTs between 19.5 °C and 23 °C; however, continued warming is expected to push spawning areas beyond suitable habitats, leading to recruitment failures [
50,
51]. These ecological disruptions pose a significant threat to the long-term sustainability of Japanese common squid stocks.
Similarly, walleye pollock and Japanese sardine, both cold-water species, have suffered severe population declines due to rising SST. Walleye pollock landings declined from 165,837 tons at their peak to just 9 tons in 2018, while Japanese sardine landings fell from 194,352 tons to 90 tons in 2021, rendering their commercial fisheries nearly unsustainable.
Previous studies indicate that walleye pollock’s optimal spawning and hatching temperatures range from 2 °C to 7 °C, with hatching success declining significantly when SST exceeds this threshold [
52,
53]. Rising SST has impaired early life stage survival, as larval mortality rates increase when water temperatures surpass physiological limits [
8,
53]. Long-term SST trend analyses in the East Sea reveal that since the 1990s, winter SST in mid-to-northern waters has consistently exceeded 7 °C, negatively affecting spawning success and larval survival [
54,
55]. Additionally, the northward shift of spawning grounds due to warming waters has reduced larval survival rates in Korean waters [
56]. Changes in ocean currents have further disrupted the transport of walleye pollock larvae to suitable nursery grounds, increasing mortality rates and recruitment failures [
54,
56]. These findings suggest that while historical fishing pressure may have contributed to the initial decline of walleye pollock, the sustained population collapse appears to be primarily driven by SST-induced ecological and oceanographic changes.
Likewise, rising SST has directly impacted the recruitment success and distribution of Japanese sardines. Japanese sardines thrive within an optimal temperature range of 10 °C to 17 °C, but continued SST rise has pushed temperatures beyond this threshold, reducing suitable spawning areas [
57]. Additionally, warming-induced disruptions in planktonic ecosystems have led to food scarcity during critical early life stages, further exacerbating recruitment failures. Shifts in phytoplankton and zooplankton communities, driven by rising SST, have significantly reduced larval food availability, increasing juvenile mortality rates [
58,
59]. These mechanisms have contributed to the ongoing decline of Japanese sardine stocks, mirroring the trend observed in walleye pollock.
Furthermore, changes in ocean circulation have altered sardine migration patterns, limiting their presence in traditional fishing grounds in Korean waters [
60]. Similarly, rising SST and associated oceanographic changes—including altered currents and planktonic shifts—have disrupted prey availability and migration in other commercially important species, such as yellow croaker (
Larimichthys polyactis) and Japanese sandfish (
Arctoscopus japonicus) [
61,
62,
63].
These findings align with previous research demonstrating that SST-driven changes in recruitment and habitat availability have been central to the decline of cold-water fisheries in the Northwest Pacific [
57,
64]. The widespread impact of rising SST across multiple species underscores its significant ecological consequences, highlighting the urgency of understanding climate-driven shifts in fisheries production.
5.3. Effects of Increasing CO2 Emissions on COFP
The increase in atmospheric CO
2 emissions is a major driver of oceanic changes, particularly ocean acidification and rising SST, both of which impose long-term ecological stress on marine ecosystems [
43]. The continuous accumulation of atmospheric CO
2 enhances radiative forcing, leading to greater ocean heat absorption and subsequent SST rise, while also driving fundamental alterations in seawater carbonate chemistry, including decreased pH and reduced carbonate ion availability [
43,
65,
66]. These chemical shifts disrupt critical biological processes in marine organisms, weaken stock resilience, and ultimately contribute to the long-term decline in fisheries production.
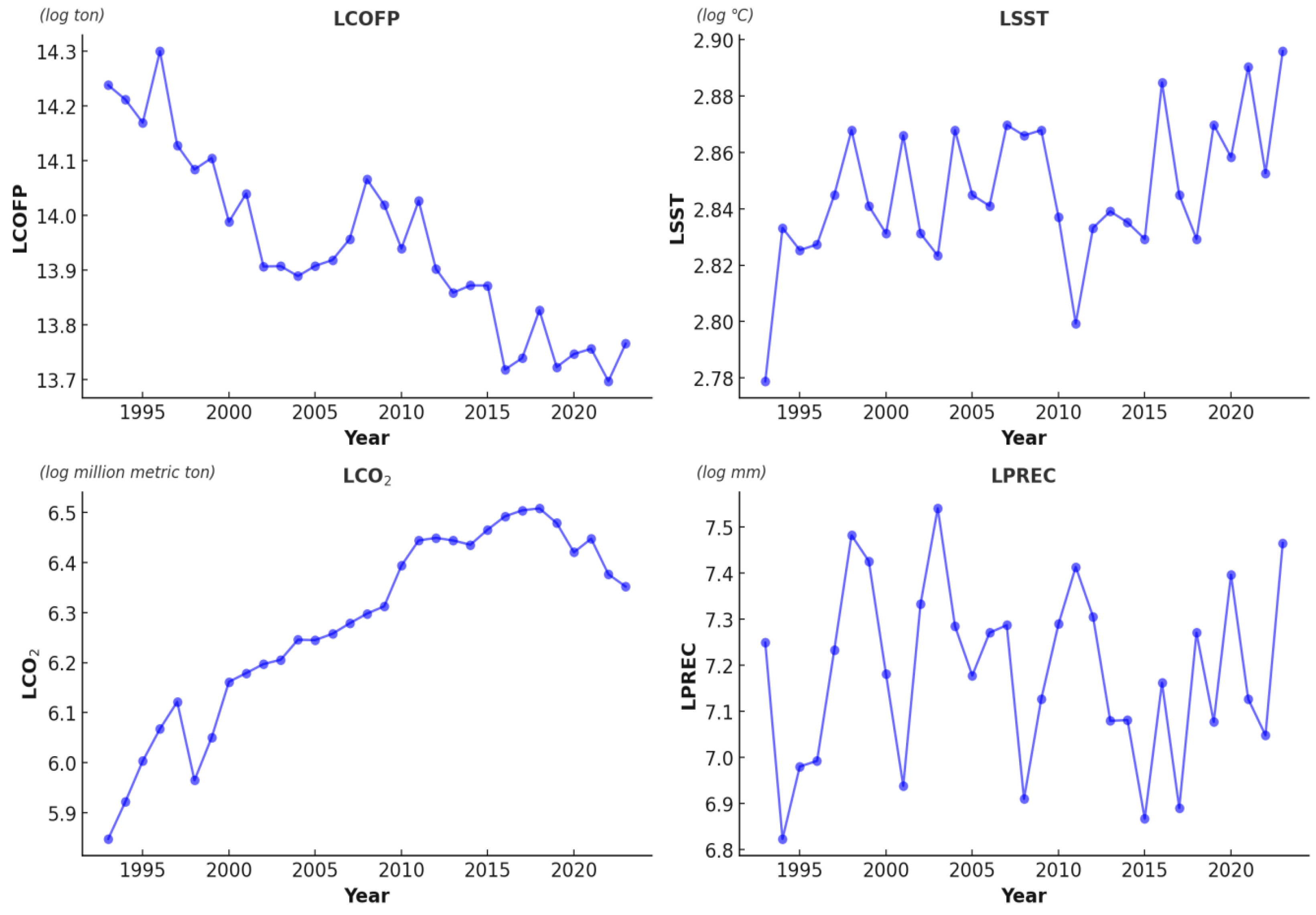
Korea’s CO
2 emissions have steadily increased over the past three decades, rising from 346.4 million metric tons in 1993 to 573.5 million metric tons in 2023 (
Figure 1). As of 2023, Korea ranks 10th globally in total CO
2 emissions and 17th in per capita emissions, reflecting its substantial contribution to global carbon loading. Given that Korea’s fisheries sector primarily relies on coastal and offshore fisheries, the intensification of ocean warming and acidification due to rising CO
2 levels is expected to exert increasing pressure on COFP. The estimated negative elasticity of CO
2 emissions (−0.82%) in this study indicates a statistically significant association with reduced fisheries production, consistent with prior research on the ecological consequences of ocean acidification.
One of the most direct consequences of ocean acidification is its detrimental impact on fish sensory and metabolic functions. Elevated CO
2 concentrations impair olfactory discrimination, predator detection, and habitat selection in fish, leading to higher mortality rates and recruitment failures [
25,
26,
37]. These disruptions may destabilize population dynamics, potentially contributing to long-term variability in fisheries production.
Beyond its physiological effects, ocean acidification also alters the structure of marine food webs. The reduction in carbonate ion availability affects calcifying organisms, such as oysters, mussels, and clams, which are key components of coastal fisheries [
33]. In particular, planktonic species that form the foundation of marine food chains are highly sensitive to acidification, leading to cascading trophic effects that limit food availability for higher trophic-level fish species [
43]. Additionally, coral reef degradation, driven by reduced calcification rates, threatens reef-associated fish populations, further compounding ecosystem disruptions [
43].
The synergistic effects of SST rise and ocean acidification further intensify ecosystem stress, leading to declines in marine biodiversity and reduced fisheries resilience. Rising ocean temperatures accelerate metabolic rates in fish, increasing energy demands, while acidification reduces their ability to process nutrients and adapt to shifting environmental conditions. These interacting stressors contribute to lower catch volumes, declining fishery stocks, and heightened economic vulnerabilities for coastal fishing communities [
67].
Given Korea’s high CO2 emissions, this study’s findings suggest that continued CO₂ emissions may intensify pressures on Korea’s fisheries sector, potentially contributing to long-term structural changes in marine ecosystems and production outcomes.
5.4. Effects of Increasing PREC on COFP
The ARDL results confirm that PREC variability is statistically significantly associated with a negative impact on COFP. Specifically, a 1% increase in LPREC corresponds to a 0.34% decline in COFP, suggesting that hydrological fluctuations may influence marine production. While the estimated negative elasticity of LPREC (−0.34%) is smaller than that of LSST (−3.52%) and LCO2 (−0.82%), precipitation-driven oceanographic variability has been linked to cascading ecological effects that could affect fisheries resources.
Precipitation variability is known to influence marine ecosystems through changes in freshwater discharge, which, in turn, can alter salinity levels, nutrient cycling, and primary production. For instance, increased precipitation may enhance riverine input and introduce nutrients that support phytoplankton growth under moderate conditions [
38]. However, excessive freshwater influx can lower salinity beyond the tolerance range of marine species, particularly stenohaline organisms, while increasing turbidity and sedimentation—both of which can reduce light penetration and constrain primary production [
38,
39]. These environmental disturbances may propagate through trophic levels, potentially affecting fish populations and overall fisheries yields.
Moreover, precipitation variability has been associated with hypoxia formation and harmful algal blooms (HABs). Enhanced terrestrial runoff during intense rainfall events elevates nitrogen and phosphorus concentrations in coastal waters, which can trigger eutrophication and promote HABs that deplete dissolved oxygen and release toxins harmful to marine life [
68]. Extreme precipitation may further intensify these disruptions by accelerating sediment loads and triggering sudden ecosystem imbalances.
Korea exhibits considerable interannual precipitation variability, primarily driven by large-scale climatic systems such as the East Asian Summer Monsoon [
41] (
Figure 1). Shifts in monsoonal patterns have increased the frequency of extreme rainfall events and prolonged droughts, both of which have been observed to coincide with reductions in marine productivity [
1]. Sudden freshwater influxes can disrupt spawning grounds, alter salinity gradients, and affect larval transport, with potential implications for recruitment success in key commercial species.
As climate change continues, rising atmospheric CO
2 concentrations are projected to alter global and regional precipitation regimes by influencing large-scale atmospheric circulation [
1]. These changes may increase the frequency and intensity of hydrological extremes, adding further stress to marine ecosystems. The combined influence of SST rise, increasing CO
2 emissions, and precipitation variability underscores the interconnected nature of climate-driven oceanographic shifts, supporting the need for an integrated and adaptive approach to fisheries management.
5.5. Policy Implications and Conclusions
The findings of this study suggest that climate-induced oceanographic changes—rising SST, increasing CO2 emissions, and PREC variability—are statistically associated with persistent declines in Korea’s COFP. These factors have collectively contributed to long-term structural shifts in fisheries resources, altering species distribution, recruitment success, and overall ecosystem stability. Without immediate policy interventions, these changes are expected to further destabilize fish stocks and compromise the sustainability of the fishing industry. Therefore, an integrated and climate-resilient approach is required to mitigate the adverse effects of climate change on Korea’s fisheries sector.
A key policy implication is the need to restructure fisheries management frameworks to accommodate climate-driven shifts in fish stocks. Traditional static total allowable catch systems may no longer be effective in regulating fisheries, as species distributions continue to shift due to SST rise. A more adaptive fisheries management approach, incorporating dynamic stock assessments, climate-responsive harvest regulations, and real-time monitoring of climate indicators, would enable more flexible and evidence-based management strategies [
69,
70,
71]. By integrating SST, ocean acidification, and precipitation variability into stock assessment models, policymakers can enhance forecasting accuracy and minimize uncertainty in fisheries resource management.
International cooperation is another critical component in addressing transboundary fisheries management challenges. Many commercially valuable species, such as walleye pollock, Japanese sardine, and Japanese common squid, exhibit migratory patterns that cross national jurisdictions, making unilateral approaches insufficient. Korea must strengthen bilateral and multilateral agreements with neighboring countries—Japan, China, and Russia—to facilitate joint stock assessments, coordinated harvest regulations, and shared climate impact data. Enhanced regional cooperation will be essential for ensuring the long-term sustainability of shared fisheries resources and mitigating potential geopolitical conflicts over shifting fish stocks.
Additionally, diversifying fisheries and promoting climate-resilient species should be prioritized to reduce vulnerability to climate-induced habitat shifts. Korea’s fisheries remain heavily reliant on a narrow range of commercially dominant species, which increases susceptibility to stock collapses. Developing and promoting alternative species that are more tolerant to SST rise and acidification could enhance industry resilience. Expanding sustainable aquaculture practices would help stabilize fisheries production while alleviating pressure on overexploited wild stocks.
In parallel with aquaculture, stock enhancement programs that involve the release of climate-resilient fish species could further support fisheries sustainability. Selective breeding and genetic improvement programs for temperature-tolerant and acidification-resistant species have gained increasing attention as adaptive strategies to climate change. Korea has already implemented large-scale seed release programs for various commercially valuable species, and further expansion of these efforts could enhance stock recovery and ecological stability [
72,
73,
74]. Developing new candidate species with high thermal and acidification tolerance, particularly those adapted to rising SST and shifting oceanographic conditions, will be essential for securing long-term fisheries sustainability.
Marine conservation and habitat restoration will also be critical in counteracting the adverse effects of climate change on fisheries production. Korea’s high CO
2 emissions contribute to ocean acidification, which weakens calcifying organisms and disrupts marine food web dynamics. Expanding Marine Protected Areas, artificial reef programs, and blue carbon ecosystem restoration projects (e.g., seagrass and salt marsh restoration) can enhance ecosystem resilience while improving water quality and providing essential nursery habitats for commercially valuable fish species [
75,
76,
77].
Lastly, climate adaptation strategies must be fully integrated into national fisheries policies to improve long-term sustainability and resilience against climate variability. Developing early warning systems for extreme climate events, such as marine heatwaves and excessive precipitation, can help policymakers and fishers anticipate and mitigate disruptions to fisheries production. Strengthening stakeholder engagement among fishers, scientists, and policymakers is also crucial for developing practical and economically viable adaptation strategies that align with both environmental conservation and industry sustainability.
Despite the significant contributions of this study, certain limitations remain that future research should address. Key ecological and anthropogenic factors—such as fishing effort, regulatory regimes, pH, and habitat conditions—were not included in the analysis. The study also relies on national-level aggregate data and linear specifications, which may overlook species-specific dynamics and nonlinear ecological responses. Future research should adopt disaggregated datasets and more flexible modeling frameworks to better capture the complex interactions between climate variability and fisheries production.
Nonetheless, this study offers one of the most comprehensive empirical evaluations to date of Korea’s coastal and offshore fisheries under climate stress, providing a valuable foundation for sector-specific adaptive strategies and policy innovation.
In conclusion, this study underscores the urgent need for climate-resilient fisheries management strategies to address the potential long-term impacts of SST rise, increasing CO2 emissions, and PREC variability on Korea’s COFP. Implementing adaptive harvest regulations, strengthening international cooperation, promoting climate-resilient species, and expanding marine conservation initiatives will be essential for safeguarding fisheries sustainability. Given the increasing frequency and intensity of climate-related stressors, a proactive and integrated approach is critical for ensuring the long-term sustainability of marine resources and protecting fisheries-dependent communities from environmental and economic instability.