Abstract
This study was the first report regarding the application of barley malt (BM) for diets of aquaculture species. Triplicate groups of grass carp Ctenopharyngodon idellus with an initial size of about 1.2 kg were selected and fed with either BM or commercial feed (CF) to apparent satiation for 8 weeks in outdoor ponds connected with a flow-through aquaculture system. The results showed that the final body weight (1651 g) was lower in the BM fish than in the CF fish (1791 g). The edible part was lower in the BM fish than in the CF fish as indicated by the viscerosomatic index. Except for ash levels, which were lower in the fillet of the BM fish than for that of the CF fish, moisture, protein, and lipid levels were not impacted by the application of BM. Water-holding capacity indicators (drop loss, frozen exudation rate, and cooking loss) of grass carp muscle were not relevant to dietary modifications. Hematoxylin-eosin (HE) staining showed that the diameter of the myofibers was decreased while density was increased in response to the application of BM, which contributed to the improvement in textural properties (hardness, gumminess, and chewiness) in the muscle of the BM fish as compared to the CF fish. Glutamic acid level was highest, followed by aspartic acid, lysine, leucine, alanine, and arginine in grass carp muscle. Except three amino acids (proline, phenylalanine, and histidine), the amounts of the other 15 amino acids, essential amino acids, semi-essential amino acids, nonessential amino acids, and delicious amino acids were not impacted by different treatments, suggesting that the application of BM had a minor effect on the amino acid composition of grass carp muscle. Oleic acid (C18:1n-9), linoleic acid (C18:2n-6), and palmitic acid (C16:0) were the most abundant fatty acids in grass carp muscle. The amounts of poly-unsaturated fatty acid (PUFA) in the muscle decreased in response to the application of BM as the diet of grass carp, and n-6 PUFAs (C18:2n-6 and C20:2n-6) rather than n-3 PUFAs accounted for this change, which is beneficial for human health. In conclusion, the application of BM had minor impacts on the proximate composition and amino acid composition but improved textural properties and decreased n-6 PUFAs in the fillet of grass carp.
Key Contribution:
The application of BM had minor impacts on the proximate composition and amino acid composition, but improved textural properties and decreased n-6 PUFAs in the fillet of grass carp.
1. Introduction
Grass carp Ctenopharyngodon idellus, one of the most important cultured freshwater fish species, serves as a rich protein source for the general population in China. In 2023, the annual production of grass carp increased by up to 5.94 million tons, accounting for about 21.4% of aquaculture production for freshwater fish in China [1]. With the improvement in people’s living standards and the rapid development of the aquaculture industry, flesh quality of fishery products has attracted growing attention from both consumers and farmers. Flesh quality is a complex set of characteristics involving intrinsic factors such as chemical composition and texture [2,3]. Nutrients are considered to be primary contributors to flesh quality, thus, dietary composition strongly affects the fillet quality of cultured fish [4,5,6,7]. Nowadays, the culture of grass carp in China mainly depends on the application of extruded artificial feeds with high-energy density, which often produces fatty fish with poor body shape and inferior flesh quality flesh [8]. To improve the flesh quality, feed-fed market-sized grass carp are usually transported to ponds with superior water quality and then subjected to several months of starvation consuming lipid storages, which is commonly accepted as the “lean fish technique” in China [9]. However, the production of “lean grass carp” is against animal welfare and is harmful to the health and immunity of grass carp, which cannot tolerate long-distance transportation. Thus, other, better solutions should be found to improve the fillet quality with minimal impact on the health of grass carp.
Malt Fructus Hordei Germinatus, also known as barley malt, is obtained by germination and drying of the mature fruits of barley, which is a common “medicinal and food” plant [10]. From a macroscopic point of view, barley malt with different lengths has different applications for human beings. For example, malt with young shoots of about 5 mm in length is generally of high medicinal value [11], while malt with germs of more than 20 mm is often used as beer malt [12]. Microscopically, barley malt itself contains active substances including vitamins [10], enzymes [13], alkaloids [14,15], and phenols [16], as well as nutrients including proteins [17], amino acids [18], and sugars [19]. Moreover, it has been shown that barley malt has antioxidant [16], anti-free radical [20], anti-inflammatory [21,22], anti-tumour [23], liver protection [24], and hypoglycemic properties [25]. To our knowledge, the application of barley malt has been mainly studied in mammals; there are scarce studies regarding its application in aquaculture species. Therefore, this study was performed to compare the flesh quality (nutrient composition and textural properties) of grass carp fed with commercial feeds and barley malt. The results of this study provide useful information on the application of barley malt in aquaculture.
2. Materials and Methods
2.1. Experimental Fish and Diets
Grass carp juveniles with an initial body weight of about 0.90 kg per fish were purchased from a local producer (Fuling, Chongqing, China). After transient sterilization with 3% NaCl solution, the fish were distributed into two outdoor rectangular ponds (0.2 ha, water depth 1.5 m) in the Nanchuan base of Chongqing Fishery Sciences Research Institute. The fish were acclimatized in our facility for two weeks, during which time they were fed with a commercial diet (Shuangliu Chia Tai Co., Ltd., Chengdu, China). The moisture, crude protein, lipid, and ash levels of the diet were 8.33%, 31.7%, 4.97%, and 8.54%, respectively. Fish were fed twice a day (9:00 and 17:00) to visualize satiation manually. Canadian malting barley was purchased from Zhengzhou Liangzhidao Trading Co., Ltd. (Zhengzhou, China), and stored in a warehouse with good ventilation. The barley was placed in a germination barrel, drenched with water for 8 h, and then drained off for 4 h. After repeating this procedure two times, the barley started to sprout under a moist environment. The fresh malt sprouts were collected to feed the grass carp when their length reached 3~5 mm. The moisture, crude protein, lipid, and ash levels of barley malt were 54.2%, 13.6%, 4.89%, and 2.97%, respectively.
2.2. Feeding Trial
After acclimatization, grass carp with an initial average size of about 1.20 kg per fish were selected and randomly divided into six outdoor rectangular cement ponds with 240 fish per pool (20 × 5 × 3 m3). The water depth of the pond was maintained at 1.5 m. These ponds were connected with a flow-through aquaculture system, and the water exchange was maintained at about 20 m3/h. Either the commercial feed (abbreviated as CF) or barley malt (abbreviated as BM) was allocated to three replicate ponds of grass carp. The fish were fed to apparent satiation twice (9:00 and 17:00) and once daily (9:00), when the water temperature was above 20 °C and 10~20 °C, respectively. The feed intake was adjusted according to the feeding behaviour of the fish, and was recorded daily. The survival and general health status of the fish were checked daily. A natural photoperiod (approximately 14/10 h of light/dark cycle) was adopted in this experiment. Water temperature was monitored daily, and water quality parameters were monitored once weekly. During the experimental period, water temperature fluctuated between 10~22.4 °C, dissolved oxygen varied between 6.0~9.0 mg/L, nitrite concentration fluctuated between 0.05~0.1 mg/L, and pH fluctuated between 6.0~7.2. Ammonia nitrogen concentration was maintained at less than 0.1 mg/L.
2.3. Sample Collection
Upon completion of the 8-week feeding trial, the fish were subject to a 24 h fasting period prior to sampling. Grass carp juveniles were counted and bulk-weighed pond by pond. Three fish were randomly selected from each pond and killed by a sharp strike on the head. The fish were dissected to obtain viscera and white muscle above the lateral line. Before dissection, body length and body weight of these fish were measured to calculate condition factors. One part of the muscle was used for nutrient composition analysis, one part was used for the determination of water-holding capacity, one part was used for texture determination, and the remainder were fixed in 4% paraformaldehyde solution.
2.4. Proximate Composition Analysis
The moisture, crude protein, crude lipid, and crude ash levels of the muscle, diets, and barley malt were determined according to AOAC [26]. Moisture was determined in an oven at 105 °C for 24 h until constant weight. Protein and lipids were determined using the Kjeldahl method (N × 6.25) and Soxhlet extraction method, respectively. Ash was determined in a muffle furnace at 550 °C for 4 h until constant weight.
2.5. Determination of Water-Holding Capacity
The muscle samples were sealed and stored at 4 °C for 24 h, then, surface moisture was drained off and the samples were weighed to calculate the drip loss. The samples were sealed and stored at −20 °C for 24 h, then thawed, drained of surface moisture and weighed to calculate the frozen exudation rate. The samples were cooked in a steamer for 5 min, then drained of surface water and weighed to calculate the cooking loss [27].
2.6. Textural Profile Analysis
The textural properties (hardness, chewiness, adhesiveness, springiness, cohesiveness, and resilience) of the muscle cubes (1 × 1 × 2 cm3) were analyzed at room temperature using the Pro CT V 1.5 Build 20 texture analyzer. A double compression cycle test was performed using a flat-bottomed cylindrical probe P/36R. The test parameters were set as follows: pre-test speed 5 mm/s, test speed 1 mm/s, return speed 5 mm/s, compression degree 30%, trigger point load 5 g, and data frequency 20 pps according to Zheng [28].
2.7. Histological Analysis
The muscle samples were fixed for at least 24 h and then sent to Wuhan Powerful Biology Company for section preparation and HE (hematoxylin-eosin) staining. The images were captured using the Olympus CX33 optical microscope (Tokyo, Japan). The diameter and density of the muscle fibres were measured using ImageJ 23.0 software.
2.8. Amino Acid and Fatty Acid Composition Analysis
The muscle samples were dried using a freeze-dryer (SCIENTZ-10YG/A, Ningbo, China). The commercial diet and muscle were sent to Guangzhou Ashare Aquatech Co., Ltd. (Guangzhou, China) for the analysis of amino acid and fatty acid composition. The amino acid composition was analyzed with reference to GB 5009.124–2016 [29] using an automated amino acid analyzer (Model 835-50, Hitachi, Japan). The fatty acid profile was analyzed with reference to GB 5009.168–2016 [30] using a Shimadzu GC2010 gas chromatography (Shimadzu, Kyoto, Japan) equipped with a hydrogen flame ionization detector and a Supelco SP-2560 gas capillary column.
2.9. Evaluation of the Nutritional Value of Muscle Amino Acids
The protein quality of muscle samples was evaluated according to the amino acid scoring criteria recommended by FAO/WHO and the amino acid standard model of egg protein proposed by the Institute of Nutrition and Food Safety at the Chinese Academy of Preventive Medical Sciences [31,32]. The amino acid score (AAS), chemical score (CS), and essential amino acid index (EAAI) were calculated as follows [33]:
In the formula, n represents the number of compared essential amino acids; a, b, c, …, j represent the level of each essential amino acid in the test protein (mg/g N); ae, be, ce, …, je represent the level of each essential amino acid in the whole egg protein (mg/g N). Unit mg/g N represents the amount of amino acid per gram of nitrogen in the test protein (the level of each essential amino acid × 100 × 6.25/the level of protein).
2.10. Statistical Analysis
The results are expressed as the mean ± SEM (standard error of the mean) of three replicates. After testing for normal distribution and homogeneity of variance, the data were analyzed using independent t-tests, with the significance level set at 0.05. All the statistical analyses were completed using SPSS 23.0 software (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Growth and Biometric Parameters
Data for growth and biometric parameters are presented in Table 1. During the feeding trial, the survival rate of experimental fish was above 97.9%. The final body weight per fish was significantly higher (p < 0.05) in grass carp fed commercial feeds (CF, 1791 g) than in fish fed barley malt (BM, 1651 g). Both the condition factor and viscerosomatic index of the CF fish were significantly lower than those of the BM fish (p < 0.05).

Table 1.
Growth and biometric parameters of grass carp fed CF and BM.
3.2. Proximate Composition and Water-Holding Capacity
Data for proximate composition and water-holding capacity of grass carp muscle are shown in Table 2. Moisture, protein, and lipid levels of grass carp muscle were not affected by different treatments (p > 0.05). The crude ash level was significantly lower in the muscle of the BM fish than in the CF fish (p < 0.05). The tested water-holding capacity parameters including drop loss, frozen exudation rate, and cooking loss were not significantly affected by dietary modifications (p > 0.05).

Table 2.
Proximate composition and water-holding capacity of grass carp muscle.
3.3. Textural Properties of Grass Carp Muscle
Data for textural properties of grass carp muscle are shown in Table 3. The hardness, gumminess, and chewiness in the muscle of the BM fish were significantly higher than for those of the CF fish (p < 0.05), while springiness and cohesiveness were not impacted by different treatments (p > 0.05).

Table 3.
Texture properties of muscle in grass carp fed CF and BM.
3.4. Histological Characteristics of Myofibers
According to Figure 1, the diameter of myofibers was significantly higher in the CF fish (112 μm) than in the BM fish (97.8 μm; p < 0.05), while the density of myofibers followed an opposite trend to that of the diameter.
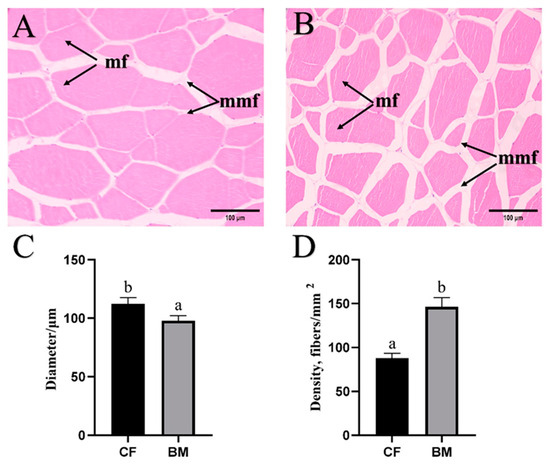
Figure 1.
Histological characteristics of myofiber in grass carp. Data are presented as mean ± SEM (n = 3). Different letters on the error bar indicate significant differences (p < 0.05). (A,B) indicate HE staining of myofiber in grass carp fed commercial feeds (CF) and barley malt (BM), respectively. mf, myofiber; mmf, matrix among myofibers. (C,D) indicate quantification of myofiber diameter and density in grass carp fed CF and BM, respectively.
3.5. Amino Acid Composition of Grass Carp Muscle
As shown in Table 4, 18 amino acids were detected in the commercial diet and grass carp muscle. The amino acid profile of grass carp muscle generally followed that of the commercial diet. Glutamic acid level (about 13%) was highest, followed by aspartic acid, lysine, leucine, alanine, and arginine in the muscle of grass carp. Proline level was significantly higher in the muscle of the BM fish than in the CF fish (p < 0.05), while phenylalanine and histidine levels followed an opposite trend to that of proline. The concentrations of the other 15 amino acids were not affected by dietary modifications. In addition, the levels of total amino acids (TAA), essential amino acids (EAA), semi-essential amino acids (SEAA), nonessential amino acids (NEAA), and delicious amino acids (DAA) were all not significantly impacted by different treatments (p > 0.05).

Table 4.
Amino acid composition in the commercial diet and grass carp muscle (% dry matter basis).
3.6. Nutritional Value of Amino Acids in Grass Carp Muscle
Data for nutritional value evaluation of essential amino acids in the muscle are shown in Table 5. Both the concentration and AAS value of the EAA in grass carp muscle were higher than those of the FAO/WHO standard. Except lysine, the concentration and CS of the other EAA were lower than those of the whole egg protein. Compared with the whole egg protein, methionine and valine were the first and second limiting amino acid, respectively. The concentration, AAS, CS, and EAAI values of EAA in the white muscle of the BM fish were lower than for those of the CF fish.

Table 5.
Nutritional value of essential amino acids in the muscle of grass carp fed CF and BM.
3.7. Fatty Acid Profiles in Grass Carp Muscle
As shown in Table 6, 17 and 19 fatty acids were detected in the commercial diet and grass carp muscle, respectively. The amino acid profile of grass carp muscle generally followed that of the commercial diet. Oleic acid (C18:1n-9), linoleic acid (C18:2n-6), and palmitic acid (C16:0) were the most abundant fatty acids in grass carp muscle. C14:0, C16:0, and C16:1n-7 levels in the muscle of the CF fish were lower than for those of the BM fish (p < 0.05), while the opposite was true for C18:2n-6 and C20:2n-6. The other 14 fatty acids in grass carp muscle were not affected by dietary modifications (p > 0.05). The amounts of total saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA) were not impacted by different treatments. The amounts of total polyunsaturated fatty acids (PUFA) in the muscle of the BM fish were significantly lower than that of the CF fish (p < 0.05), and the changes in the amount of n-6 PUFAs rather than n-3 PUFAs accounted for this phenomenon. The ratio of n-3/n-6 PUFA in grass carp muscle was not impacted by dietary modifications.

Table 6.
Fatty acid composition in the commercial diet and grass carp muscle (% dry matter basis).
4. Discussion
Sensory properties and nutritional value are important factors in determining the flesh quality of aquatic products. In this study, barley malt was fed to market-sized grass carp for 8 weeks, and its effects on the sensory properties and nutrient composition of the flesh were evaluated. To our knowledge, this was the first report regarding the application of barley malt for diets of aquatic species. In this study, the growth performance was 7.81% lower in grass carp fed with barley malt (BM, 1651 g) than in fish fed with commercial feeds (CF, 1791 g), which might be associated with the feeding ratio and dietary protein intake. Although the fish were fed to apparent satiation, the moisture level of BM was far greater than that of CF, while the opposite was true for protein level. Thus, the actual feeding ratio and protein intake were lower in the BM fish than in the CF fish. Both the condition factor and viscerosomatic index were higher in the BM fish than in the CF fish, suggesting that the application of BM might decrease the proportion of edible parts in grass carp. The reason might be attributed to the higher carbohydrate levels in the BM, which could be efficiently converted into lipids for storage around the mesentery in grass carp [34]. Though the protein level of the BM (13.6%) was far less than that of CF (31.7%), the protein level was a little lower in the muscle of the CF fish (17.8%) than in the CF fish (18.6%), and the difference was not significant. Given that BM cost at least 30% less than CF, the abovementioned results suggested that BM has the potential to replace CF in grass carp. The lower ash level in BM (2.97%) as compared with CF (8.54%) should be the main cause for the lower ash percentage in the muscle of the BM fish than in the CF fish. Similarly to our study, lower ash levels were also evidenced in the muscle of grass carp fed with green grass than fish fed with commercial feed [35].
Water-holding capacity, as an important guarantee for freshness and intrinsic flavour, indicates the muscle’s ability to retain and bind water [36,37]. In this study, drip loss, frozen exudation rate, and water loss-rate in grass carp muscle were not affected by dietary modifications, suggesting that the application of BM as the diet of grass carp did not affect the water-holding capacity of the flesh. Texture is one of the most important flesh quality characteristics, which affects the mechanical processing of the flesh and its acceptability by consumers. Hardness is a good biomarker for the freshness of the fish fillet [7]. In general, the changing pattern of flesh hardness is consistent with that of chewiness [6,38]. Besides hardness and chewiness, gumminess was also higher in the muscle of the BM fish than in the CF fish in this study, suggesting that the application of BM as the diet of grass carp improved texture property of the flesh. In line with our study, hardness, chewiness, and gumminess of the flesh were also improved in grass carp fed green grass or fava beans as compared to fish fed with commercial feeds [39,40,41]. As the basic unit of muscle structure, the size and density of the myofibers are important factors in determining muscle texture [42]. In general, hardness of the flesh is inverse to the diameter of myofibers and is proportional to myofiber density within a certain range [43]. In this study, the application of BM as the diet of grass carp decreased the diameter but increased the density of myofibers, which improved the chewiness and hardness of the flesh as compared with those of the CF fish.
The quantity and quality of amino acids are important indicators for the nutritional value of fish fillet [44]. The AAS, CS, and EAAI are commonly used to evaluate the protein quality of the flesh [45,46]. In accordance with the results of Li et al. [47], glutamic acid was the highest, followed by aspartic acid, lysine, and leucine in grass carp muscle. In this study, only histidine, phenylalanine, and proline levels were affected, while the amounts of TAA, EAA, SEAA, NEAA, and DAA levels in the muscle of grass carp were not affected by dietary modifications. These results suggested that the application of BM as the diet of grass carp had a minor impact on the amino acid composition of the flesh. It was previously reported that proline levels increased in grass carp muscle in response to dietary vitamin E supplementation [48]. The increase in muscle proline levels in the BM fish might be attributed to the fact that BM was rich in vitamin E [49]. In this study, EAA/TAA ratios were 44.2% and 44.4%, while EAA/NEAA ratios in the muscle of the CF and BM fish were 92.7% and 93.0%, respectively. The EAA/TAA ratio of grass carp muscle in this study exceeded 40%, while the EAA/NEAA ratio exceeded 60% of the ideal protein model recommended by FAO/WHO [32]. In addition, the AAS value of all the EAAs in grass carp muscle of both treatments was above 1. These results suggest that grass carp muscle is a protein source of high quality for human beings. According to the CS value, the first two limiting amino acids of grass carp muscle were methionine + cysteine and valine in this study, which was similar to previous reports [50,51]. It was noteworthy that muscle lysine levels in both treatments were higher than that of the FAO/WHO standard and whole egg protein, suggesting that the consumption of grass carp muscle could attenuate the lysine deficiency of cereal-based diets for consumers [44,52].
Fatty acid profiles of fish fillets are susceptible to the influence of food and water environments [53,54,55,56,57]. It was previously reported that the amounts of n-6 PUFAs in the muscle of grass carp in natural habitats of rivers and lakes are lower than those of farmed grass carp fed compound feeds [47,51]. In this study, the amounts of PUFAs in the muscle were decreased in response to the application of BM as the diet of grass carp, and n-6 PUFAs (C18:2n-6 and C20:2n-6) rather than n-3 PUFAs accounted for this change. It is generally accepted that the consumption of n-6 PUFAs has an increased risk of tumours to human health [58]. From this perspective, the consumption of fillets from the BM fish should be more beneficial to human health as compared with the CF fish. BM not only contains many conventional nutrients but also vitamin E, which is less abundant in plant-derived raw materials [49]. In addition to the lower n-6 PUFAs from BM as compared with the CF, vitamin E abundance might also contribute to the reduction in n-6 PUFAs in the fillet of the BM fish, as dietary vitamin E supplementation was effective to decrease n-6 PUFAs in grass carp muscle [48].
5. Conclusions
In conclusion, the application of BM had minor impacts on the proximate composition and amino acid composition but improved textural properties and decreased n-6 PUFAs in the fillet of grass carp.
Author Contributions
Conceptualization, C.H. and Y.C.; methodology, Y.C.; software, H.L.; validation, Z.H., H.L. and D.M.; formal analysis, Z.H.; investigation, H.G.; resources, P.F.; data curation, W.J.; writing—original draft preparation, Z.H.; writing—review and editing, Z.H.; visualization, H.L.; supervision, Y.C.; project administration, C.H.; funding acquisition, Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Incentive Project for Chongqing Scientific Research Institutes (CSTB2023JXJL-YFX0083) from Chongqing Science and Technology Bureau.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Animal Ethics Committee of Southwest University.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request.
Acknowledgments
The authors were greatly appreciated for the help of sampling by the workers from Chongqing Fishery Sciences Research Institute and graduate students from southwest University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ministry of Agriculture and Rural Affairs. China Fishery Statistics Yearbook; China Agriculture Press: Beijing, China, 2023; pp. 24–25.
- Fauconneau, B.; Alami-Durante, H.; Laroche, M.; Marcel, J.; Vallot, D. Growth and meat quality relations in carp. Aquaculture 1995, 129, 265–297. [Google Scholar] [CrossRef]
- Johnston, I.A. Muscle development and growth: Potential implications for flesh quality in fish. Aquaculture 1999, 177, 99–115. [Google Scholar] [CrossRef]
- Grigorakis, K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: A review. Aquaculture 2007, 272, 55–75. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernández-Segovia, I.; Serra, J.A.; Barat, J.M. Comparison of wild and cultured sea bass (Dicentrarchus labrax) quality. Food Chem. 2010, 119, 1514–1518. [Google Scholar] [CrossRef]
- Asghari, M.; Shabanpour, B.; Pakravan, S. Evaluation of some qualitative variations in frozen fillets of beluga (Huso huso) fed by different carbohydrate to lipid ratios. J. Food Sci. Technol. 2014, 51, 430–439. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Zhao, H.; Chong, J.; Li, D.; Xia, J. Integrated multiple-omics reveals the regulatory mechanism underlying the effects of artificial feed and grass feeding on growth and muscle quality of grass carp (Ctenopharyngodon idellus). Aquaculture 2023, 562, 738808. [Google Scholar] [CrossRef]
- Chen, C.; Lu, S.H.; Gao, Y.Y. Problems and key technologies of marketable fish slimming processing and breeding. South China Agric. 2021, 15, 31–35. (In Chinese) [Google Scholar]
- Liu, W.W.; Kong, N.; Liu, S.; Wang, X.; Dong, H.J.; Geng, W. Review on chemical constituents and functional activities of hordei fructus germinatus. Food Drug 2023, 25, 384–390. (In Chinese) [Google Scholar]
- Dong, H. Discussion on milk returning of raw stir-fried malt. Clin. J. Tradit. Chin. Med. 1999, 4, 297. (In Chinese) [Google Scholar]
- Ling, J.H. Studies on the Chemical Constituents and the Processing Procedures of Malt. Master’s Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2007. (In Chinese). [Google Scholar]
- Yun, J.M.; Han, P.; Wu, H.B. Dynamic analysis of enzymatic activity in barley malt during drying process. Food Sci. 2009, 30, 166–169. (In Chinese) [Google Scholar]
- An, J.; Chen, Y.G. Determination of the total alkaloids in malt by acid dye colorimetry method. J. Guangdong Pharm. Univ. 2014, 30, 590–594. (In Chinese) [Google Scholar]
- Tao, J.H.; Gong, X.Y.; Zou, J.L.; Chen, Y.G. Simultaneous determination of alkaloids and tricin in malt extract by HPLC. Tradit. Chin. Drug Res. Clin. Pharmacol. 2020, 31, 102–104. (In Chinese) [Google Scholar]
- Maillard, M.N.; Soum, M.H.; Boivin, P.; Berset, C. Antioxidant activity of barley and malt: Relationship with phenolic content. LWT-Food Sci. Technol. 1996, 29, 238–244. [Google Scholar] [CrossRef]
- Sharma, P.; Kotari, S.L. Barley: Impact of processing on physicochemical and thermal properties—A review. Food Rev. Int. 2017, 33, 359–381. [Google Scholar] [CrossRef]
- Nie, C.; Wang, C.L.; Wang, Y.R.; Zhou, G.T. Influence of malting conditions on the amino acid compositions of malt. China Brew. 2010, 29, 89–92. (In Chinese) [Google Scholar]
- Yang, X.Y. Research on the Components and Hypoglycemic Activity of Soluble Carbohydrate from Barley Malt with Different Germination Days. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2018. (In Chinese). [Google Scholar]
- Ikram, S.; Zhang, H.; Ming, H.; Wang, J. Recovery of major phenolic acids and antioxidant activity of highland barley brewer’s spent grains extracts. J. Food Process. Preserv. 2020, 44, e14308. [Google Scholar] [CrossRef]
- Kanauchi, O.; Oshima, T.; Andoh, A.; Shioya, M.; Mitsuyama, K. Germinated barley foodstuff ameliorates inflammation in mice with colitis through modulation of mucosal immune system. Scand. J. Gastroenterol. 2008, 43, 1346–1352. [Google Scholar] [CrossRef]
- Kanauchi, O.; Serizawa, I.; Araki, Y.; Suzuki, A.; Andoh, A.; Fujiyama, Y.; Mitsuyama, K.; Takaki, K.; Toyonaga, A.; Sata, M.; et al. Germinated barley foodstuff, a prebiotic product, ameliorates inflammation of colitis through modulation of the enteric environment. J. Gastroenterol. 2003, 38, 134–141. [Google Scholar] [CrossRef]
- Rao, S.; Chinkwo, K.; Santhakumar, A.; Johnston, S.; Blanchard, C. Apoptosis induction pathway in human colorectal cancer cell line SW480 exposed to cereal phenolic extracts. Molecules 2019, 24, 2465. [Google Scholar] [CrossRef]
- Quan, M.; Li, Q.; Zhao, P.; Tian, C. Chemical composition and hepatoprotective effect of free phenolic extract from barley during malting process. Sci. Rep. 2018, 8, 4460. [Google Scholar] [CrossRef]
- Yang, Y.C.; Xu, D.P. Hypoglycemic effect and structural determination of polysaccharides from barley malt. J. Food Sci. Biotechnol. 2012, 31, 1087–1092. (In Chinese) [Google Scholar]
- AOAC. Official Methods of Analysis of Official Analytical Chemists International, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Cheng, H.H. Muscular Nutritional Components and Meat Quality of Grass Carp (Ctenopharyngodon idellus) Cultured Under the Model of Cultivating Fish with Grass. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2018. (In Chinese). [Google Scholar]
- Zheng, J.M. Effects of Water Flow and Starvation on the Muscle Quality of Triploid Crucian Carp. Master’s Thesis, Southwest University, Chongqing, China, 2023. (In Chinese). [Google Scholar]
- GB 5009.124-2016; Determination of Amino Acids in Food Safety National Standards. National Standardization Management Committee: Beijing, China, 2016.
- GB 5009.168–2016; Determination of Fatty Acids in Food Safety National Standards. National Standardization Management Committee: Beijing, China, 2016.
- Pellett, P.L.; Young, V.R.; UN University. Nutritional Evaluation of Protein Foods; The United National University Publishing Company: Tokyo, Japan, 1980; pp. 26–29. [Google Scholar]
- Institute of Nutrition and Hygiene; National Academy of Preventive Sciences. List of Food Ingredients; People’s Medical Publishing House: Beijing, China, 1991. [Google Scholar]
- Wang, S.E. Study of Nutritional Composition, Volatile Compound and Texture Property of Tilapia. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2011. (In Chinese). [Google Scholar]
- Chen, Y.J.; Tian, L.X.; Yang, H.J.; Chen, P.F.; Yuan, Y.; Liu, Y.J.; Liang, G.Y. Effect of protein and starch level in practical extruded diets on growth, feed utilization, body composition, and hepatic transaminases of juvenile grass carp, Ctenopharyngodon idella. J. World Aquac. Soc. 2012, 43, 187–197. [Google Scholar] [CrossRef]
- Bi, X.M.; Yu, E.M.; Wang, G.J.; Yu, D.G.; Gong, W.B.; Xie, J. Comparison and analysis of nutrition composition of grass carp raised with grass and artificial feed. Guangdong Agric. Sci. 2011, 38, 132–134. (In Chinese) [Google Scholar]
- Li, L.; Zhou, J.S.; He, Y.L.; Yang, Y.H.; Wang, L.Z.; Yang, J.N.; Lu, L. Comparative Study of Muscle Physicochemical Characteristics in Common Cyprinus carpio, Silurus asotus and Ctenopharyngodon idellus. J. Hydroecol. 2013, 34, 82–86. (In Chinese) [Google Scholar]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wen, H.; Tian, J.; Jiang, M.; Liu, W.; Yang, C.; Yu, L.; Lu, X. Effect of stocking density on growth performance, serum biochemical parameters, and muscle texture properties of genetically improved farm tilapia, Oreochromis niloticus. Aquac. Int. 2018, 26, 1247–1259. [Google Scholar] [CrossRef]
- Li, B.S.; Leng, X.J.; Li, X.Q.; Li, J.L. Effects of feeding broad bean on growth and flesh quality of grass carp Ctenopharyngodon idellus. J. Fish. Sci. China 2008, 15, 1042–1049. (In Chinese) [Google Scholar]
- Guan, L.; Zhu, J.R.; Li, X.Q.; Leng, X.J. Muscle characteristics comparison between grass carp and crisped grass carp. J. Shanghai Ocean. Univ. 2011, 20, 748–753. (In Chinese) [Google Scholar]
- Zhao, H.H.; Xia, J.G.; Zhang, X.; He, X.G.; Li, L.; Tang, R.; Chi, W.; Li, D.P. Diet affects muscle quality and growth traits of grass carp (Ctenopharyngodon idellus): A comparison between grass and artificial feed. Front. Physiol. 2018, 9, 283. [Google Scholar] [CrossRef]
- Shao, T. Quality Changes and Mechanism of Crisp Grass Carp (Ctenopharyngodon idellus C.et V) During Crispness Formation Process. Master’s Thesis, Southwest University, Chongqing, China, 2023. (In Chinese). [Google Scholar]
- Johnston, I.A.; Alderson, R.; Sandham, C.; Dingwall, A.; Mitchell, D.; Selkirk, C.; Nickell, D.; Baker, R.; Robertson, B.; Whyte, D.; et al. Muscle fibre density in relation to the colour and texture of smoked Atlantic salmon (Salmo salar L.). Aquaculture 2000, 189, 335–349. [Google Scholar] [CrossRef]
- Wang, X.; Han, Y. Comparison of the proximate composition, amino acid composition and growth-related muscle gene expression in diploid and triploid rainbow trout (Oncorhynchus mykiss) muscles. J. Elem. 2017, 22, 1179–1191. [Google Scholar] [CrossRef]
- Aronal, A.P.; Huda, N.; Ahmad, R. Amino acid and fatty acid profiles of Peking and Muscovy duck meat. Int. J. Poult. Sci. 2012, 11, 229–236. [Google Scholar] [CrossRef]
- Pyz-Łukasik, R.; Paszkiewicz, W. Species variations in the proximate composition, amino acid profile, and protein quality of the muscle tissue of grass carp, bighead carp, siberian sturgeon, and wels catfish. J. Food Qual. 2018, 2018, 2625401. [Google Scholar] [CrossRef]
- Li, Z.Y.; Ding, H.X.; Zhang, L.; Tu, Z.C. Comparative analysis on the nutritional quality of grass carp muscle from different habitats. Food Ferment. Ind. 2021, 47, 133–139. (In Chinese) [Google Scholar]
- Yao, K. Effects of vitamin E on growth performance and flesh quality of sub-adult grass carp and its mechanism. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2023. (In Chinese). [Google Scholar]
- Tong, E.J.; Zhang, C.Q.; Du, Y.J.; Liu, L.; Lu, J.H.; Chen, X. Analysis on the nutrients of malted barley and discussion on its application in food industry. Food Ind. 2023, 44, 323–327. (In Chinese) [Google Scholar]
- Ding, Y.Q.; Liu, Y.M.; Xiong, S.B. The comparative study on nutritional components between the muscle of Elopichthys bambusa and Ctenopharyngodon idellus. Acta Nutr. Sin. 2011, 33, 196–198. (In Chinese) [Google Scholar]
- Cheng, H.L.; Jiang, F.; Peng, Y.X.; Xu, X.H.; Dong, Z.G.; Xu, X.; Guo, Z.Q.; Sun, J.B.; Wang, J.Z.; Wu, G.S. Comparison of nutrient composition of muscles of wild and farmed grass carp, Ctenopharyngodon idellus. Food Sci. 2013, 34, 266–270. (In Chinese) [Google Scholar]
- Koch, J.F.; Rawles, S.D.; Webster, C.D.; Cummins, V.; Kobayashi, Y.; Thompson, K.R.; Gannam, A.L.; Twibell, R.G.; Hyde, N.M. Optimizing fish meal-free commercial diets for Nile tilapia, Oreochromis niloticus. Aquaculture 2016, 452, 357–366. [Google Scholar] [CrossRef]
- Lenas, D.; Chatziantoniou, S.; Nathanailides, C.; Triantafillou, D. Comparison of wild and farmed sea bass (Dicentrarchus labrax L) lipid quality. Procedia Food Sci. 2011, 1, 1139–1145. [Google Scholar] [CrossRef]
- Xu, H.; Turchini, G.M.; Francis, D.S.; Liang, M.; Mock, T.S.; Rombenso, A.; Ai, Q. Are fish what they eat? A fatty acid’s perspective. Prog. Lipid Res. 2020, 80, 101064. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Feng, L.; Wu, P.; Liu, Y.; Ren, H.M.; Jin, X.W.; Jiang, J.; Kuang, S.Y.; Li, S.W.; Tang, L.; et al. Modification of beneficial fatty acid composition and physicochemical qualities in the muscle of sub-adult grass carp (Ctenopharyngodon idella): The role of lipids. Aquaculture 2022, 561, 738656. [Google Scholar] [CrossRef]
- Montenegro, L.F.; Descalzo, A.M.; Cunzolo, S.A.; Pérez, C.D. Modification of the content of n-3 highly unsaturated fatty acid, chemical composition, and lipid nutritional indices in the meat of grass carp (Ctenopharyngodon idella) fed alfalfa (Medicago sativa) pellets. J. Anim. Sci. 2020, 98, skaa084. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, Y.; Han, Z.; Zheng, Y.; Wang, X.; Zhang, M.; Li, H.; Xu, J.; Chen, X.; Ding, Z.; et al. Comparative effects of dietary soybean oil and fish oil on the growth performance, fatty acid composition and lipid metabolic signaling of grass carp, Ctenopharyngodon idella. Aquac. Rep. 2022, 22, 101002. [Google Scholar] [CrossRef]
- Wei, N.; Mi, M.T. Roles of different n-6/n-3 polyunsaturated fatty acid ratio in gap junctional intercellular communication in breast cancer cell lines. J. Army Med. Univ. 2006, 28, 338–341. (In Chinese) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).