Gene Expression and Antioxidant Characteristics of Rainbow Trout (Oncorhynchus mykiss) Eggs Used for Meiotic Gynogenesis
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics
2.2. Eggs and Sperm
2.3. Irradiation of Sperm, Egg Activation and Diploidization
2.4. mRNA Extraction
2.5. Quantitative Real-Time PCR Analysis
2.6. Antioxidant Enzyme Activity Analysis
2.7. Statistical Analysis
3. Results
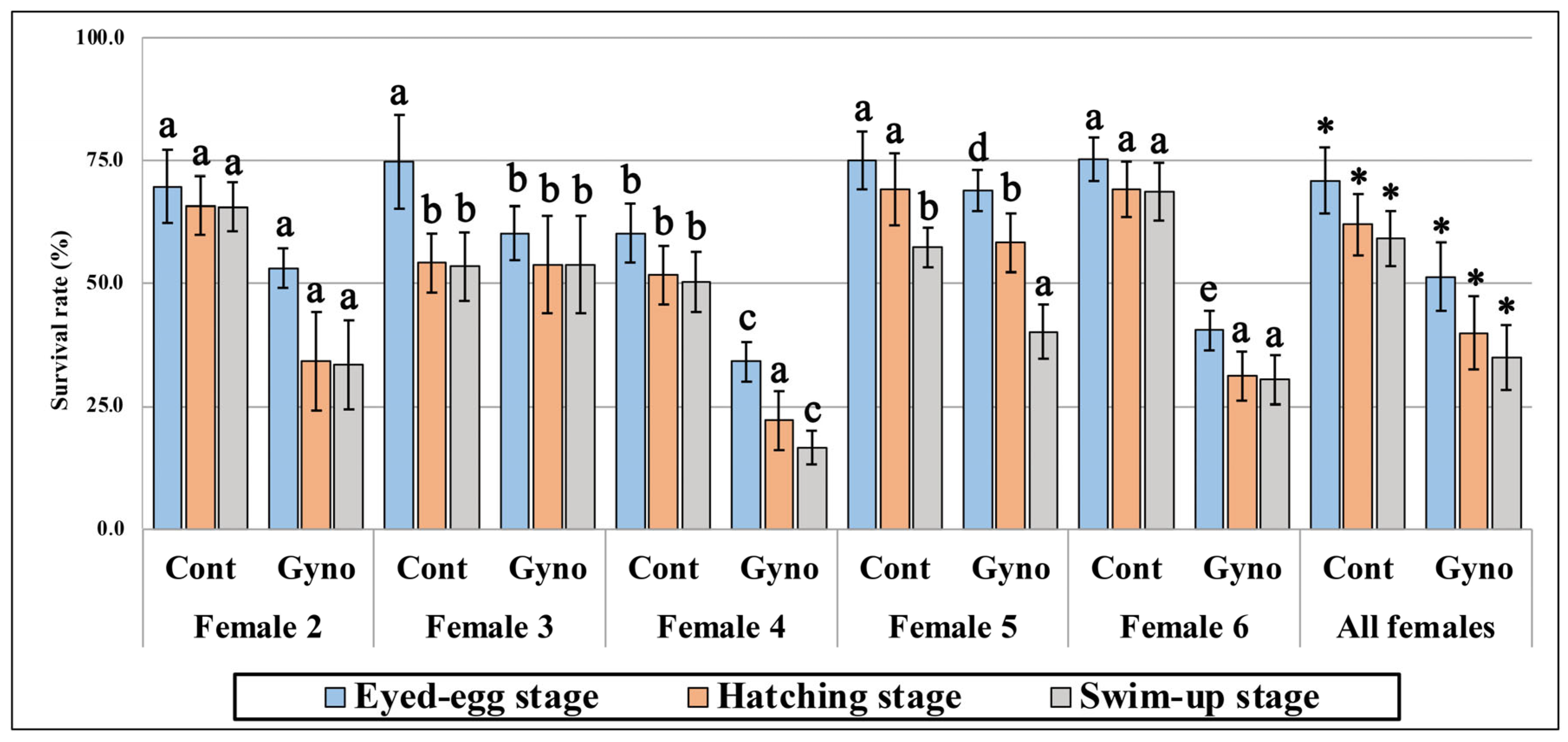
3.1. Survival of Embryos Developing in Eggs Fertilized with Non- and UV-Irradiated Sperm
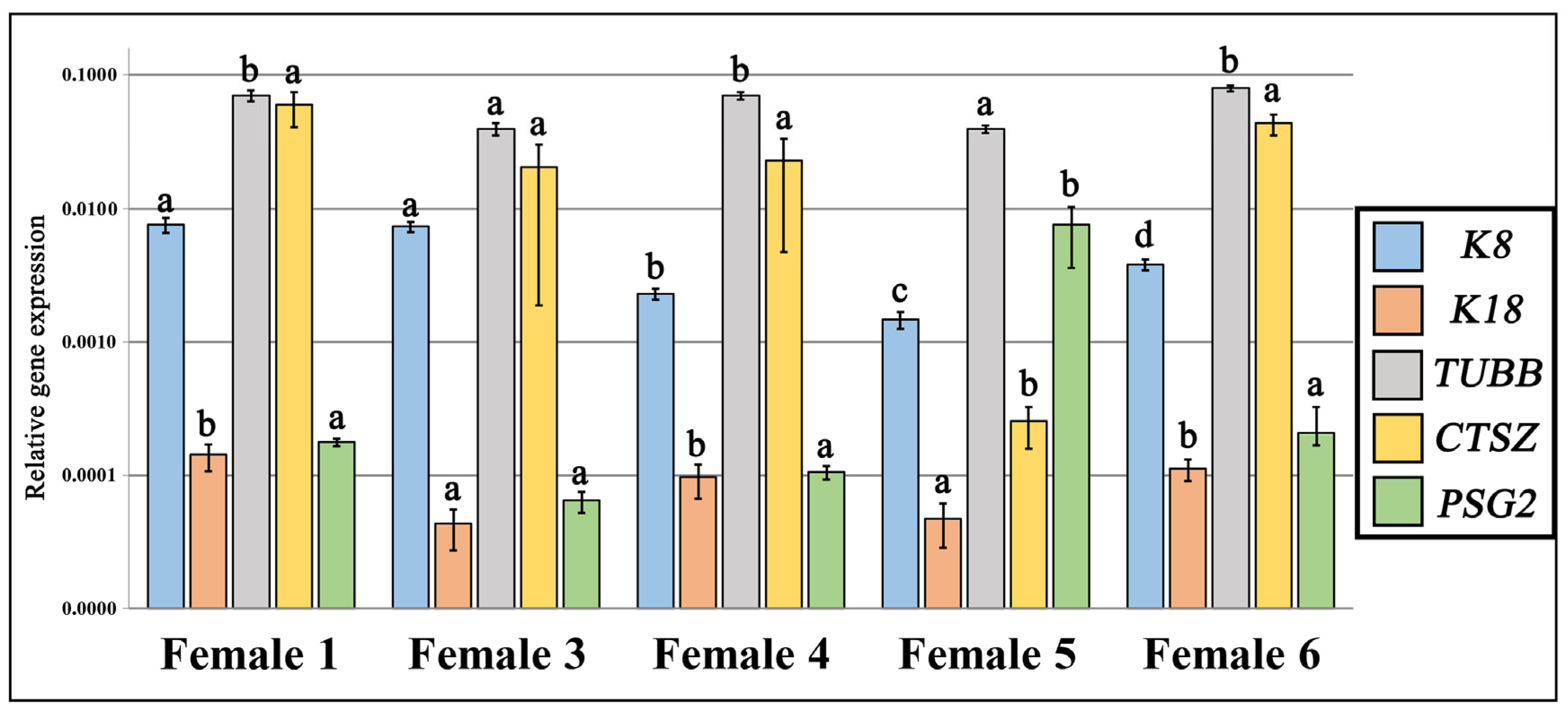
3.2. Maternal mRNA Transcription
3.3. Activity of Antioxidant Enzymes in Eggs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, M.A.; Brandon, R.A. Gynogenesis and hybridization between Ambystoma platineum and Ambystoma texanum in Illinois. Copeia 1984, 1984, 324–337. [Google Scholar] [CrossRef]
- Gumm, J.M.; Thaker, M. Association preferences of unisexual Amazon mollies (Poecilia formosa): Differential response to swords based on sex of the bisexual parental species. Behaviour 2009, 146, 907–921. [Google Scholar] [CrossRef]
- Grismer, J.L.; Grismer, L.L. Who’s your mommy? Identifying maternal ancestors of asexual species of Leiolepis Cuvier, 1829 and the description of a new endemic species of asexual Leiolepis Cuvier, 1829 from Southern Vietnam. Zootaxa 2010, 2433, 47–61. [Google Scholar] [CrossRef]
- Neaves, W.B.; Baumann, P. Unisexual reproduction among vertebrates. Trends Genet. 2011, 27, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Pandian, T.J.; Koteeswaran, R. Ploidy induction and sex control in fish. Hydrobiologia 1998, 384, 167–243. [Google Scholar] [CrossRef]
- Campos-Ramos, R.; Harvey, S.C.; McAndrew, B.J.; Penman, D.J. An investigation of sex determination in the Mozambique tilapia, Oreochromis mossambicus, using synaptonemal complex analysis, FISH, sex reversal and gynogenesis. Aquaculture 2003, 221, 125–140. [Google Scholar] [CrossRef]
- Chen, S.-L.; Tian, Y.-S.; Yang, J.-F.; Shao, C.-W.; Ji, X.-S.; Zhai, J.-M.; Liao, X.-L.; Zhuang, Z.-M.; Su, P.-Z.; Xu, J.-Y.; et al. Artificial gynogenesis and sex determination in half-smooth tongue sole (Cynoglossus semilaevis). Mar. Biotechnol. 2008, 11, 243–251. [Google Scholar] [CrossRef]
- Fopp-Bayat, D.; Hliwa, P.; Ocalewicz, K. Presence of gynogenetic males suggests a female heterogamety in sterlet Acipenser ruthenus L. Anim. Reprod. Sci. 2018, 189, 110–118. [Google Scholar] [CrossRef]
- Arai, K. Genetic improvement of aquaculture finfish species by chromosome manipulation techniques in Japan. Aquaculture 2001, 197, 205–228. [Google Scholar] [CrossRef]
- Harris, M.P.; Henke, K.; Hawkins, M.B.; Witten, P.E. Fish is Fish: The use of experimental model species to reveal causes of skeletal diversity in evolution and disease. J. Appl. Ichthyol. 2014, 30, 616–629. [Google Scholar] [CrossRef]
- Jagiełło, K.; Zalewski, T.; Dobosz, S.; Michalik, O.; Ocalewicz, K. High rate of deformed larvae among gynogenetic brown trout (Salmo trutta m. fario) doubled haploids. BioMed Res. Int. 2017, 2017, 2975187. [Google Scholar] [CrossRef]
- Zhang, X.; Mizukoshi, M.; Zhang, H.; Tan, E.; Igarashi, Y.; Suzuki, Y.; Mitsuyama, S.; Kinoshita, S.; Saito, K.; Watabe, S.; et al. Ultrahigh-density linkage map construction using low-coverage whole-genome sequencing of a doubled haploid population: Case study of Torafugu (Takifugu rubripes). Genes 2018, 9, 120. [Google Scholar] [CrossRef]
- Komen, H.; Thorgaard, G.H. Androgenesis, gynogenesis and the production of clones in fishes: A review. Aquaculture 2007, 269, 150–173. [Google Scholar] [CrossRef]
- Jagiełło, K.; Dobosz, S.; Zalewski, T.; Polonis, M.; Ocalewicz, K. Developmental competence of eggs produced by rainbow trout Doubled Haploids (DHs) and generation of the clonal lines. Reprod. Domest. Anim. 2018, 53, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Naruse, K.; Ijiri, K.; Shima, A.; Egami, N. The production of cloned fish in the medaka (Oryzias latipes). J. Exp. Zool. 1985, 236, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Komen, J.; Bongers, A.B.J.; Richter, C.J.J.; Van Muiswinkel, W.B.; Huisman, E.A. Gynogenesis in common carp (Cyprinus carpio L.): II. The production of homozygous gynogenetic clones and F1 hybrids. Aquaculture 1991, 92, 127–142. [Google Scholar] [CrossRef]
- Quillet, E.; Garcia, P.; Guyomard, R. Analysis of the production of all homozygous lines of rainbow trout by gynogenesis. J. Exp. Zool. 1991, 257, 367–374. [Google Scholar] [CrossRef]
- Ocalewicz, K. Quality of fish eggs and production of androgenetic and gynogenetic doubled haploids (DHs). Fish Physiol. Biochem. 2024, 50, 1947–1957. [Google Scholar] [CrossRef]
- Bobe, J.; Labbé, C. Egg and sperm quality in fish. Gen. Comp. Endocrinol. 2010, 165, 535–548. [Google Scholar] [CrossRef]
- Migaud, H.; Bell, G.; Cabrita, E.; McAndrew, B.; Davie, A.; Bobe, J.; Herráez, M.P.; Carrillo, M. Gamete quality and broodstock management in temperate fish. Rev. Aquac. 2013, 5, S194–S223. [Google Scholar] [CrossRef]
- Bobe, J. Egg quality in fish: Present and future challenges. Anim. Front. 2015, 5, 66–72. [Google Scholar] [CrossRef]
- Aegerter, S.; Jalabert, B.; Bobe, J. Large scale real-time PCR analysis of mRNA abundance in rainbow trout eggs in relationship with egg quality and post-ovulatory ageing. Mol. Reprod. Dev. 2005, 72, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, E.; Fostier, A.; Bobe, J. Characterization of rainbow trout egg quality: A case study using four different breeding protocols, with emphasis on the incidence of embryonic malformations. Theriogenology 2007, 67, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Polonis, M.; Błaszczyk, A.; Jagiełło, K.; Panasiak, L.; Dobosz, S.; Ocalewicz, K. Inter-clutch egg differences and androgenesis in rainbow trout (Oncorhynchus mykiss, Walbaum 1792). Oceanol. Hydrobiol. Stud. 2021, 50, 160–168. [Google Scholar] [CrossRef]
- Samarin, A.M.; Sampels, S.; Policar, T.; Rodina, M.; Hematyar, N. mRNA abundance changes during in vitro oocyte ageing in African catfish Clarias gariepinus (Burchell, 1822). Aquac. Res. 2017, 49, 1037–1045. [Google Scholar] [CrossRef]
- Goryczko, K.; Dososz, S.; Mäkinen, T.; Tomasik, L. UV-irradiation of rainbow trout sperm as a practical method for induced gynogenesis. J. Appl. Ichthyol. 1991, 7, 136–146. [Google Scholar] [CrossRef]
- Billard, R. Utilisation d’un syst’eme tris-glycocolle pour tamponner le dilueurd’ins’emination pour truite. Bull. Français Piscic. 1997, 264, 102–112. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Carrizo, V.; Valenzuela, C.A.; Zuloaga, R.; Aros, C.; Altamirano, C.; Valdés, J.A.; Molina, A. Effect of cortisol on the immune-like response of rainbow trout (Oncorhynchus mykiss) myotubes challenged with Piscirickettsia salmonis. Veter-Immunol. Immunopathol. 2021, 237, 110240. [Google Scholar] [CrossRef]
- Meiler, K.A.; Cleveland, B.; Radler, L.; Kumar, V. Oxidative stress-related gene expression in diploid and triploid rainbow trout (Oncorhynchus mykiss) fed diets with organic and inorganic zinc. Aquaculture 2021, 533, 736149. [Google Scholar] [CrossRef]
- Yamaha, E.; Otani, S.; Minami, A.; Arai, K. Dorso-ventral axis perturbation in goldfish embryos caused by heat- and pressure-shock treatments for chromosome set manipulation. Fish. Sci. 2002, 68, 313–319. [Google Scholar] [CrossRef]
- Ocalewicz, K.; Gurgul, A.; Polonis, M.; Dobosz, S. Preliminary identification of candidate genes related to survival of gynogenetic rainbow trout (Oncorhynchus mykiss) based on comparative transcriptome analysis. Animals 2020, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Ocalewicz, K.; Gurgul, A.; Dobosz, S.; Jasielczuk, I.; Szmatoła, T.; Semik-Gurgul, E.; Kucharski, M.; Rożyński, R. Transcriptome Profiling Reveals Differences Between Rainbow Trout Eggs with High and Low Potential for Gynogenesis. Genes 2025, 16, 803. [Google Scholar] [CrossRef]
- Mantovani, G.; Macciò, A.; Madeddu, C.; Mura, L.; Gramignano, G.; Lusso, M.R.; Murgia, V.; Camboni, P.; Ferreli, L.; Mocci, M.; et al. The impact of different antioxidant agents alone or in combination on reactive oxygen species, antioxidant enzymes and cytokines in a series of advanced cancer patients at different sites: Correlation with disease progression. Free Radic. Res. 2003, 37, 213–223. [Google Scholar] [CrossRef]
- Goud, A.P.; Goud, P.T.; Diamond, M.P.; Gonik, B.; Abu-Soud, H.M. Reactive oxygen species and oocyte aging: Role of superoxide, hydrogen peroxide, and hypochlorous acid. Free. Radic. Biol. Med. 2008, 44, 1295–1304. [Google Scholar] [CrossRef]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Tarín, J.J.; Pérez-Albalá, S.; Cano, A. Consequences on offspring of abnormal function in ageing gametes. Hum. Reprod. Updat. 2000, 6, 532–549. [Google Scholar] [CrossRef]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef]
- Samarin, A.M.; Østbye, T.-K.K.; Ruyter, B.; Sampels, S.; Burkina, V.; Blecha, M.; Gela, D.; Policar, T. Alteration of mRNA abundance, oxidation products and antioxidant enzyme activities during oocyte ageing in common carp Cyprinus carpio. PLoS ONE 2019, 14, e0212694. [Google Scholar] [CrossRef]
- Lanes, C.; Fernandes, J.; Kiron, V.; Babiak, I. Profiling of key apoptotic, stress, and immune-related transcripts during embryonic and postembryonic development of Atlantic cod (Gadus morhua L.). Theriogenology 2012, 78, 1583–1596.e2. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R.; Waldenström, J. With reference to reference genes: A Systematic review of endogenous controls in gene expression studies. PLoS ONE 2015, 10, e0141853. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Martin, K.; Dixon, D.; Hernandez, A.G.; Weber, G.M. Transcriptome analysis of egg viability in rainbow trout, Oncorhynchus mykiss. BMC Genom. 2019, 20, 319. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.M.; Birkett, J.; Martin, K.; Dixon, D.; Gao, G.; Leeds, T.D.; Vallejo, R.L.; Ma, H. Comparisons among rainbow trout, Oncorhynchus mykiss, populations of maternal transcript profile associated with egg viability. BMC Genom. 2021, 22, 448. [Google Scholar] [CrossRef]
- Rise, M.L.; Nash, G.W.; Hall, J.R.; Booman, M.; Hori, T.S.; Trippel, E.A.; Gamperl, A.K. Variation in embryonic mortality and maternal transcript expression among Atlantic cod (Gadus morhua) broodstock: A functional genomics study. Mar. Genom. 2014, 18, 3–20. [Google Scholar] [CrossRef]
- Lim, H.Y.G.; Alvarez, Y.D.; Gasnier, M.; Wang, Y.; Tetlak, P.; Bissiere, S.; Wang, H.; Biro, M.; Plachta, N. Keratins are asymmetrically inherited fate determinants in the mammalian embryo. Nature 2020, 585, 404–409. [Google Scholar] [CrossRef]
- Plancha, C.E. Cytokeratin dynamics during oocyte maturation in the hamster requires reaching of metaphase I. Differentiation 1996, 60, 87–98. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsuzaki, M.; Suzuki, H. Intermediate Filament Keratin Dynamics During Oocyte Maturation Requires Maturation/M-Phase Promoting Factor and Mitogen-Activated Protein Kinase Kinase Activities in the Hamster. Reprod. Domest. Anim. 2009, 45, e184–e188. [Google Scholar] [CrossRef]
- Janke, C.; Magiera, M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef]

| Gene Name | Primer Sequence | Amplicon Size (bp) | Reference |
|---|---|---|---|
| Keratin 8 (K8) | F: CTCATTAAGAAGGACGTGGATGAG R: CGCAGCTCCTCCTCATAGATC | 116 | [22] |
| Keratin 18 (K18) | F: GTACGAGCACCTGCTCAACATC R: GAGCATCCTGGAGCTTGAAGTC | 98 | [22] |
| Tubulin β (TUBB) | F: GTGCATCTTCAGGCTGGACAGT R: GTATGTACCAGTTGGGTCAATG | 96 | [22] |
| Cathepsin Z (CTSZ) | F: GACCGATCAGCTGTGGGATC R: GACACAATGTGTTGATGTAG | 102 | [22] |
| Tubulin polyglutamylase complex subunit 2 (PSG2) | F: CCTAATTGGCGAGACCATTAAG R: TCGAACTTGAGCTGGAAGTG | 81 | [22] |
| β-actin (Actb) | F: GCCGGCCGCGACCTCACAGACTAC R: CGGCCGTGGTGGTGAAGCTGTAGC | 73 | [30] |
| Elongation factor 1-alpha (Elf1a) | F: TTAAGCAACCATGGGAAAGG R: TACCTGCCGGTCTCAAACTT | 264 | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocalewicz, K.; Pałucha, K.; Błaszczyk, A.; Kuciński, M.; Dobosz, S.; Panasiak, L.; Rożyński, R. Gene Expression and Antioxidant Characteristics of Rainbow Trout (Oncorhynchus mykiss) Eggs Used for Meiotic Gynogenesis. Fishes 2025, 10, 585. https://doi.org/10.3390/fishes10110585
Ocalewicz K, Pałucha K, Błaszczyk A, Kuciński M, Dobosz S, Panasiak L, Rożyński R. Gene Expression and Antioxidant Characteristics of Rainbow Trout (Oncorhynchus mykiss) Eggs Used for Meiotic Gynogenesis. Fishes. 2025; 10(11):585. https://doi.org/10.3390/fishes10110585
Chicago/Turabian StyleOcalewicz, Konrad, Karolina Pałucha, Agata Błaszczyk, Marcin Kuciński, Stefan Dobosz, Ligia Panasiak, and Rafał Rożyński. 2025. "Gene Expression and Antioxidant Characteristics of Rainbow Trout (Oncorhynchus mykiss) Eggs Used for Meiotic Gynogenesis" Fishes 10, no. 11: 585. https://doi.org/10.3390/fishes10110585
APA StyleOcalewicz, K., Pałucha, K., Błaszczyk, A., Kuciński, M., Dobosz, S., Panasiak, L., & Rożyński, R. (2025). Gene Expression and Antioxidant Characteristics of Rainbow Trout (Oncorhynchus mykiss) Eggs Used for Meiotic Gynogenesis. Fishes, 10(11), 585. https://doi.org/10.3390/fishes10110585






