Dietary Inclusion of Micro-Algal Astaxanthin on Gut Health of Rainbow Trout Oncorhynchus mykiss: Insights from Gut Morphology, Physiological Indices and Microbiota Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Fish Breeding and Treatment
2.3. Sample Collection
2.4. Gut Histology
2.5. Analysis of Antioxidant and Immune Parameters
2.6. Gene Expression Analysis
2.7. DNA Extraction and Sequencing of Intestinal Microbiota
2.8. Data Statistical Analysis
3. Results
3.1. Dietary Astaxanthin Improves Intestinal Morphology of Rainbow Trout
3.2. Antioxidant Responses of Guts Were Induced by Dietary Astaxanthin
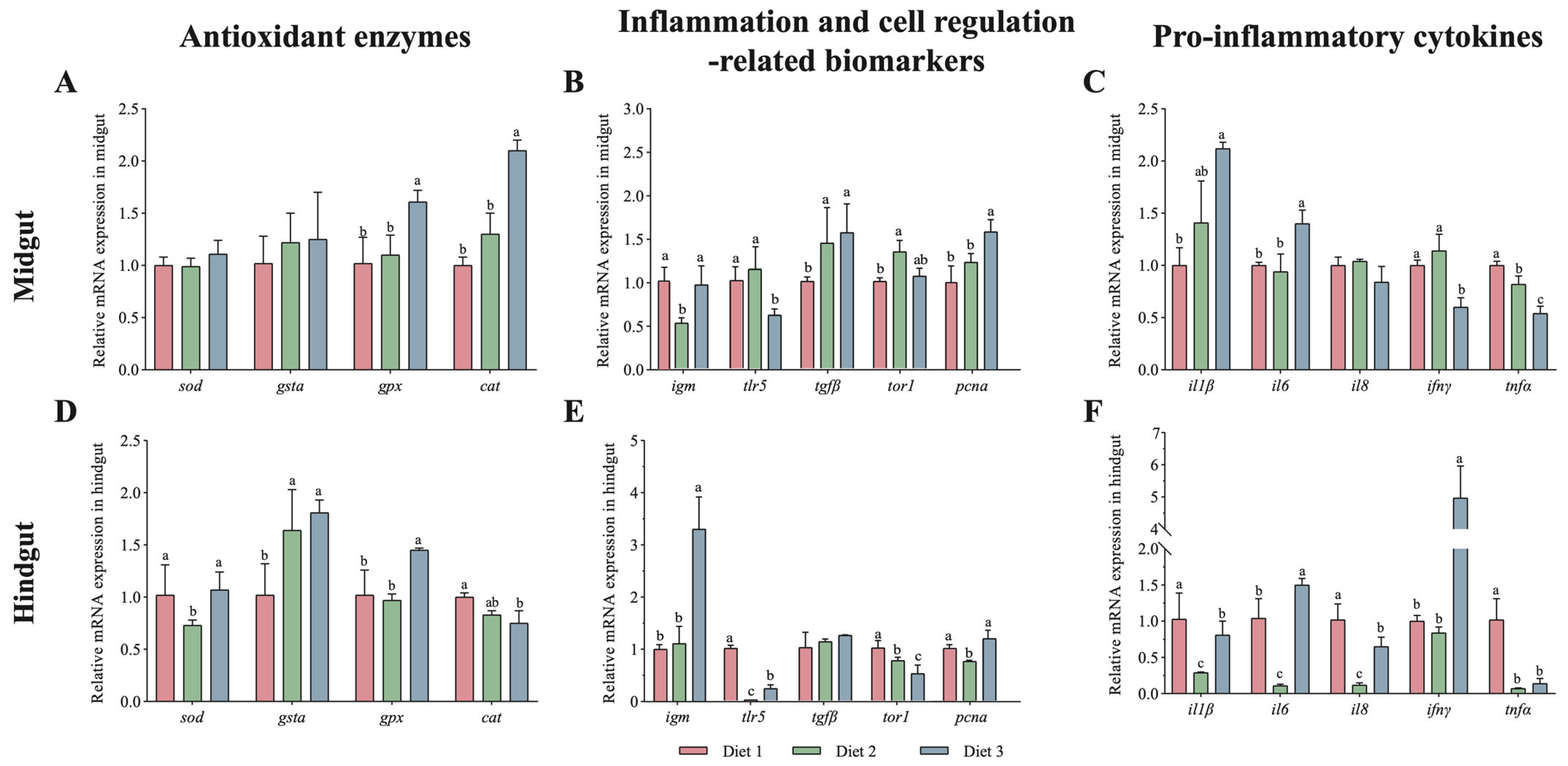
3.3. Immune Responses of Guts Were Induced by Dietary Astaxanthin
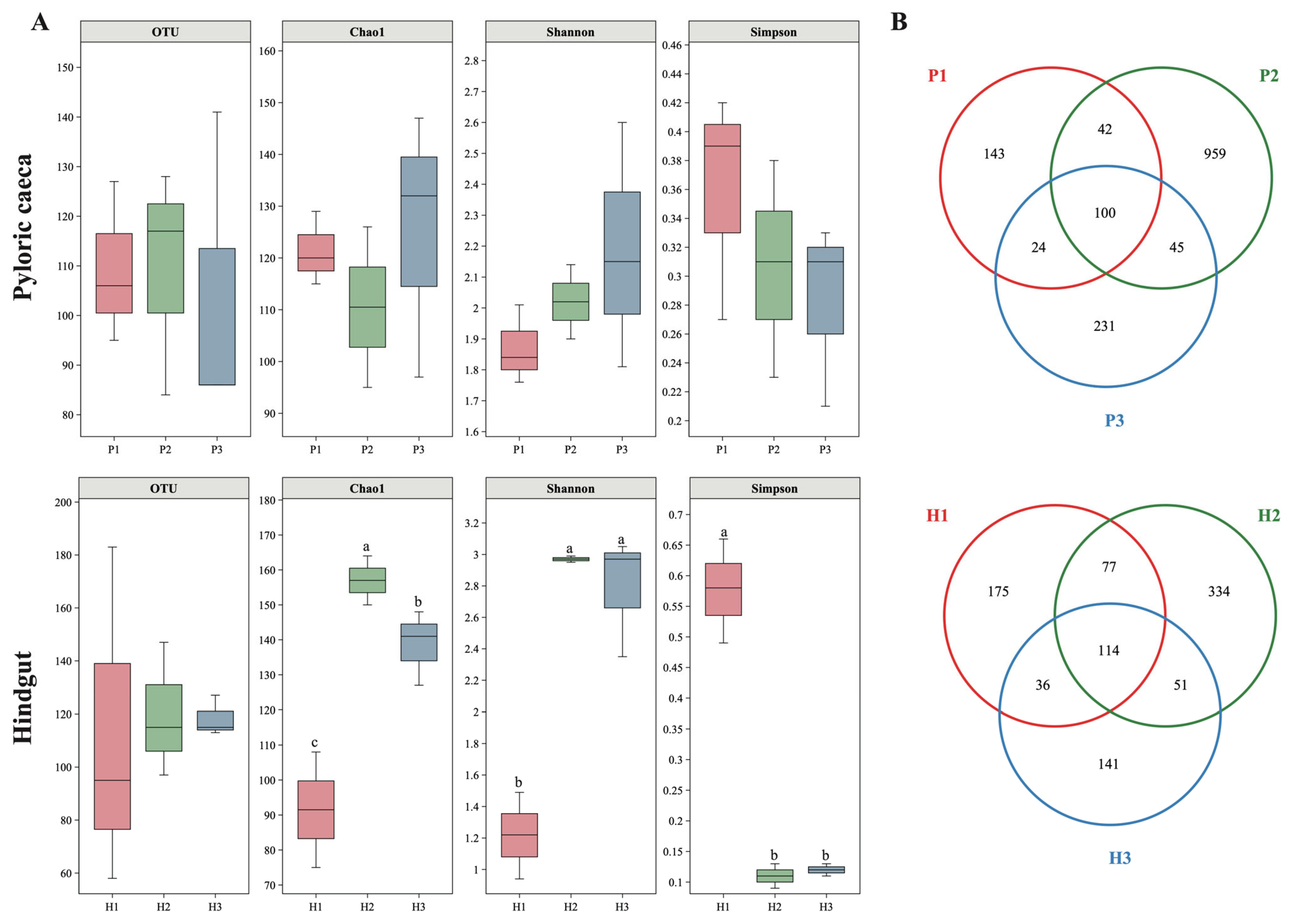
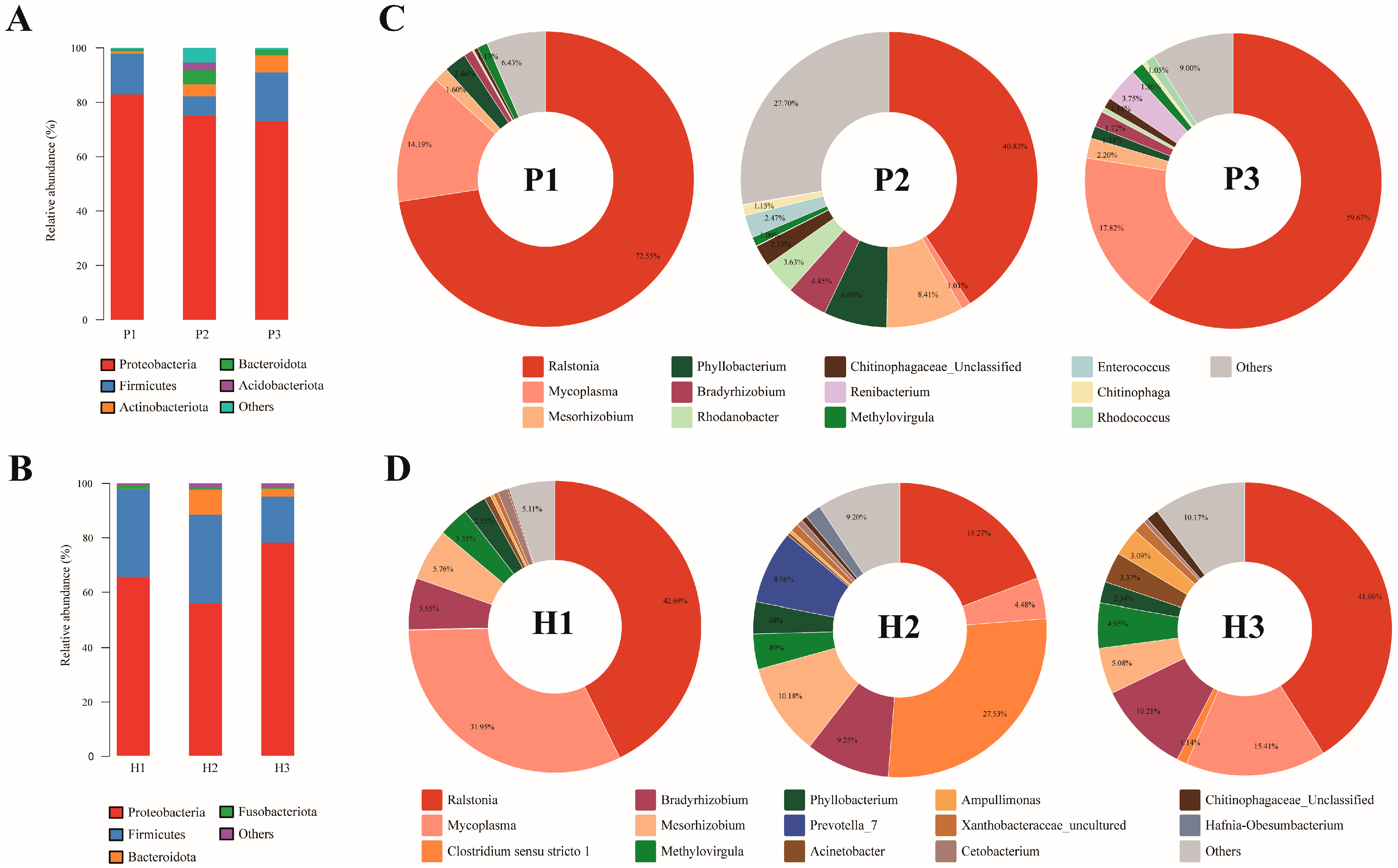
3.4. Dietary Astaxanthin Alters the Gut Microbiota Diversity and Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dan, X.M.; Zhang, T.W.; Li, Y.W.; Li, A.X. Immune responses and immune-related gene expression profile in orange-spotted grouper after immunization with Cryptocaryon irritans vaccine. Fish Shellfish Immunol. 2013, 34, 885–891. [Google Scholar] [CrossRef]
- Ali, M.; Nicholson, H.L. Increasing zebrafish (Danio rerio) numbers in a limited tank space reduces night-time fish sleep-like state and induces aggressive behaviour. Depress. Anxiety 2018, 1, 1003. [Google Scholar]
- Portz, D.E.; Woodley, C.M.; Cech, J.J. Stress-associated impacts of short-term holding on fishes. Rev. Fish Biol. Fish. 2006, 16, 125–170. [Google Scholar] [CrossRef]
- Lock, E.J.; Waagbo, R.; Bonga, S.W.; Flik, G. The significance of vitamin D for fish: A review. Aquac. Nutr. 2010, 16, 100–116. [Google Scholar] [CrossRef]
- Hernandez, L.H.; Hardy, R.W. Vitamin A functions and requirements in fish. Aquac. Res. 2020, 51, 3061–3071. [Google Scholar] [CrossRef]
- Wade, N.M.; Gabaudan, J.; Glencross, B.D. A review of carotenoid utilisation and function in crustacean aquaculture. Rev. Aquac. 2017, 9, 141–156. [Google Scholar] [CrossRef]
- Besen, K.P.; Melim, E.W.H.; da Cunha, L.; Favaretto, E.D.; Moreira, M.; Fabregat, T.E.P. Lutein as a natural carotenoid source: Effect on growth, survival and skin pigmentation of goldfish juveniles (Carassius auratus). Aquac. Res. 2019, 50, 2200–2206. [Google Scholar] [CrossRef]
- Abd El-Gawad, E.A.; Wang, H.P.; Yao, H. Diet supplemented with synthetic carotenoids: Effects on growth performance and biochemical and immunological parameters of yellow perch (Perca flavescens). Front. Physiol. 2019, 10, 1056. [Google Scholar] [CrossRef]
- Mansour, A.T.; El-Feky, M.M.M.; El-Beltagi, H.S.; Sallam, A.E. Synergism of dietary co-supplementation with lutein and bile salts improved the growth performance, carotenoid content, antioxidant capacity, lipid metabolism, and lipase activity of the marbled spinefoot rabbitfish, Siganus rivulatus. Animals 2020, 10, 1643. [Google Scholar] [CrossRef]
- Xie, W.; Deng, H.G.; Li, K.; Ma, Y.C.; Gao, M.R.; Duan, H.; Sui, L.Y. Dietary supplementation of archaeal carotenoids improved antioxidative capacity and regulated immune-related gene expression of golden trout Oncorhynchus mykiss against challenge. Aquac. Res. 2022, 53, 5053–5062. [Google Scholar] [CrossRef]
- Elbahnaswy, S.; Elshopakey, G.E. Recent progress in practical applications of a potential carotenoid astaxanthin in aquaculture industry: A review. Fish Physiol. Biochem. 2023, 14, 100685. [Google Scholar] [CrossRef]
- Long, X.W.; Wang, L.; Li, Y.P.; Sun, W.H.; Wu, X.G. Effects of long-term Haematococcus pluvialis astaxanthin feeding on the growth, coloration, and antioxidant capacity of commercial-sized Oncorhynchus mykiss. Aquac. Rep. 2023, 30, 101603. [Google Scholar] [CrossRef]
- Boe, M.R.; Vo, T.T.M.; Hansen, A.K.G.; Lerfall, J. Effect of natural carotenoids obtained from Haematococcus pluvialis, Paracoccus carotinifaciens, and Phaffia rhodozyma on flesh pigmentation and related biochemical mechanisms in Atlantic salmon (Salmo salar L.). Aquaculture 2025, 596, 741743. [Google Scholar] [CrossRef]
- Sha, Y.P.; Chen, Y.H.; Dong, J.; Gao, X.F.; Yuan, H.T.; Zhang, J.X.; Gao, Y.N.; Li, X.J. Effects of astaxanthin microencapsulated from Haematococcus pluvialis on the growth, muscle quality, antioxidant system, and related gene expression of Cyprinus carpio. Aquac. Int. 2025, 33, 110. [Google Scholar] [CrossRef]
- Wang, L.; Long, X.W.; Li, Y.P.; Zhang, Y.; Sun, W.H.; Wu, X.G. Effects of three sources of astaxanthin on the growth, coloration, and antioxidant capacity of rainbow trout (Oncorhynchus mykiss) during long-term feeding. Fishes 2024, 9, 174. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Chen, X.X.; Too, H.P. Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Appl. Microbiol. Biotechnol. 2020, 104, 5725–5737. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Oushani, A.K.; Enferadi, M.H. Effects of Haematococcus pluvialis supplementation on antioxidant system and metabolism in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2012, 38, 413–419. [Google Scholar] [CrossRef]
- Long, X.W.; Wu, X.G.; Zhao, L.; Liu, J.G.; Cheng, Y.X. Effects of dietary supplementation with Haematococcus pluvialis cell powder on coloration, ovarian development and antioxidation capacity of adult female Chinese mitten crab, Eriocheir sinensis. Aquaculture 2017, 473, 545–553. [Google Scholar] [CrossRef]
- Wang, W.L.; Liu, M.T.; Fawzy, S.; Xue, Y.C.; Wu, M.Q.; Huang, X.X.; Yi, G.F.; Lin, Q. Effects of dietary Phaffia rhodozyma astaxanthin on growth performance, carotenoid analysis, biochemical and immune-physiological parameters, intestinal microbiota, and disease resistance in Penaeus monodon. Front. Microbiol. 2021, 12, 762689. [Google Scholar] [CrossRef]
- Kalinowski, C.T.; Larroquet, L.; Veron, V.; Robaina, L.; Izquierdo, M.S.; Panserat, S.; Kaushik, S.; Fontagne-Dicharry, S. Influence of dietary astaxanthin on the hepatic oxidative stress response caused by Episodic Hyperoxia in rainbow trout. Antioxidants 2019, 8, 626. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, C.F.; Cao, X.M.; Zhu, J.M.; He, J.; Wu, P.; Ye, Y.T. Supplementation of dietary astaxanthin alleviated oxidative damage induced by chronic high pH stress, and enhanced carapace astaxanthin concentration of Chinese mitten crab Eriocheir sinensis. Aquaculture 2018, 483, 230–237. [Google Scholar] [CrossRef]
- Jiang, X.D.; Zu, L.; Wang, Z.Y.; Cheng, Y.X.; Yang, Y.H.; Wu, X.G. Micro-algal astaxanthin could improve the antioxidant capability, immunity and ammonia resistance of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immun. 2020, 102, 499–510. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chien, Y.H.; Pan, C.H. Effects of dietary supplementation of carotenoids on survival, growth, pigmentation, and antioxidant capacity of characins, Hyphessobrycon callistus. Aquaculture 2006, 261, 641–648. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, X.Q.; Leng, X.J.; Zhang, C.L.; Han, Z.Y.; Zhang, F.G. Effects of dietary astaxanthins on pigmentation of flesh and tissue antioxidation of rainbow trout (Oncorhynchus mykiss). Aquac. Int. 2013, 21, 579–589. [Google Scholar] [CrossRef]
- Wang, C.; Qin, S.; Sun, F.J.; Shao, Y.P.; Du, R.; Gao, Z.Q.; Cui, Y.L. Effects of feeding Haematococcus pluvialis powder on astaxanthin accumulation, biochemical compositions, antioxidant activity, and gut microbial community in juvenile crucian carp (Carassius auratus L.). Aquac. Rep. 2024, 36, 102109. [Google Scholar] [CrossRef]
- Song, X.L.; Wang, L.; Li, X.Q.; Chen, Z.Z.; Liang, G. Dietary astaxanthin improved the body pigmentation and antioxidant function, but not the growth of discus fish (Symphysodon spp.). Aquac. Res. 2017, 48, 1359–1367. [Google Scholar] [CrossRef]
- Carmen Tatiana, K.; Monica, B.B.; Silvia, T.; Matthew, S.; Laurence, L.; Vincent, V.; Stephane, P.; Maria Soledad, I.; Sadasivam, J.K.; Stephanie, F.-D. More than an antioxidant: Role of dietary astaxanthin on lipid and glucose metabolism in the liver of rainbow trout (Oncorhynchus mykiss). Antioxidants 2023, 12, 136. [Google Scholar] [CrossRef]
- Ringø, E.; Zhou, Z.; Vecino, J.L.G.; Wadsworth, S.; Romero, J. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquac. Nutr. 2015, 22, 219–282. [Google Scholar] [CrossRef]
- Pratap, K.; Majzoub, M.E.; Taki, A.C.; Hernandez, S.M.; Magnusson, M.; Glasson, C.R.K.; de Nys, R.; Thomas, T.; Lopata, A.L.; Kamath, S.D. The algal polysaccharideulvan and carotenoid astaxanthin both positively modulate gut microbiota in mice. Foods 2022, 11, 565. [Google Scholar] [CrossRef]
- Ou, W.H.; Liao, Z.B.; Yu, G.J.; Xu, H.G.; Liang, M.Q.; Mai, K.S.; Zhang, Y.J. The effects of dietary astaxanthin on intestinal health of juvenile tiger puffer Takifugu rubripes in terms of antioxidative status, inflammatory response and microbiota. Aquac. Nutr. 2019, 25, 466–476. [Google Scholar] [CrossRef]
- Ma, F.; Ma, R.L.; Zou, Y.L.; Zhao, L. Effect of astaxanthin on the antioxidant capacity and intestinal microbiota of tsinling lenok trout (Brachymystax lenok tsinlingensis). Mar. Biotechnol. 2022, 24, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Ministry of Agriculture Fisheries and Fishery Administration Bureau. China Fishery Statistical Yearbook 2024; Agriculture Press: Beijing, China, 2024.
- Mansfield, G.S.; Desai, A.R.; Nilson, S.A.; Van Kessel, A.G.; Drew, M.D.; Hill, J.E. Characterization of rainbow trout (Oncorhynchus mykiss) intestinal microbiota and inflammatory marker gene expression in a recirculating aquaculture system. Aquaculture 2010, 307, 95–104. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Hao, Y.T.; Zhang, L.H.; Gao, X.W.; Xu, Y.H.; Wang, J.J.; Hanafiah, F.; Khor, W.; Sun, Y.F.; Wu, C.B. Profiling the gut structure and microbiota, and identifying two dominant bacteria belonging to the Weissella genus in mandarin fish (Siniperca chuatsi) fed an artificial diet. Front. Microbiol. 2024, 15, 1486501. [Google Scholar] [CrossRef]
- Fang, H.H.; Xie, J.J.; Zhao, W.; Liu, Z.L.; Liu, Y.J.; Tian, L.X.; Niu, J. Study supplementation of astaxanthin in high-fat diet on growth performance, antioxidant ability, anti-inflammation, non-specific immunity and intestinal structure of juvenile Trachinotus ovatus. Aquac. Nutr. 2021, 27, 2575–2586. [Google Scholar] [CrossRef]
- Poljšak, B.; Jamnik, P.; Raspor, P.; Pesti, M. Oxidation-antioxidation-reduction processes in the cell: Impacts of environmental pollution. Encycl. Environ. Health 2011, 300–306. [Google Scholar] [CrossRef]
- Duan, Y.F.; Zhang, J.S.; Dong, H.B.; Wang, Y.; Liu, Q.S.; Li, H. Oxidative stress response of the black tiger shrimp Penaeus monodon to Vibrio parahaemolyticus challenge. Fish Shellfish Immun. 2015, 46, 354–365. [Google Scholar] [CrossRef]
- Chen, X.M.; Gao, C.S.; Du, X.Y.; Yao, J.M.; He, F.F.; Niu, X.T.; Wang, G.Q.; Zhang, D.M. Effects of dietary astaxanthin on the growth, innate immunity and antioxidant defence system of Paramisgurnus dabryanus. Aquac. Nutr. 2020, 26, 1453–1462. [Google Scholar] [CrossRef]
- Ettefaghdoost, M.; Haghighi, H. Impact of different dietary lutein levels on growth performance, biochemical and immuno-physiological parameters of oriental river prawn (Macrobrachium nipponense). Fish Shellfish Immunol. 2021, 115, 86–94. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.Q.; Wang, X.L.; Ren, Q.; Zhang, G.S.; Liang, F.F.; Yin, S.W. Immune responses of two superoxide dismutases (SODs) after lipopolysaccharide or Aeromonas hydrophila challenge in pufferfish, Takifugu obscurus. Aquaculture 2016, 459, 1–7. [Google Scholar] [CrossRef]
- Zhu, X.W.; Hao, R.J.; Zhang, J.P.; Tian, C.X.; Hong, Y.C.; Zhu, C.H.; Li, G.L. Dietary astaxanthin improves the antioxidant capacity, immunity and disease resistance of coral trout (Plectropomus leopardus). Fish Shellfish Immunol. 2022, 122, 38–47. [Google Scholar] [CrossRef]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef]
- Lim, K.H.; Staudt, L.M. Toll-like receptor signaling. Cold Spring Harb. Perspect. Biol. 2013, 5, a011247. [Google Scholar] [CrossRef]
- Li, M.Y.; Gao, C.S.; Du, X.Y.; Zhao, L.; Zhang, D.M. Effect of sub-chronic exposure to selenium and astaxanthin on Channa argus: Bioaccumulation, oxidative stress and inflammatory response. Chemosphere 2019, 244, 125546. [Google Scholar] [CrossRef]
- Yasui, Y.; Hosokawa, M.; Mikami, N.; Miyashita, K.; Tanaka, T. Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cytokines. Chem. Biol. Interact. 2011, 193, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ikeda, Y.; Ohigashi, H. Aggregated ursolic acid, a natural triterpenoid, binds to CD36 for inducing interleukin-1β release from murine peritoneal macrophages. Cancer Res. 2007, 67, 3383. [Google Scholar]
- Wei, L.; Wu, J.; Zhao, L.; Chen, Y.X.; Yang, P. CD36 knockdown alleviates lipid accumulation induced by tumor necrosis factor-α and interleukin-6 in HepG2 cell. J. Army Med. Univ. 2018, 40, 1833–1838. [Google Scholar]
- Sakudoh, T.; Kuwazaki, S.; Iizuka, T.; Narukawa, J.; Yamamoto, K.; Uchino, K.; Sezutsu, H.; Banno, Y.; Tsuchida, K. CD36 homolog divergence is responsible for the selectivity of carotenoid species migration to the silk gland of the silkworm Bombyx mori. J. Lipid Res. 2013, 54, 482–495. [Google Scholar] [CrossRef]
- Nassir, F.; Wilson, B.; Han, X.L.; Gross, R.W.; Abumrad, N.A. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J. Biol. Chem. 2007, 282, 19493–19501. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Carmody, R.N.; Gerber, G.K.; Luevano, J.M., Jr.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef]
- Desai, A.R.; Links, M.G.; Hill, J.E. Effects of plant-based diets on the distal gut microbiome of rainbow trout (Oncorhynchus mykiss). Aquaculture 2012, 350, 134–142. [Google Scholar] [CrossRef]
- Rimoldi, S.; Antonini, M.; Gasco, L.; Moroni, F.; Terova, G. Intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) may be improved by feeding a Hermetia illucens meal/low-fishmeal diet. Fish Physiol. Biochem. 2021, 47, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Lyons, P.P.; Turnbull, J.F.; Dawson, K.A.; Crumlish, M. Effects of low-level dietary microalgae supplementation on the distal intestinal microbiome of farmed rainbow trout Oncorhynchus mykiss (Walbaum). Aquac. Res. 2016, 48, 2438–2452. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.P.; Clement, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Tejera, D.; Limongi, G.; Bertullo, M.; Cancela, M. Ralstonia pickettii bacteremia in hemodialysis patients: A report of two cases. Rev. Bras. Ter. Intensiv. 2016, 28, 195–198. [Google Scholar]
- Chi, C.Y.; Fung, C.P.; Wong, W.W.; Liu, C.Y. Brevundimonas bacteremia: Two case reports and literature review. Scand. J. Infect. Dis. 2004, 36, 59–61. [Google Scholar] [CrossRef]
- Holben, W.E.; Williams, P.; Gilbert, M.A.; Saarinen, M.; Sarkilahti, L.K.; Apajalahti, J.H.A. Phylogenetic analysis of intestinal microflora indicates a novel mycoplasma phylotype in farmed and wild salmon. Microbiol. Ecol. 2002, 44, 175–185. [Google Scholar] [CrossRef]
- Brown, R.M.; Wiens, G.D.; Salinas, I. Analysis of the gut and gill microbiome of resistant and susceptible lines of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immun. 2019, 86, 497–506. [Google Scholar] [CrossRef]
- Ciric, M.; Waite, D.; Draper, J.; Jones, J.B. Characterization of mid-intestinal microbiota of farmed Chinook salmon using 16S rRNA gene metabarcoding. Arch. Biol. Sci. 2019, 71, 577–587. [Google Scholar] [CrossRef]
- Mora-Sanchez, B.; Balcazar, J.L.; Perez-Sanchez, T. Effect of a novel postbiotic containing lactic acid bacteria on the intestinal microbiota and disease resistance of rainbow trout (Oncorhynchus mykiss). Biotechnol. Lett. 2020, 42, 1957–1962. [Google Scholar] [CrossRef]
- Heinritz, S.N.; Mosenthin, R.; Weiss, E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr. Res. Rev. 2013, 26, 191–209. [Google Scholar] [CrossRef]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Wang, M.; Ma, H.T.; Guan, S.Y.; Luo, T.; Zhao, C.C.; Cai, G.P.; Zheng, Y.B.; Jia, X.Y.; Di, J.B.; Li, R.Z.; et al. Astaxanthin from Haematococcus pluvialis alleviates obesity by modulating lipid metabolism and gut microbiota in mice fed a high-fat diet. Food Funct. 2021, 12, 9719–9738. [Google Scholar] [CrossRef]
- Iljazovic, A.; Amend, L.; Galvez, E.J.C.; Oliveira, R.D.; Strowig, T. Modulation of inflammatory responses by gastrointestinal Prevotella spp.—From associations to functional studies. Int. J. Med. Microbiol. 2021, 311, 151472. [Google Scholar] [CrossRef]

| Items | Experimental Diets | ||
|---|---|---|---|
| Diet 1 | Diet 2 | Diet 3 | |
| Moisture (% dry diet) | 10.10 | 10.21 | 10.13 |
| Crud protein (% dry diet) | 42.45 | 41.56 | 42.02 |
| Crud lipid (% dry diet) | 23.65 | 23.82 | 23.63 |
| Ash (% dry diet) | 6.91 | 6.93 | 6.88 |
| Astaxanthin (mg/kg dry diet) | 0.00 | 18.57 | 31.25 |
| Indices | Experimental Diets | ||
|---|---|---|---|
| Diet 1 | Diet 2 | Diet 3 | |
| IW (kg) | 0.67 ± 0.04 | 0.67 ± 0.02 | 0.65 ± 0.02 |
| FW (kg) | 2.19 ± 0.16 | 2.18 ± 0.07 | 2.30 ± 0.12 |
| WG (kg) | 1.52 ± 0.13 | 1.51 ± 0.05 | 1.65 ± 0.10 |
| FCR | 0.85 ± 0.08 | 0.84 ± 0.01 | 0.87 ± 0.03 |
| SGR (%/d) | 0.99 ± 0.03 | 0.97 ± 0.02 | 1.03 ± 0.02 |
| SR (%) | 94.00 ± 2.67 | 91.56 ± 4.73 | 89.78 ± 4.02 |
| Target Gene | Primer Sequence (5′→3′) | Accession Number | |
|---|---|---|---|
| sod1 | F: | GTAGTCGTGGCTCAATGGTAAG | XM_021590204.2 |
| R: | GCTTTATATTCTGCGGGTCATT | ||
| cat | F: | TGATGTCACACAGGTGCGTA | TC99600 |
| R: | GTGGGCTCAGTGTTGTTGAG | ||
| gpx1 | F: | AAATTGCCATTCCCCTCCGA | XM_021569971.2 |
| R: | TCCATCAGGACTGACCAGGA | ||
| gsta | F: | CAGAGGACAGCTCCCTGCTT | NM_001160559.1 |
| R: | CTGAACCGGCTCTCCAGGTA | ||
| igm | F: | CACTTCATCAGATGGTCCAGTCC | X83372.1 |
| R: | ACAGTCCCATTGCTCCAGTCC | ||
| pcna | F: | TGTGACCGCAACCTCGCAATGG | XM_036936092.1 |
| R: | CACGGCAGATACGGGCAAACTCC | ||
| tgfβ | F: | AGATAAATCGGAGAGTTGCTGTG | X99303.1 |
| R: | CCTGCTCCACCTTGTGTTGT | ||
| tor1 | F: | ATGGTTCGATCACTGGTCATCA | EU179853 |
| R: | TCCACTCTTGCCACAGAGAC | ||
| tlr5 | F: | CTTACAGGAAACTCTATTCGC | NM_001124208 |
| R: | CTGTTAGCAAAGCCCAAGAGG | ||
| il1β | F: | ACATTGCCAACCTCATCATCG | AJ278242 |
| R: | TTGAGCAGGTCCTTGTCCTTG | ||
| il6 | F: | ACTCCCCTCTGTCACACACC | DQ866150 |
| R: | GGCAGACAGGTCCTCCACTA | ||
| il8 | F: | GCTGCATTGAGACGGAGAGC | HG917307 |
| R: | CCAGACAAATCTCCTGACCG | ||
| ifnγ | F: | CTGTTCAACGGAAACCCTGT | NM_001160503 |
| R: | AACACCCTCCGATCACTGTC | ||
| tnfα | F: | AAGCAGCCATCCATTTAGAGG | AJ277604 |
| R: | GTGTGTGGGATGAGGATTTGG | ||
| β-actin | F: | ATCCTGACAGAGCGCGGTTACAGT | AJ43815 |
| R: | TGCCCATCTCCTGCTCAAAGTCAA | ||
| Histological Parameters | Experimental Diets | ||
|---|---|---|---|
| Diet 1 | Diet 2 | Diet 3 | |
| Pyloric caeca | |||
| Tunica muscularis thickness (μm) | 164.45 ± 43.37 | 201.42 ± 45.54 | 196.76 ± 23.82 |
| Villus height (μm) | 970.41 ± 133.38 | 980.82 ± 68.47 | 974.33 ± 53.46 |
| Villus width (μm) | 135.04 ± 18.05 | 137.42 ± 11.03 | 136.91 ± 8.77 |
| Midgut | |||
| Tunica muscularis thickness (μm) | 399.87 ± 84.53 | 359.25 ± 66.60 | 366.82 ± 87.72 |
| Villus height (μm) | 841.24 ± 149.48 | 956.50 ± 278.26 | 838.84 ± 215.50 |
| Villus width (μm) | 146.67 ± 19.35 | 162.81 ± 16.03 | 154.71 ± 6.88 |
| Hindgut | |||
| Tunica muscularis thickness (μm) | 208.37 ± 17.90 b | 314.14 ± 20.34 a | 307.83 ± 26.89 a |
| Villus height (μm) | 1421.73 ± 220.55 | 1330.62 ± 249.59 | 1381.74 ± 110.28 |
| Villus width (μm) | 186.04 ± 41.87 | 163.20 ± 13.27 | 170.42 ± 26.86 |
| Items | Midgut | Hindgut | ||||
|---|---|---|---|---|---|---|
| Diet 1 | Diet 2 | Diet 3 | Diet 1 | Diet 2 | Diet 3 | |
| Antioxidant parameters | ||||||
| T-AOC (U/mgprot) | 1.65 ± 0.47 a | 1.16 ± 0.27 b | 1.11 ± 0.14 b | 3.38 ± 0.85 | 3.46 ± 0.62 | 3.14 ± 0.54 |
| T-SOD (U/mgprot) | 540.46 ± 65.62 b | 596.94 ± 11.29 a | 617.20 ± 57.73 b | 266.66 ± 37.25 | 284.24 ± 31.08 | 283.07 ± 27.67 |
| CAT (U/mgprot) | 4.06 ± 0.92 | 3.86 ± 0.78 | 3.46 ± 0.87 | 0.77 ± 0.15 | 0.67 ± 0.16 | 0.50 ± 0.36 |
| MDA (nmol/mgprot) | 24.29 ± 5.71 a | 15.18 ± 2.83 b | 17.62 ± 3.51 b | 18.01 ± 4.00 | 15.98 ± 2.29 | 17.89 ± 3.92 |
| Immunological parameters | ||||||
| ACP (U/gprot) | 440.94 ± 116.25 | 482.88 ± 87.03 | 490.73 ± 42.66 | 258.08 ± 23.55 | 310.05 ± 29.06 | 288.61 ± 43.88 |
| AKP (U/gprot) | 3594.70 ± 381.41 a | 1966.01 ± 563.35 b | 2699.26 ± 521.64 b | 2005.84 ± 262.77 | 2222.77 ± 507.79 | 1701.58 ± 431.36 |
| LZM (U/mgprot) | 6.71 ± 1.95 b | 4.76 ± 1.09 b | 9.57 ± 2.43 a | 47.99 ± 7.27 b | 39.82 ± 9.49 b | 64.30 ± 13.91 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Long, X.; Li, Y.; Zhang, Y.; Sun, W.; Wu, X. Dietary Inclusion of Micro-Algal Astaxanthin on Gut Health of Rainbow Trout Oncorhynchus mykiss: Insights from Gut Morphology, Physiological Indices and Microbiota Diversity. Fishes 2025, 10, 505. https://doi.org/10.3390/fishes10100505
Zhang M, Long X, Li Y, Zhang Y, Sun W, Wu X. Dietary Inclusion of Micro-Algal Astaxanthin on Gut Health of Rainbow Trout Oncorhynchus mykiss: Insights from Gut Morphology, Physiological Indices and Microbiota Diversity. Fishes. 2025; 10(10):505. https://doi.org/10.3390/fishes10100505
Chicago/Turabian StyleZhang, Min, Xiaowen Long, Yaopeng Li, Yong Zhang, Weihong Sun, and Xugan Wu. 2025. "Dietary Inclusion of Micro-Algal Astaxanthin on Gut Health of Rainbow Trout Oncorhynchus mykiss: Insights from Gut Morphology, Physiological Indices and Microbiota Diversity" Fishes 10, no. 10: 505. https://doi.org/10.3390/fishes10100505
APA StyleZhang, M., Long, X., Li, Y., Zhang, Y., Sun, W., & Wu, X. (2025). Dietary Inclusion of Micro-Algal Astaxanthin on Gut Health of Rainbow Trout Oncorhynchus mykiss: Insights from Gut Morphology, Physiological Indices and Microbiota Diversity. Fishes, 10(10), 505. https://doi.org/10.3390/fishes10100505







