Garlic Powder Evaluation as Feed Additive on Nile Tilapia (Oreochromis niloticus L.) Growth Performance, Feed Utilization, Gill Parasitic Treatment, and Monogenean Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Ingredient Preparation and Laboratory Analyses
2.2. Formulation, Diet Preparation, and Laboratory Analyses
2.3. Fish Rearing and Growth Performance
2.4. Sample Preparation and Conservation
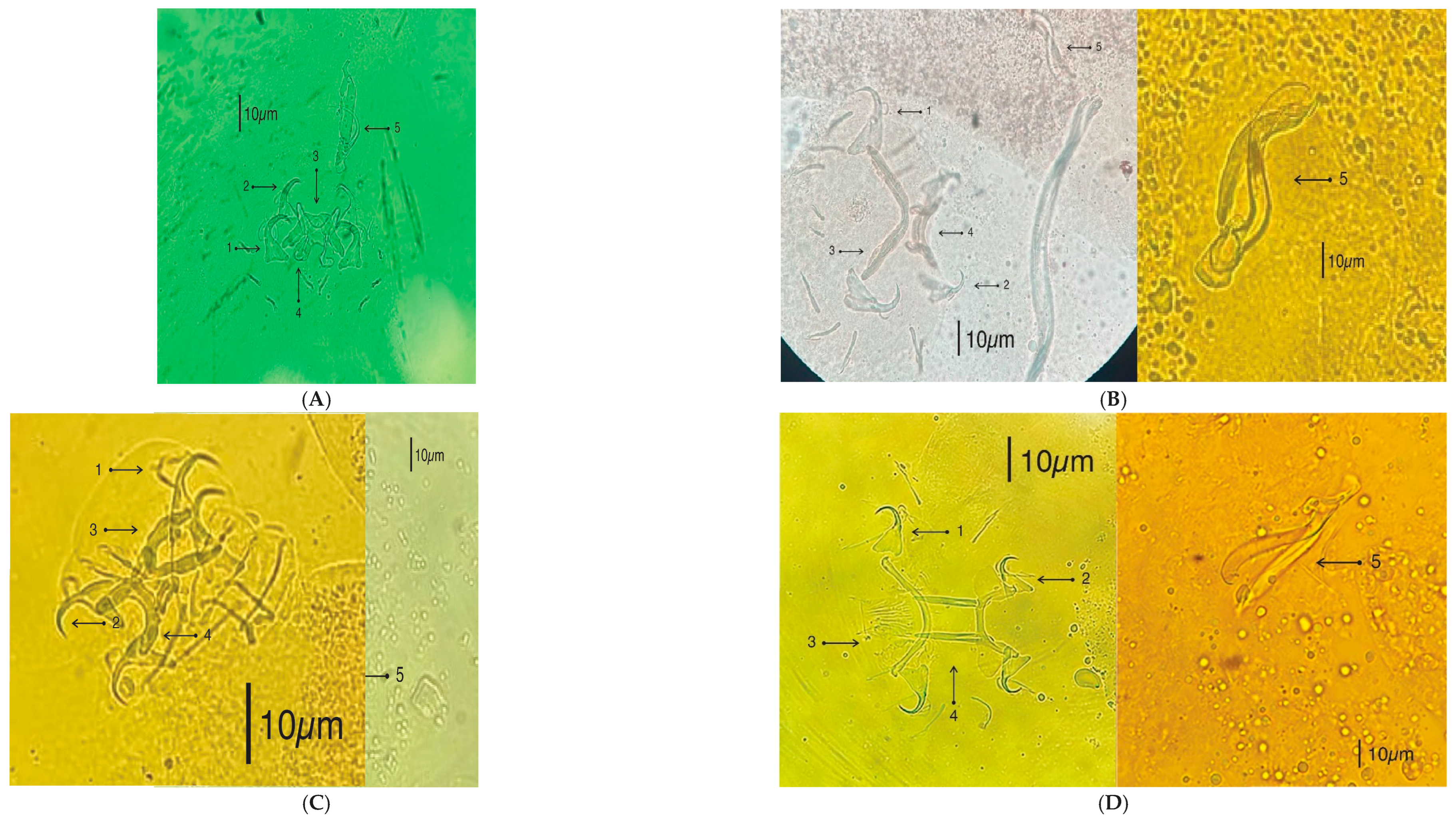
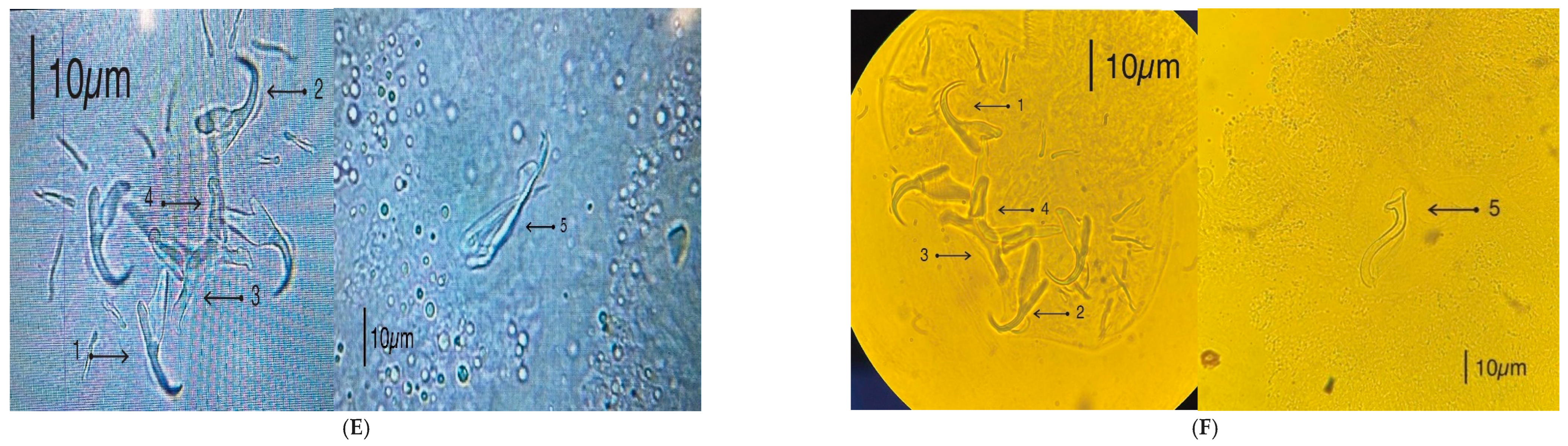
2.5. Microscopy and Illustrations
2.6. Infection Parameters of Fish
2.7. Monogenean Genetic Diversity
2.7.1. DNA Extraction, PCR Conditions, and Sequencing of Amplicons
2.7.2. Sequence Editing and Analysis
2.8. Statistical Analyses
3. Results
3.1. Growth Trial
3.2. Parasite Infection Levels and Efficacy
3.3. Morphological Identification of Monogenean
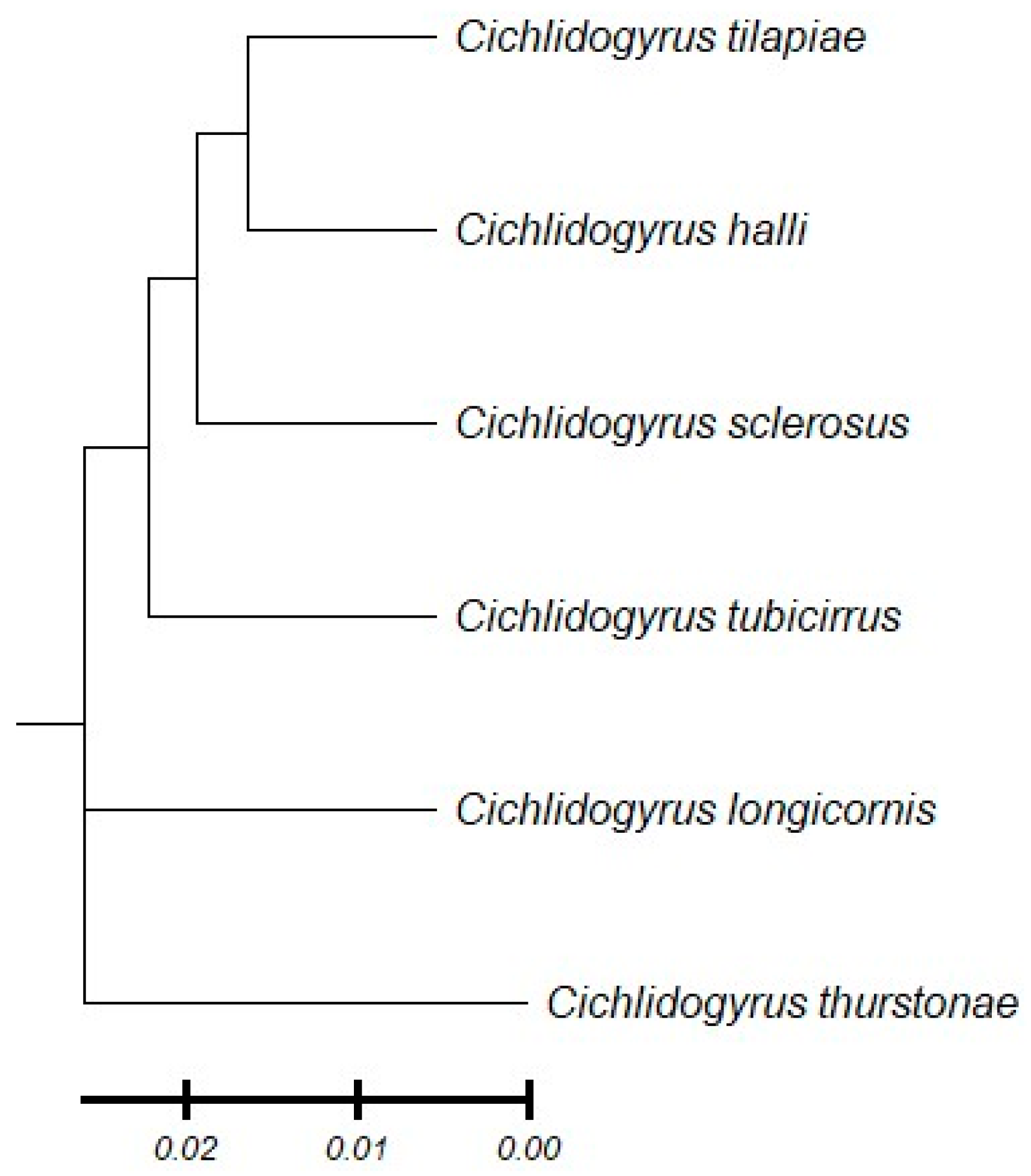
3.4. Molecular Identification and Genetic Diversity
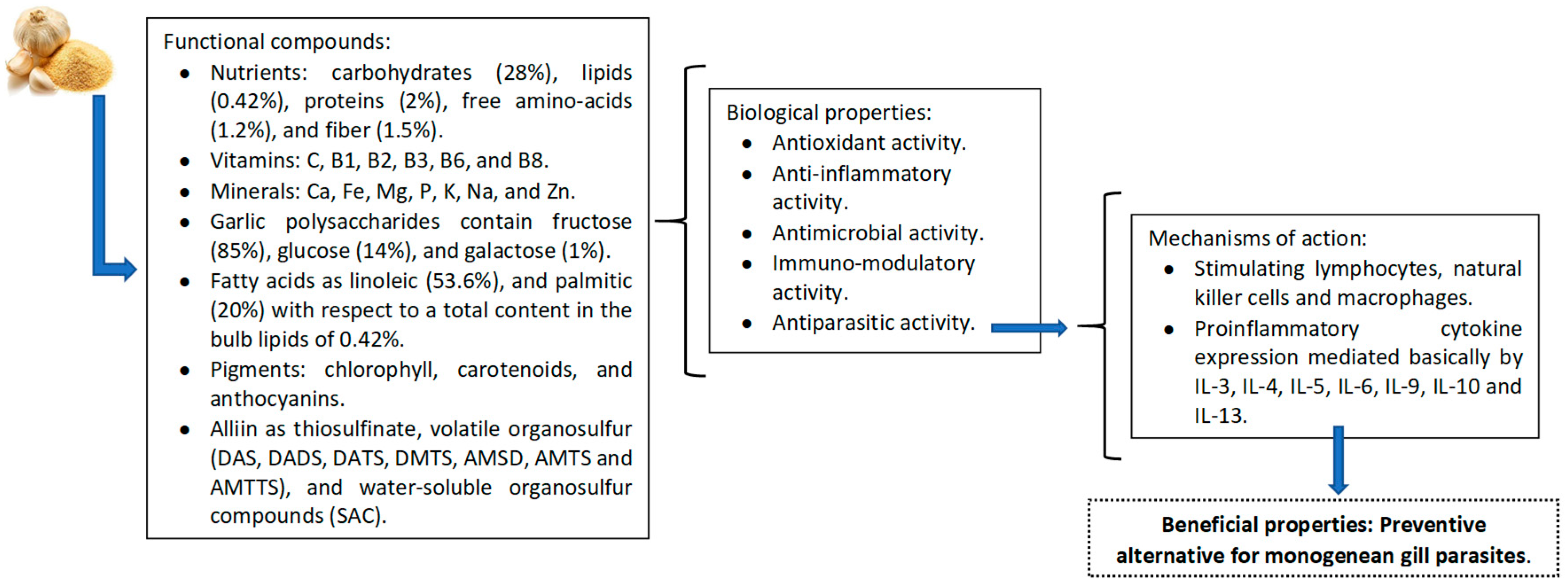
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2023–2032; OECD: Paris, France, 2023; Available online: https://www.fao.org/documents/card/es?details=CC6361EN (accessed on 3 February 2024).
- Gutiérrez-Leyva, R.; Rodríguez-González, H.; Carrillo-Domínguez, S.; Ulloa, J.A.; Ramírez-Ramírez, J.C.; Rosas-Ulloa, P.; Bautista-Rosales, P.U.; Civera-Cerecedo, R. Canary seed, Phalaris canariensis, has higher nutritional value than giant kelp seaweed, Macrocystis pyrifera, as feed ingredient in diets for Nile tilapia, Oreochromis niloticus. J. World Aquac. Soc. 2023, 54, 666–685. [Google Scholar] [CrossRef]
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.; Ambasankar, K.; Muralidhar, M.; Dayal, J.S. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/documents/card/en?details=cc0461en (accessed on 22 February 2024).
- Noor El-Deen, A.I.; Abd El-Hady, O.K.; Kenawy, A.M.; Mona, S.Z. Study of the prevailing external parasitic diseases in cultured freshwater tilapia (Oreochromis niloticus) Egypt. Life Sci. J. 2015, 12, 30–37. [Google Scholar]
- Islam, S.I.; Rodkhum, C.; Taweethavonsawat, P. An overview of parasitic co-infections in tilapia culture. Aquac. Int. 2024, 32, 899–927. [Google Scholar] [CrossRef]
- Shinn, A.P.; Avenant-Oldewage, A.; Bondad-Reantaso, M.G.; Cruz-Laufer, A.J.; García-Vásquez, A.; Hernández-Orts, J.S.; Kuchta, R.; Longshaw, M.; Metselaar, M.; Pariselle, A.; et al. A global review of problematic and pathogenic parasites of farmed tilapia. Rev. Aquac. 2023, 15, 92–153. [Google Scholar] [CrossRef]
- Paredes-Trujillo, A.; Velázquez-Abunader, I.; Papiol, V.; del Rio-Rodriguez, R.E.; Vidal-Martínez, V.M. Negative effect of ectoparasite burdens on the condition factor from farmed tilapia Oreochromis niloticus in the Yucatan, Mexico. Vet. Parasitol. 2021, 292, 109393. [Google Scholar] [CrossRef]
- Hussein, M.S.; El-Zaiat, A.M.; El-Saiad, S.M. Effects of garlic and onion oil extracts as a natural growth promoter on growth performance, nutrient utilization, whole body composition and hematological parameters of Nile tilapia (Oreochromis niloticus) fingerlings. J. Egypt. Acad. Soc. Environ. D 2016, 17, 141–155. [Google Scholar]
- Abdel-Hakim, N.; Lashin, M.; Ashry, A.; Al-Azab, A.D. Effect of fresh or dried garlic as a natural feed supplement on growth performance and nutrients utilization of the Nile Tilapia (Oreochromis niloticas). Egypt. J. Aquat. Biol. Fish. 2010, 14, 19–38. [Google Scholar] [CrossRef]
- Metwally, M.A.A. Effects of garlic (Allium sativum) on some antioxidant activities in tilapia nilotica (Oreochromis niloticus). World J. Fish Mar. Sci. 2009, 1, 56–64. [Google Scholar]
- Shalaby, A.M.; Khattab, Y.A.; Abdel Rahman, A.M. Effects of Garlic (Allium sativum) and chloramphenicol on growth performance, physiological parameters and survival of Nile tilapia (Oreochromis niloticus). J. Venom. Anim. Toxins Incl. Trop. Dis. 2006, 12, 172–201. [Google Scholar] [CrossRef]
- Reda, R.; Khalil, A.A.; Elhady, M.; Tayel, S.I.; Ramadan, E.A. Anti-parasitic activity of garlic (Allium sativum) and onion (Allium cepa) extracts against Dactylogyrus spp. (Monogenean) in Nile tilapia (Oreochromis niloticus): Hematology, immune response, histopathological investigation, and inflammatory cytokine genes of gills. BMC Vet. Res. 2024, 20, 334. [Google Scholar]
- Abd El-Galil, M.A.; Aboelhadid, S.M. Trials for the control of trichodinosis and gyrodactylosis in hatchery reared Oreochromis niloticus fries by using garlic. Vet. Parasitol. 2012, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Gutiérrez, R.; Lago-Lestón, A.; Vargas-Albores, F.; Cicala, F.; Martínez-Porchas, M. Exploring the garlic (Allium sativum) properties for fish aquaculture. Fish Physiol. Biochem. 2021, 47, 1179–1198. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, S.; Onbaşılar, E.E.; Reisli, Z.; Yalçın, S. Effect of garlic powder on the performance, egg traits and blood parameters of laying hens. J. Sci. Food Agric. 2006, 86, 1336–1339. [Google Scholar] [CrossRef]
- Lawson, L.D.; Wang, Z.J. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: Use in measuring allicin bioavailability. J. Agric. Food Chem. 2005, 53, 1974–1983. [Google Scholar] [CrossRef]
- Lee, J.Y.; Gao, Y. Review of the application of garlic, Allium sativum, in aquaculture. J. World Aquac. Soc. 2012, 43, 447–458. [Google Scholar] [CrossRef]
- Yoo, M.; Lee, S.; Kim, S.; Shin, D. Optimizing conditions for E-and Z-ajoene formation from garlic juice using response surface methodology. Food Sci. Nutr. 2014, 2, 605–611. [Google Scholar] [CrossRef]
- Garlic Market Outlook 2022–2026. Available online: https://www.reportlinker.com/clp/global/2543 (accessed on 15 April 2024).
- Erazo Pagador, G.; Dumaran Paciente, H.R.; Caloyloy, B.J. Behavior Changes and LC50 of Dried Garlic (Allium sativum) Acute Toxicity in Nile Tilapia (Oreochromis niloticus) Juvenile. Philipp. Agric. Sci 2023, 106, 3. [Google Scholar] [CrossRef]
- Muahiddah, N.; Diamahesa, W.A. The Use Of Garlic (Allium sativum) As An Immunostimulant In Aquaculture. J. Fish Health 2023, 3, 11–18. [Google Scholar] [CrossRef]
- Serna-Ardila, M.; Londoño-Maya, M.D.; Arias-Monsalve, C.S.; Londoño-Franco, L.F.; Pineda-Santis, H.R. Effect of pharmacological and homeopathic substances on Trichodina sp in larvae of red tilapia Oreochromis sp. in farming. Rev. De Investig. Vet. Perú 2022, 33, 3. [Google Scholar] [CrossRef]
- Rojas-Garcia, C.R.; Jimenez-Garcia, I.; Mendoza-Franco, E. Ecto-parasitic infection in Nile tilapia (Oreochromis niloticus) fry during male reversal in Veracruz, México. Int. Aquat. Res. 2020, 12, 3. [Google Scholar]
- El Deen, A.I.N.; Razin, A.M. Application of some medicinal Plants to eliminate Trichodina sp. in tilapia (Oreochromis niloticus). Rep. Opin. 2009, 1, 1–5. [Google Scholar]
- Chitmanat, C.; Tongdonmuan, K.; Nunsong, W. The use of crude extracts from traditional medicinal plants to eliminate Trichodina sp. in tilapia (Oreochromis niloticus) fingerlings. Songklanakarin J. Sci. Technol. 2005, 27, 359–364. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Arlington, TX, USA, 1995. [Google Scholar]
- SEGOB. Diario Oficial de la Federación, México. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5402531&fecha=31/07/2015#gsc.tab=0 (accessed on 23 February 2023).
- Harris, P.D.; Cable, J. Techniques for the Study of Monogeneans. In Parasitic Helminths of Fishes; Moravec, F.N., Justine, R.A., Bray, R.A., Eds.; CRC Press, LLC: Boca Raton, FL, USA, 2000; pp. 159–180. [Google Scholar]
- Vidal-Martínez, V.M.; Aguirre-Macedo, M.L.; Scholz, T.; González-Solís, D.; Mendoza-Franco, E.F. Atlas of the Helminth Parasites of Cichlid Fish of Mexico; Studies of the Academy of Sciences of the Czech Republic: Praha, Czech Republic, 2001; pp. 1–165. [Google Scholar]
- Pariselle, A.; Euzet, L. Systematic revision of dactylogyridean parasites (Monogenea) from cichlid fishes in Africa, the Levant and Madagascar. Zoosystema 2009, 31, 849–898. [Google Scholar] [CrossRef]
- Paperna, I. Studies on monogenetic trematodes in Israel. 2. Monogenetic trematodes of cichlids. Isr. J. Aquac. Bamidgeh 1960, 12, 20–33. [Google Scholar]
- Paperna, I.; Thurston, J.P. Monogenetic trematodes collected from cichlid fish in Uganda; including the description of five new species of Cichlidogyrus. Rev. Zool. Bot. Afr. 1969, 79, 15–33. [Google Scholar]
- Ergens, R. Nine species of the genus Cichlidogyrus Paperna, 1960 (Monogenea: Ancyrocephalinae) from Egyptian fishes. Folia Parasitol. 1981, 28, 205–214. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Jatobá, A.; Stockhausen, L.; da Silva, L.R.; de Andrade, J.I.A. Therapeutic bath of mint hydrolate in the control of monogenea for four tilapia species. Bol. Inst. Pesca 2023, 49, 1–7. [Google Scholar] [CrossRef]
- Boom, R.; Sol, C.J.; Salimans, M.M.; Jansen, C.L.; Wertheim-van Dillen, P.M.; van der Noordaa, J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990, 28, 495–503. [Google Scholar] [CrossRef]
- Ek-Huchim, J.P.; Jimenez-Garcia, I.; Pérez-Vega, J.A.; Rodríguez-Canul, R. Non-lethal detection of DNA from Cichlidogyrus spp. (Monogenea, Ancyrocephalinae) in gill mucus of the Nile tilapia Oreochromis niloticus. Dis. Aquat. Org. 2012, 98, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy; Freeman: San Francisco, CA, USA, 1973; pp. 1–573. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Faruk, M.A.Z.; Munira, S.; Hasan, M.S.; Manu, M.M.R.; Khatun, M.A.; Yeasmin, T. Dietary Effects of Garlic (Allium sativum) Powder on Growth Performance of Commercial Broiler. J. Adv. Vet. Res. 2023, 13, 1537–1542. [Google Scholar]
- Agbetuyi, O.A.; Ekeocha, A.H.; Aganga, A.A. Dry matter yield and egg organoleptic attributes of Moringa oleifera and Allium sativum fed laying hens. Res. Sq. 2023, in press. [Google Scholar]
- Kahouli, A.; Kaboul, N.; Rekike, F. Effectiveness of Garlic Powder as Nutritional in Ouled-djellal Ewes. Mal. J. Anim. Sci. 2023, 26, 34–40. [Google Scholar]
- Toghdory, A.; Ghoorchi, T.; Asadi, M.; Zareie, J. The effect of adding garlic powder in milk on performance, nutrient digestibility, diarrhea status and blood parameters in suckling Dalagh lambs. Anim. Prod. 2023, 25, 389–398. [Google Scholar]
- Thuy, N.T.; Ha, N.C. Effect of adding guava leaf (Psidium guajava) and garlic (Allium sativum) powders in diets on growth performance and diarrhea incidence of weaned piglets. Livest. Res. Rural Dev. 2023, 35. Available online: https://lrrd.cipav.org.co/lrrd35/2/3512nthi.html (accessed on 6 November 2024).
- Gutiérrez-Leyva, R.; Ulloa, J.A.; Ramírez-Ramírez, J.C.; Bautista-Rosales, P.U.; Rosas-Ulloa, P.; Silva-Carrillo, Y.; Ramírez-Acevedo, E.A.; Camarena-Herrera, M.E. Evaluation of the intensive production of juvenile tilapia under greenhouse conditions: Profitability analysis and aspects of its applicability. Rev. Bio Cienc. 2020, 7, 1–23. [Google Scholar]
- Xie, S.; Cui, Y.; Yang, Y.; Liu, J. Energy budget of Nile tilapia (Oreochromis niloticus) in relation to ration size. Aquaculture 1997, 154, 57–68. [Google Scholar] [CrossRef]
- Güroy, D.; Emre, N.; Yalım, F.B.; Karadal, O.; Kaya, D.; Arifoğlu, N. Interaction of dietary garlic (Allium sativum), onion (Allium cepa), and probiotic on the growth performance and health status of juvenile rainbow trout (Oncorhynchus mykiss). Aquac. Int. 2024, 32, 4515–4528. [Google Scholar] [CrossRef]
- Ukenye, E.A.; Megbowon, I.; Oguntade, O.R.; Ayo-Olalusi, C.I.; Mojekwu, T.; Edah, B.; Sokenu, B.A.; Joseph, J.B.; Adeyemi, Y.B.; Jubrin, H.; et al. Growth Performance and Immunity Analysis of Clarias gariepinus Juveniles Fed with Moringa leaf, Ginger and Garlic Powder. Niger. Agric. J. 2023, 54, 275–280. [Google Scholar]
- Diab, A.S.; Aly, S.M.; John, G.; Abde-Hadi, Y.; Mohammed, M.F. Effect of garlic, black seed and Biogen as immunostimulants on the growth and survival of Nile tilapia, Oreochromis niloticus (Teleostei: Cichlidae), and their response to artificial infection with Pseudomonas fluorescens. Afr. J. Aquat. Sci. 2008, 33, 63–68. [Google Scholar] [CrossRef]
- Vásquez Ocmín, M.C.; Ayarza Rengifo, J.A.; Tuesta Rojas, C.A.; Murrieta Morey, G.A. Report of Cichlidogyrus tilapiae (Monogenoidea: Dactylogyridae) in Oreochromis niloticus «tilapia» (Cichliformes: Cichlidae) collected in a fishpond in the Peruvian Amazon. Rev. Investig. Vet. Perú 2022, 33, e22743. [Google Scholar] [CrossRef]
- Marques, J.F.; Cabral, H.N. Effects of sample size on fish parasite prevalence, mean abundance and mean intensity estimates. J. Appl. Ichthyol. 2007, 23, 158–162. [Google Scholar] [CrossRef]
- Khidr, A.A.A.; Said, A.E.; Samak, O.A.A.; Sheref, S.E.A. The impacts of ecological factors on prevalence, mean intensity and seasonal changes of the monogenean gill parasite, Microcotyloides sp., infesting the Terapon puta fish inhabiting coastal region of Mediterranean Sea at Damietta region. J. Basic Appl. Zool. 2012, 65, 109–115. [Google Scholar] [CrossRef]
- Suliman, E.A.M.; Al-Harbi, A.H. Prevalence and seasonal variation of ectoparasites in cultured Nile tilapia Oreochromis niloticus in Saudi Arabia. J. Parasit. Dis. 2016, 40, 1487–1493. [Google Scholar] [CrossRef][Green Version]
- Yavuzcan Yildiz, H.; Bekcan, S. Control of ectoparasitosis in carp (Cyprinus carpio) induced by Gyrodactylus elegans (Monogenea) with garlic (Allium sativum) and onion (Allium cepa) extracts. Ecocycles 2020, 6, 10–17. [Google Scholar] [CrossRef]
- Maniat, M.; Ghotbeddin, N.; Ghatrami, E.R. Effect of garlic on growth performance and body composition of benni fish (Mesopotamichthys sharpeyi). Int. J. Biosci. 2014, 5, 269–277. [Google Scholar]
- Mohammad, M.A. Effect of adding garlic Allium sativum powder in diet on hematological, biochemical and histopathological criteria of common carp Cyprinus carpio L. Iraqi J. Agric. Sci. 2023, 54, 1040–1049. [Google Scholar] [CrossRef]
- Oliveira, R.P.; Macedo, A.M.; Chiari, E.; Pena, S.D.J. An alternative approach to evaluating the intraspecific genetic variability of parasites. Parasitol. Today 1997, 13, 196–200. [Google Scholar] [CrossRef]
- Feist, S.W.; Longshaw, M. Histopathology of fish parasite infections–importance for populations. J. Fish Biol. 2008, 73, 2143–2160. [Google Scholar] [CrossRef]
- Maged, A.E.; Rehab, R.; Rasheed, N.; Ibrahim, I.; Saad, H.M.; Batiha, G.E.S.; Abou Zaid, A.A. New Insights on the Effects of Monogenean Gill Parasites on Naturally Infested Scoberomorus commerson: Host Response, Electron Microscopy, and Histopathological Studies. Damanhour J. Vet. Sci. 2024, 11, 20–25. [Google Scholar] [CrossRef]
- Barzegar, M.; Raissy, M.; Shamsi, S. Protozoan parasites of Iranian freshwater fishes: Review, composition, classification, and modeling distribution. Pathogens 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Fridman, S.; Sinai, T.; Zilberg, D. Efficacy of garlic based treatments against monogenean parasites infecting the guppy (Poecilia reticulata (Peters)). Vet. Parasitol. 2014, 203, 51–58. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; García-Recio, E.; Ruiz, C.; De Luna-Bertos, E.; Illescas-Montes, R.; Costela-Ruiz, V.J. Biological properties and therapeutic applications of garlic and its components. Food Funct. 2022, 13, 2415–2426. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Tsiaganis, M.C.; Laskari, K.; Melissari, E. Fatty acid composition of Allium species lipids. J. Food Compos. Anal. 2006, 19, 620–627. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef]
- Čolić, M.; Vučević, D.; Kilibarda, V.; Radičević, N.; Savić, M. Modulatory effects of garlic extracts on proliferation of T-lymphocytes in vitro stimulated with concanavalin A. Phytomedicine 2002, 9, 117–124. [Google Scholar] [CrossRef]
- Cubas, A.C. Citoquinas y Receptores en la Interacción hospedero-parásito. An. Fac. Med. 2000, 61, 68–77. [Google Scholar] [CrossRef][Green Version]
- Radwan, M.; El-Sharkawy, M.A.; Alabssawy, A.N.; Ghanem, S.F.; Mohammadein, A.; Al Malki, J.S.; Al-Thomali, W.S.; Manaa, E.A.; Soliman, R.A.; Yassir, S.; et al. The synergy between serious parasitic pathogens and bacterial infestation in the cultured Nile tilapia (Oreochromis niloticus): A severe threat to fish immunity, causing mass mortality and significant economic losses. Aquac. Int. 2023, 31, 2421–2449. [Google Scholar] [CrossRef]

| Feed Additive or Ingredient Levels a | Experimental Conditions b | Feed Quality c | Growth Performance d | Feed Utilization e | Antiparasitic Activity f | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WG | SGR | HIS | FI | FCR | FE | PER | Genus | E | P | ||||
| GO = 0, 5, and 10 g/kg. | IBW = 14 g/fish, EU = earthen pond cages (2 × 4 × 1 m), SD = 50 fish/cage, WT = 27.1 °C, FR = 3% of BW/day, and EP = 120 days. | CP = 249.7 g/kg, L = 31.3 g/kg, and GE = 14.2 MJ/kg. | ↑ | ↑ | ↓ | ↑ | ↓ | NE | ↑ | NE | NE | NE | Hussein et al. [9]. |
| FG = 0, 3, and 5 g/kg. DG = 0, 3, and 5 g/kg. | IBW = 0.26 g/fish, EU = concrete ponds (7.5 × 2.25 × 0.70 m), SD = 60 fish/replicate, FR = 4% of BW/day, and EP = 154 days. | CP = 330 g/kg, L = 111 g/kg, and GE = 18.8 MJ/kg. | ↑ | ↑ | ↓ | SR | ↓ | NE | ↑ | NE | NE | NE | Abdel-Hakim et al. [10]. |
| GP = 150 mg/kg. GO = 32 g/kg. | IBW = 20–21 g/fish, FR = 3% of BW/day, SD = 10 fish/replicate, and EP = 90 days. | CP = 60.9 g/kg and L = 19.9 g/kg. | ↑ | ↑ | ↓ | ↓ | ↓ | ↑ | ↑ | NE | NE | NE | Metwally [11]. |
| GP = 0, 10, 20, 30, and 40 g/kg. | EU = glass aquaria (75 × 40 × 50 cm) of 100 L, WT = 26–27 °C, SD = 20 fish/aquaria, FR = 3% of BW/day, and EP = 90 days. | CP = 340–352 g/kg, L = 83–88 g/kg, and GE = 18.8–18.9 MJ/kg. | ↑ | ↑ | SR | ↑ | ↓ | ↑ | ↑ | NE | NE | NE | Shalaby et al. [12]. |
| GE = 0.0, 0.02, 0.04, 0.06, 0.08, 0.1, 0.12, 0.14, 0.16, and 0.18 µg/mL. | IBW = 40–60 g/fish, SD = 10 fish/replicate, EU = 96-L glass aquaria, and EP = 4 days. | NE | NE | NE | NE | NE | NE | NE | NE | Dactylogyrus | 85.7–100 | NE | Reda et al. [13]. |
| GO = 1, 1.5, 2, 2.5, and 3 PPT. GC = 3 PPT and 300 mg/L. | IBW = 5-, 15-, and 30-day-old fries, SD = 5000 fish/pond, EU = hatchery earthen ponds (3 m length × 2 m width × 1 m water depth), and EP = 7 days. | NE | NE | NE | NE | NE | NE | NE | NE | Gyrodactylus | NE | 17–29 | Abd El-Galil and Aboelhadid [14]. |
| Ingredients (% “as Is”) | Control | GP1% | GP2% | GP3% |
|---|---|---|---|---|
| Garlic powder 1 | 0 | 1 | 2 | 3 |
| Soybean paste 2 | 26 | 26 | 26 | 26 |
| Wheat flour 3 | 30 | 30 | 30 | 25 |
| Sweet potato flour 4 | 12 | 11 | 10 | 14 |
| Tuna by-product silage 5 | 20 | 20 | 20 | 20 |
| Vitamin–mineral premix 6 | 2 | 2 | 2 | 2 |
| Salmon oil 5 | 6 | 6 | 6 | 6 |
| Unflavored gelatin powder (binder) 7 | 4 | 4 | 4 | 4 |
| Total | 100 | 100 | 100 | 100 |
| Dry matter (%) | 91.7 ± 0.5 | 90.6 ± 0.4 | 89.8 ± 1.2 | 91.5 ± 0.6 |
| Crude protein (%) | 33.5 ± 5.7 | 32.1 ± 0.3 | 32.4 ± 1.5 | 32.1 ± 1.9 |
| Ethereal extract (%) | 6.3 ± 1.1 | 6.5 ± 2.6 | 6.3 ± 3.4 | 6.4 ± 0.2 |
| Gross energy (MJ/kg) | 18.7 ± 0.0 | 18.9 ± 0.1 | 18.8 ± 0.1 | 18.7 ± 0.1 |
| Control | GP1% | GP2% | GP3% | Mean | |
|---|---|---|---|---|---|
| Temperature (°C) | 26.3 ± 0.1 | 26.9 ± 0.3 | 26.9 ± 0.4 | 27.2 ± 0.2 | 26.8 |
| Dissolved oxygen (mg/L) | 6.1 ± 0.1 | 6.2 ± 0.1 | 5.9 ± 0.2 | 5.9 ± 0.1 | 6.0 |
| pH | 7.8 ± 0.2 | 7.8 ± 0.1 | 7.7 ± 0.2 | 7.6 ± 0.1 | 7.7 |
| Total ammonia-nitrogen (mg/L) | 0.21 ± 0.04 | 0.21 ± 0.08 | 0.18 ± 0.00 | 0.21 ± 0.04 | 0.20 |
| Nitrite (mg/L) | 0.36 ± 0.05 | 0.34 ± 0.08 | 0.25 ± 0.08 | 0.19 ± 0.13 | 0.29 |
| Nitrate (mg/L) | 13.3 ± 1.7 | 11.1 ± 4.8 | 11.1 ± 1.0 | 8.4 ± 3.3 | 11.0 |
| Control | GP1% | GP2% | GP3% | Mean | |
|---|---|---|---|---|---|
| Survival (%) | 95.2 ± 8.3 a | 100 ± 0.0 a | 90.5 ± 0.0 a | 100 ± 16.5 a | 96.4 |
| Initial weight (g) | 38.7 ± 1.4 a | 41.2 ± 1.6 a | 40.5 ± 4.3 a | 38.9 ± 1.4 a | 39.8 |
| Final weight (g) | 82.9 ± 7.0 a | 92.3 ± 5.2 a | 82.6 ± 1.6 a | 84.4 ± 2.5 a | 85.6 |
| Total length (cm) | 15.2 ± 0.9 a | 15.7 ± 0.3 a | 15.2 ± 0.9 a | 18.5 ± 5.9 a | 16.1 |
| Weight gain (g) | 44.2 ± 6.3 a | 51.1 ± 4.0 a | 42.1 ± 2.9 a | 45.6 ± 3.8 a | 45.8 |
| Apparent feed intake (g/fish/day) | 4.0 ± 0.1 a | 4.2 ± 0.1 a | 4.2 ± 0.3 a | 4.0 ± 0.1 a | 4.1 |
| Specific growth rate (%/day) | 2.5 ± 0.2 a | 2.7 ± 0.1 a | 2.4 ± 0.3 a | 2.6 ± 0.2 a | 2.6 |
| Feed conversion ratio | 2.8 ± 0.3 a | 2.5 ± 0.2 a | 3.0 ± 0.5 a | 2.7 ± 0.3 a | 2.8 |
| Protein efficiency ratio | 1.1 ± 0.1 a | 1.3 ± 0.1 a | 1.0 ± 0.2 a | 1.2 ± 0.1 a | 1.2 |
| Condition factor | 2.4 ± 0.3 a | 2.4 ± 0.2 a | 2.4 ± 0.4 a | 2.2 ± 0.4 a | 2.3 |
| Treatment | Parasitological Index | |||
|---|---|---|---|---|
| Prevalence (%) | Mean Intensity | Mean Abundance | ||
| Initial point | mean ± SD | 21.9 ± 28.2 | 26.8 ± 34.4 | 7.0 ± 9.0 |
| C. thurstonae | 11 | 26 | 2.7 | |
| Control | C. tilapiae | 11 | 56 | 5.9 |
| C. sclerosus | 68 | 16.4 | 11.2 | |
| C. halli | 11 | 13.5 | 1.4 | |
| mean ± SD | 25 ± 28.5 | 28.0 ± 19.4 | 5.3 ± 4.4 | |
| GP1% | C. thurstonae | 33 | 13.8 | 4.0 |
| C. sclerosus | 33 | 9.7 | 2.8 | |
| C. halli | 22 | 22.8 | 4.3 | |
| C. tubicirrus | 6 | 41.0 | 2.0 | |
| C. longicornis | 6 | 12.0 | 0.6 | |
| mean ± SD | 20 ± 13.5 | 19.9 ± 18.2 | 2.7 ± 1.5 | |
| GP2% | C. thurstonae | 10 | 25.0 | 2.4 |
| C. sclerosus | 38 | 11.9 | 4.5 | |
| C. halli | 19 | 33.0 | 6.3 | |
| C. tubicirrus | 14 | 17.0 | 2.4 | |
| C. longicornis | 19 | 20.8 | 4.0 | |
| mean ± SD | 20 ± 10.7 | 21.5 ± 8.0 | 3.9 ± 1.6 | |
| GP3% | C. thurstonae | 11 | 2.5 | 0.2 |
| C. sclerosus | 53 | 16.9 | 8.0 | |
| C. halli | 16 | 9.0 | 1.3 | |
| C. tubicirrus | 21 | 10.0 | 1.9 | |
| mean ± SD | 25 ± 18.9 | 9.6 ± 5.9 | 2.9± 3.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado-Moreno, S.M.; Gutiérrez-Leyva, R.; Carmona-Gasca, C.A.; Martínez-González, S.; Ramírez-Ramírez, J.C.; De La Cruz-Moreno, C.O.; Borrayo-González, J.J.F. Garlic Powder Evaluation as Feed Additive on Nile Tilapia (Oreochromis niloticus L.) Growth Performance, Feed Utilization, Gill Parasitic Treatment, and Monogenean Diversity. Fishes 2025, 10, 34. https://doi.org/10.3390/fishes10010034
Salgado-Moreno SM, Gutiérrez-Leyva R, Carmona-Gasca CA, Martínez-González S, Ramírez-Ramírez JC, De La Cruz-Moreno CO, Borrayo-González JJF. Garlic Powder Evaluation as Feed Additive on Nile Tilapia (Oreochromis niloticus L.) Growth Performance, Feed Utilization, Gill Parasitic Treatment, and Monogenean Diversity. Fishes. 2025; 10(1):34. https://doi.org/10.3390/fishes10010034
Chicago/Turabian StyleSalgado-Moreno, Socorro Marisa, Ranferi Gutiérrez-Leyva, Carlos Alfredo Carmona-Gasca, Sergio Martínez-González, José Carmen Ramírez-Ramírez, Carlos Omar De La Cruz-Moreno, and Juan José Fernando Borrayo-González. 2025. "Garlic Powder Evaluation as Feed Additive on Nile Tilapia (Oreochromis niloticus L.) Growth Performance, Feed Utilization, Gill Parasitic Treatment, and Monogenean Diversity" Fishes 10, no. 1: 34. https://doi.org/10.3390/fishes10010034
APA StyleSalgado-Moreno, S. M., Gutiérrez-Leyva, R., Carmona-Gasca, C. A., Martínez-González, S., Ramírez-Ramírez, J. C., De La Cruz-Moreno, C. O., & Borrayo-González, J. J. F. (2025). Garlic Powder Evaluation as Feed Additive on Nile Tilapia (Oreochromis niloticus L.) Growth Performance, Feed Utilization, Gill Parasitic Treatment, and Monogenean Diversity. Fishes, 10(1), 34. https://doi.org/10.3390/fishes10010034







