Report on Intersex and Abnormal Mature Aquacultured Walleye Pollock, Gadus chalcogrammus
Abstract
1. Introduction
2. Materials and Methods
2.1. Parental Broodstock and Spawning
2.2. The Embryos and F1 Generation Measurement
2.3. Fish and Sampling
2.4. Histological Characterization
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Todd, E.V.; Liu, H.; Muncaster, S.; Gemmell, N.J. Bending genders: The biology of natural sex change in fish. Sex. Dev. 2016, 10, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.S.-W.; Zhang, Z.; Qin, M.; Ge, W. Knockout of zebrafish ovarian aromatase gene (cyp19a1a) by TALEN and CRISPR/Cas9 leads to all-male offspring due to failed ovarian differentiation. Sci. Rep. 2016, 6, 37357. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.A.M.; Peichel, C.L. Are homologies in vertebrate sex determination due to shared ancestry or to limited options? Genome Biol. 2010, 11, 205. [Google Scholar]
- McClelland, K.; Bowles, J.; Koopman, P. Male sex determination: Insights into molecular mechanisms. Asian J. Androl. 2012, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Furukawa, F.; Mukai, K.; Yazawa, T.; Kitano, T. Peroxisome proliferator-activated receptor alpha is involved in the temperature-induced sex differentiation of a vertebrate. Sci. Rep. 2020, 10, 11672. [Google Scholar] [CrossRef]
- Römer, U.; Beisenherz, W. Environmental determination of sex in Apistogramma (Cichlidae) and two other freshwater fishes (Teleost). J. Fish Biol. 1996, 48, 714–725. [Google Scholar]
- Ramee, S.W.; Lipscomb, T.N.; DiMaggio, M.A. Evaluation of the effect of larval stocking density, salinity, and temperature on stress response and sex differentiation in the Dwarf Gourami and Rosy Barb. Aquac. Rep. 2020, 16, 100287. [Google Scholar] [CrossRef]
- Robertson, C.E.; Wright, P.A.; Köblitz, L.; Bernier, N.J. Hypoxia-inducible factor-1 mediates adaptive developmental plasticity of hypoxia tolerance in zebrafish, Danio rerio. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140637. [Google Scholar] [CrossRef]
- Pfennig, F.; Kurth, T.; Meißner, S.; Standke, A.; Hoppe, M.; Zieschang, F.; Reitmayer, C.; Göbel, A.; Kretzschmar, G.; Gutzeit, H.O. The social status of the male Nile tilapia (Oreochromis niloticus) influences testis structure and gene expression. Reproduction 2012, 143, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Atz, J.W. Intersexuality in fishes. In Intersexuality in Vertebrates Including Man; Armstrong, C.N., Marshall, A.J., Eds.; Academic Press: London, UK, 1964; pp. 145–232. [Google Scholar]
- Pauly, D. Darwin’s Fishes: An Encyclopedia of Ichthyology, Ecology, and Evolution; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Sadovy, M.Y.; Liu, M. Functional hermaphroditism in fishes. Copeia 2008, 1, 136–156. [Google Scholar]
- Zhukova, K.; Privalikhin, A.M. First record of intersexuality in walleye pollock Theragra chalcogramma (Pallas, 1814). Mar. Biodivers. Rec. 2015, 8, 89–91. [Google Scholar] [CrossRef]
- Caprioli, R.; Costa, C.; Cataldi, E.; Boglione, C.; Cataudella, S. First record of female germ cells in the testis of a wild Mediterranean northern bluefin tuna Thunnus thynnus L. 1758. J. Fish Biol. 2007, 71, 620–622. [Google Scholar] [CrossRef]
- Macías, D.; Saber, S.; Osuna, A.M.; Cruz-Castán, R.M.; Gómez-Vives, M.J.; Báez, J.C. First record of intersexuality in Euthynnus alletteratus in the Mediterranean Sea: Histological description. Marine Biodiv. Rec. 2014, 7, e3. [Google Scholar] [CrossRef]
- Sawada, Y.; Seoka, M.; Okada, T.; Miyashita, S.; Murata, O.; Kumai, H. Hermaphroditism in a captive-raised Pacific bluefin tuna. J. Fish. Biol. 2022, 60, 263–265. [Google Scholar]
- Hiatt, T.; Dalton, M.; Felthoven, R.; Fissel, B.; Garber-Yonts, B.; Haynie, A.; Kasperski, S.; Lew, D.; Package, C.; Sepez, J.; et al. Economic status of the ground fish fisheries off Alaska. In Stock Assessment and Fishery Evaluation Report for the Ground Fish Fisheries of the Gulf of Alaska and Bering Sea/Aleutian Islands Area; North Pacific Fishery Management Council: Anchorage, AK, USA, 2010; Volume 2009, p. 254. [Google Scholar]
- Livingston, P.A. Importance of predation by groundfish, marine mammals and birds on walleye Pollock Theragra chalcogramma and Pacific herring Clupea pallasi in the eastern Bering Sea. Mar. Ecol. Prog. Ser. 1993, 102, 205–215. [Google Scholar] [CrossRef]
- Napp, J.M.; Kendall, A.W., Jr.; Schumacher, J.D. A synthesis of biological and physical processes affecting the feeding environment of larval walleye pollock (Theragra chalcogramma) in the eastern Bering Sea. Fish. Oceanogr. 2000, 9, 147–162. [Google Scholar] [CrossRef]
- Sinclair, E.H.; Vlietstra, L.S.; Johnson, D.S.; Zeppelin, T.K.; Byrd, G.V.; Springer, A.M.; Ream, R.R.; Hunt, G.L. Patterns in prey use among fur seals and seabirds in the Pribilof Islands. Deep Sea Res. II 2008, 55, 1897–1918. [Google Scholar] [CrossRef]
- FAO. Global Fishery and Aquaculture Production. 2022. Available online: http://www.fao.org/fishery/statistics/en (accessed on 10 April 2024).
- Hinckley, S. The reproductive biology of walleye pollock, Theragra chalcogramma, in the Bering Sea with reference to spawning stock structure. Fish. Bull. 1987, 85, 481–498. [Google Scholar]
- Kim, S.S.; Lee, C.J.; Yoo, H.K.; Choi, J.; Byun, S.G.; Kim, W.J.; Lim, H.J.; Park, J.S. Effect of water temperature on walleye pollock (Gadus chalcogrammus) embryos, larvae and juveniles: Survival, HSP70 expression, and physiological responses. Aquaculture 2022, 554, 738136. [Google Scholar] [CrossRef]
- Haugen, T.; Andersson, E.; Norberg, B.; Taranger, G.L. The production of hermaphrodites of Atlantic cod (Gadus morhua) bymasculinization with orally administered 17-a-methyltestosterone, and subsequent production of all-female cod populations. Aquaculture 2010, 31, 248–254. [Google Scholar]
- Kirubakaran, T.G.; Andersen, Ø.; Rosa, M.C.; Andersstuen, T.; Hallan, K.; Peter Kent, M.P.; Lien, S. Characterization of a male specific region containing a candidate sex determining gene in Atlantic cod. Sci. Rep. 2019, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Mires, D. Production of aquatic animals. In The Tilapias; Nash, C.E., Novotony, A.J., Eds.; Elsevier: New York, NY, USA, 1995; pp. 133–152. [Google Scholar]
- Piferrer, F.; Beaumont, A.; Falguière, J.-C.; Flajšhans, M.; Haffray, P.; Colombo, L. Polyploid fish and shellfish: Production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 2009, 293, 125–156. [Google Scholar] [CrossRef]
- Gunderson, D.R.; Dygert, P.H. Reproductive effort as a predictor of natural mortality rate. J. Cons. Int. Explor. Mer. 1988, 44, 200–209. [Google Scholar] [CrossRef]
- Browen-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.; Lowerre-Barbieri, S.K. A Standardized termonology for describing reproductive development in fishes. Mar. Coast. Fish. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Nolan, M.; Jobling, S.; Brighty, G.; Sumpter, J.P.; Tyler, C.R. A histological description of intersexuality in the roach. J. Fish Biol. 2001, 58, 160–176. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Kidd, K.A.; Sumpter, J.P. Chemically induced alterations to gonadal differentiation in fish, fish diseases and disorders. In Non-Infectious Disorders, 2nd ed.; CABI: Wallingford, UK, 2010; Volume 2, pp. 144–165. [Google Scholar]
- Schmitt, C.J.; Hinck, J.E.; Blazer, V.S.; Denslow, N.D.; Dethloff, G.M.; Bartish, T.M.; Coyle, J.J.; Tillitt, D.E. Environmental contaminants and biomarker responses in fish from the Rio Grande and its U.S. tributaries: Spatial and temporal trends. Sci. Total Environ. 2005, 350, 161–193. [Google Scholar] [CrossRef] [PubMed]
- Bahamonde, P.A.; Munkittrick, K.R.; Martyniuk, C.J. Intersex in teleost fish: Are we distinguishing endocrine disruption from natural phenomena? Gen. Comp. Endocrinol. 2013, 192, 25–35. [Google Scholar] [CrossRef]
- Kinnison, M.T.; Unwin, M.J.; Jara, F. Macroscopic intersexuality in salmonid fishes. N. Z. J. Mar. Freshwat. Res. 2000, 34, 125–134. [Google Scholar] [CrossRef]
- Privalikhin, A.M. Resorption of developing oocytes as a mechanism of formation of individual and population fecundity in walleye pollock Theragra chalcogramma (Gadidae). J. Ichthyol. 2003, 43, 454–463. [Google Scholar]
- Baird, T.A.; Olla, B.L. Social and reproductive behavior of a captive group of walleye pollock, Theragra chalcogramma. Environ. Biol. Fish. 1991, 30, 295–301. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.P.; Kruse, G.H. Classification of ovarian stages of Walleye Pollock Theragra chalcogramma. In Resiliency of Gadid Stocks to Fishing and Climate Change; Alaska Sea Grant College Program AK-SG-08-01; Alaska Sea Grant: Anchorage, AK, USA, 2008; pp. 1–20. [Google Scholar]
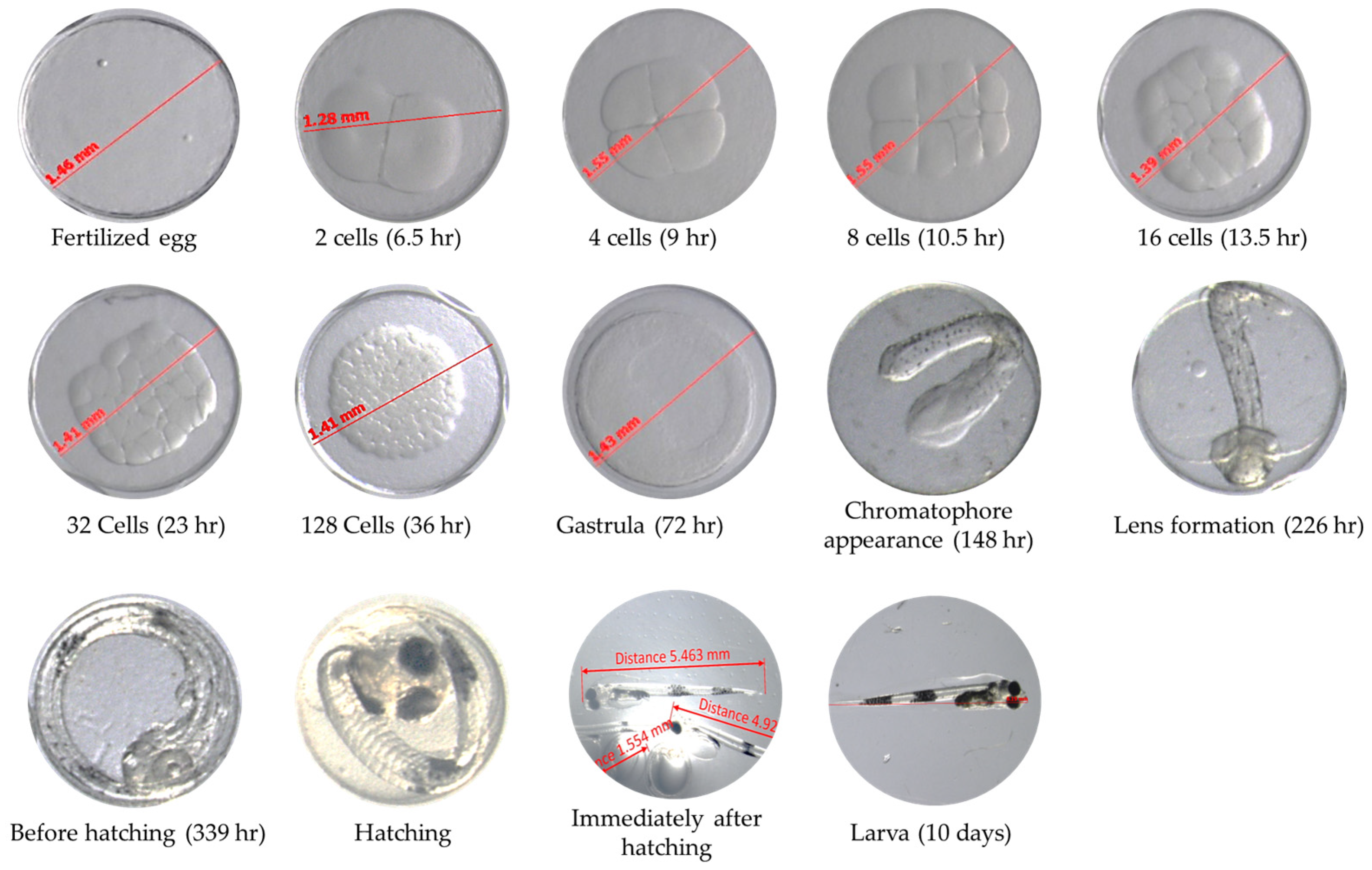

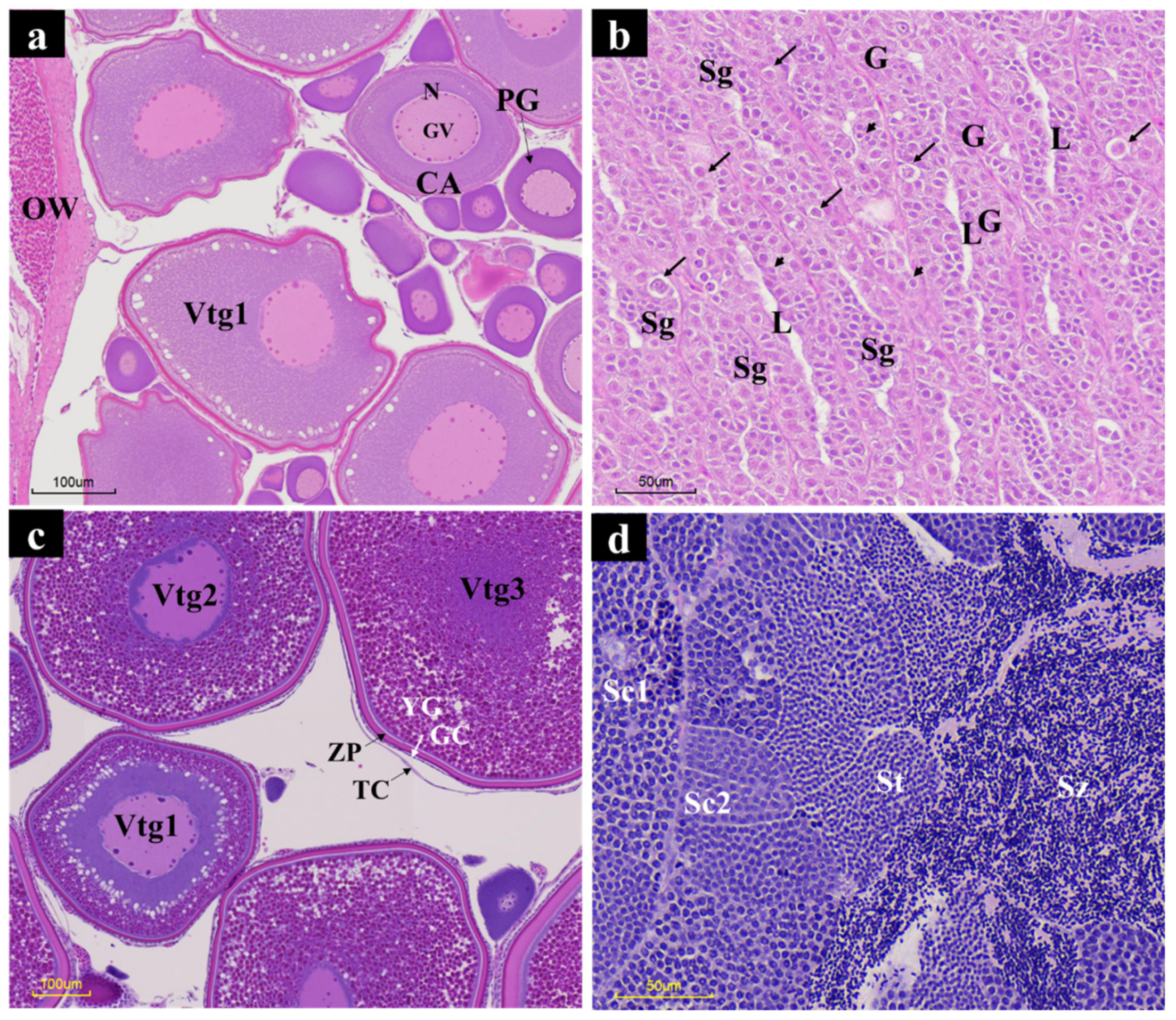
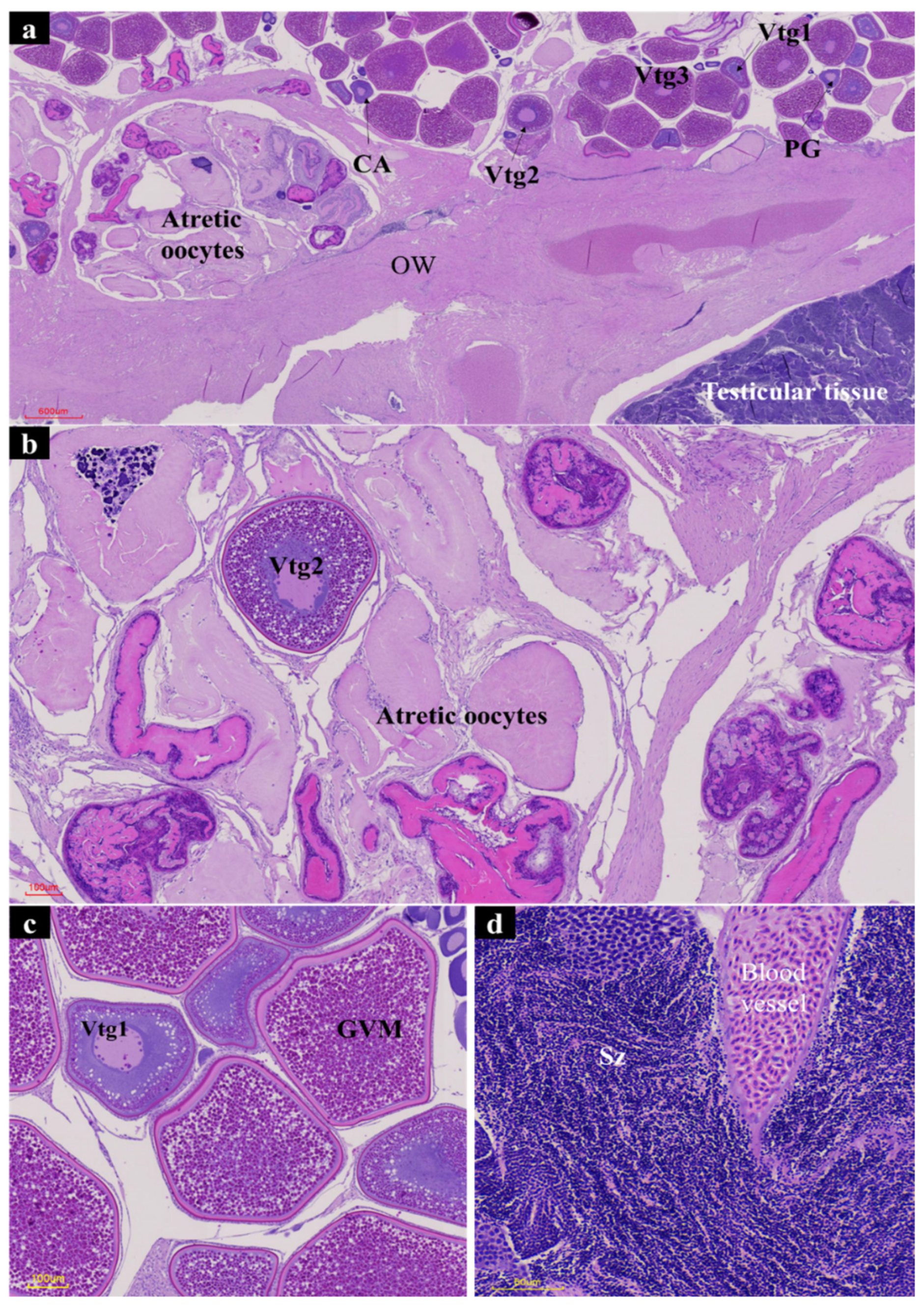

| Age | Length (cm) | Weight (g) |
|---|---|---|
| 1 day | 0.49 ± 0.007 | - |
| 100 days | 1.76 ± 0.015 | 0.047 ± 0.006 |
| 180 days | 10.83 ± 2.1 | 11.2 ± 2.9 |
| 360 days | 19.7 ± 1.1 | 53.2 ± 17.4 |
| 2 years | 39.3 ± 5.3 | 535.4 ± 175.1 |
| 3 years | 46.5 ± 4.2 | 882.7 ± 283.2 |
| 4 years | 51.3 ± 4.2 | 1340.8 ± 468.3 |
| 5 years | 59.7 ± 3.7 | 2211.7 ± 453.6 |
| 6 years | 62.2 ± 4.4 | 2189.1 ± 599.4 |
| Hatched (Generation)/Age | Length (cm) | Weight (g) | GSI (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | p Value | Female | Male | p Value | Ovary | Intersex | |
| * 2015 (F1)/6 | 60.4 ± 4.9 | 57.6 ± 4.2 | 0.125 | 2226.4 ± 649.5 | 1903.5 ± 483.5 | 0.009 | 26.5 ± 3.3 | 23.6 |
| * 2017 (F2)/4 | 51.9 ± 2.6 | 47.3 ± 2.8 | 0.699 | 1163.3 ± 153.4 | 961.2 ± 203.5 | 0.319 | 14.2 ± 4.1 | 14.4 |
| ** 2022 (F2)/1.4 | 28.4 ± 2.8 | 26.5 ± 3.6 | 0.316 | 184.8 ±59.3 | 161.0 ± 36.5 | 0.719 | 1.93 ± 1.4 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.-K.; Woo, S.-J.; Lee, K.-W.; Joo, M.-S.; Kim, K.-D.; Park, J.-J.; Kim, S.-S. Report on Intersex and Abnormal Mature Aquacultured Walleye Pollock, Gadus chalcogrammus. Fishes 2025, 10, 35. https://doi.org/10.3390/fishes10010035
Yoo H-K, Woo S-J, Lee K-W, Joo M-S, Kim K-D, Park J-J, Kim S-S. Report on Intersex and Abnormal Mature Aquacultured Walleye Pollock, Gadus chalcogrammus. Fishes. 2025; 10(1):35. https://doi.org/10.3390/fishes10010035
Chicago/Turabian StyleYoo, Hae-Kyun, Soo-Ji Woo, Ki-Wook Lee, Min-Soo Joo, Kyeong-Duck Kim, Jung-Jun Park, and So-Sun Kim. 2025. "Report on Intersex and Abnormal Mature Aquacultured Walleye Pollock, Gadus chalcogrammus" Fishes 10, no. 1: 35. https://doi.org/10.3390/fishes10010035
APA StyleYoo, H.-K., Woo, S.-J., Lee, K.-W., Joo, M.-S., Kim, K.-D., Park, J.-J., & Kim, S.-S. (2025). Report on Intersex and Abnormal Mature Aquacultured Walleye Pollock, Gadus chalcogrammus. Fishes, 10(1), 35. https://doi.org/10.3390/fishes10010035





