Impact of Lowering TSH Cut-Off on Neonatal Screening for Congenital Hypothyroidism in Minas Gerais, Brazil
Abstract
1. Introduction
2. Materials and Methods
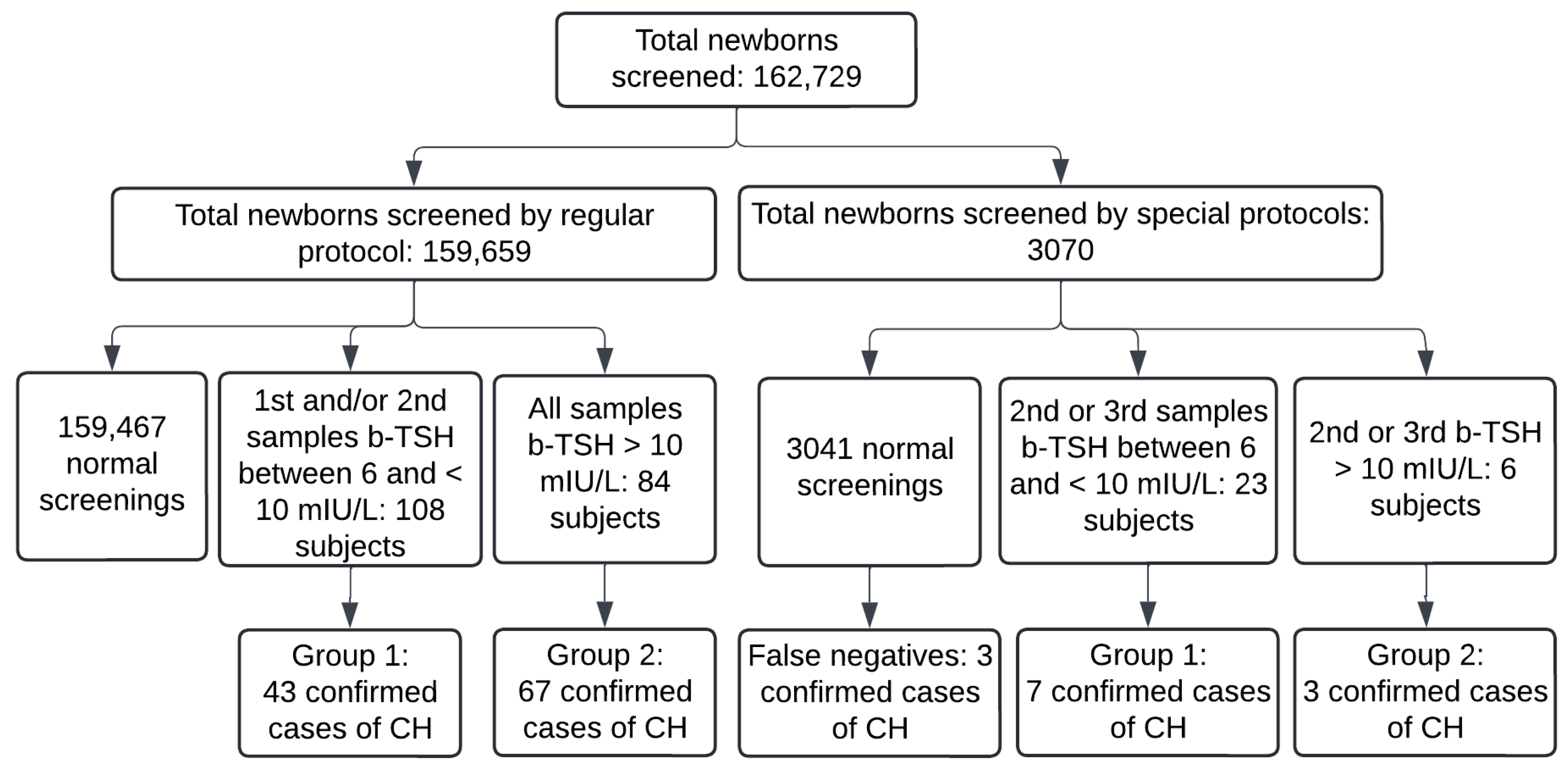
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trotsenburg, P.V.; Stoupa, A.; Léger, J.; Rohrer, T.; Peters, C.; Fugazzola, L.; Cassio, A.; Heinrichs, C.; Beauloye, V.; Pohlenz, J.; et al. Congenital Hypothyroidism: A 2020–2021 Consensus Guidelines Update—An ENDO-European Reference Network (ERN) initiative endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021, 31, 387–419. [Google Scholar] [CrossRef] [PubMed]
- Wassner, A.J.; Brown, R.S. Congenital hypothyroidism: Recent advances. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, K.; Minamitani, K.; Anzo, M.; Adachi, M.; Ishii, T.; Onigata, K.; Kusuda, S.; Harada, S.; Horikawa, R.; Minagawa, M.; et al. Guidelines for Mass Screening of Congenital Hypothyroidism (2014 revision) Mass Screening Committee, Japanese Society for Pediatric Endocrinology, and Japanese Society for Mass Screening. Clin. Pediatr. Endocrinol. 2015, 24, 107–133. [Google Scholar] [PubMed]
- Chiesa, A.; Prieto, L.; Mendez, V.; Papendieck, P.; Calcagno, M.D.L.; Gruñeiro-Papendieck, L. Prevalence and etiology of congenital hypothyroidism detected through an argentine neonatal screening program (1997–2010). Horm. Res. Paediatr. 2013, 80, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Barry, Y.; Bonaldi, C.; Goulet, V.; Coutant, R.; Léger, J.; Paty, A.C.; Delmas, D.; Cheillan, D.; Roussey, M. Increased incidence of congenital hypothyroidism in France from 1982 to 2012: A nationwide multicenter analysis. Ann Epidemiol 2016, 26, 100–105.e4. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Brooke, I.; Heales, S.; Ifederu, A.; Langham, S.; Hindmarsh, P.; Cole, T.J. Defining the newborn blood spot screening reference interval for TSH: Impact of ethnicity. J. Clin. Endocrinol. Metab. 2016, 101, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.H.; Kato, T.; Harada, S.; Inomata, H.; Aoki, K. Time Trend and Geographic Distribution of Treated Patients with Congenital Hypothyroidism Relative to the Number of Available Endocrinologists in Japan. J. Pediatr. 2010, 157, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Heather, N.L.; Derraik, J.G.B.; Webster, D.; Hofman, P.L. The impact of demographic factors on newborn TSH levels and congenital hypothyroidism screening. Clin. Endocrinol. 2019, 91, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.B.; Cutfield, W.S.; Webster, D.; Carll, J.; Derraik, J.G.B.; Jefferies, C.; Gunn, A.J.; Hofman, P.L. Etiology of increasing incidence of congenital hypothyroidism in New Zealand from 1993–2010. J. Clin. Endocrinol. Metab. 2012, 97, 3155–3160. [Google Scholar] [CrossRef]
- Harris, K.B.; Pass, K.A. Increase in congenital hypothyroidism in New York State and in the United States. Mol. Genet. Metab. 2007, 91, 268–277. [Google Scholar] [CrossRef]
- Olivieri, A.; Fazzini, C.; Medda, E. Multiple factors influencing the incidence of congenital hypothyroidism detected by neonatal screening. Horm. Res. Paediatr. 2015, 83, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Korada, S.M.; Pearce, M.; Ward, M.P.; Avis, E.; Turner, S.; Wastell, H.; Cheetham, T. Difficulties in selecting an appropriate neonatal thyroid stimulating hormone (TSH) screening threshold. Arch. Dis. Child. 2010, 95, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Langham, S.; Hindmarsh, P.; Krywawych, S.; Peters, C. Screening for Congenital Hypothyroidism: Comparison of Borderline Screening Cut-Off Points and the Effect on the Number of Children Treated with Levothyroxine. Eur. Thyroid. J. 2013, 2, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Cherella, C.E.; Wassner, A.J. Congenital hypothyroidism: Insights into pathogenesis and treatment. Int. J. Pediatr. Endocrinol. 2017, 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Deladoëy, J.; Van Vliet, G. The changing epidemiology of congenital hypothyroidism: Fact or artifact? Expert. Rev. Endocrinol. Metab. 2014, 9, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Deladoëy, J.; Ruel, J.; Giguère, Y.; Van Vliet, G. Is the incidence of congenital hypothyroidism really increasing? A 20-year retrospective population-based study in Québec. J. Clin. Endocrinol. Metab. 2011, 96, 2422–2429. [Google Scholar] [CrossRef] [PubMed]
- Connelly, K.J.; Lafranchi, S.H. Detection of neonates with mild congenital hypothyroidism (primary) or isolated hyperthyrotropinemia: An increasingly common management dilemma. Expert. Rev. Endocrinol. Metab. 2014, 9, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.; Monte, O.; Tomimori, E.K.; Catarino, R.M.; Sterza, T.; Rocha, T.; Pereira, K.C.C.; Mattos, H.S.; Fagundes, L.B.; Liberato, M.M.; et al. Sonographic evaluation of the thyroid size in neonates. J. Clin. Ultrasound 2015, 43, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde. Protocolo Clínico e Diretrizes Terapêuticas para Hipotireoidismo Congênito; Portaria SAS/MS no. 1161, de 18 de Novembro de 2015; Ministério da Saúde: Brasilia, Brasil, 2015; pp. 1–8. [Google Scholar]
- Barone, B.; Lopes, C.d.L.S.; Tyszler, L.S.; do Amaral, V.B.; Zarur, R.H.C.; Paiva, V.N.; Leite, D.B.; Meirelles, R.M. Avaliação do valor de corte de TSH em amostras de filtro na triagem neonatal para diagnóstico de hipotireoidismo congênito no programa “Primeiros Passos”—IEDE/RJ. Arq. Bras. Endocrinol. Metabol. 2013, 57, 57–61. [Google Scholar] [CrossRef]
- Nascimento, M.L.; Nascimento, A.L.; Dornbusch, P.; Ohira, M.; Simoni, G.; Cechinel, E.; Linhares, R.M.M.; Lee, J.V.D.S.; Silva, P.C.A. Impact of the reduction in TSH cutoff level to 6 mIU/L in neonatal screening for congenital hypothyroidism in Santa Catarina: Final results. Arch. Endocrinol. Metab. 2020, 64, 816–823. [Google Scholar] [CrossRef]
- Matos, D.M.; Ramalho, R.J.R.; Carvalho, B.M.; Almeida, M.A.C.T.; Passos, L.F.D.; Vasconcelos, T.T.S.; Melo, E.V.; Oliveira, C.R.P.; Santos, E.G.; Resende, K.F.; et al. Evolution to permanent or transient conditions in children with positive neonatal TSH screening tests in Sergipe, Brazil. Arch. Endocrinol. Metab. 2016, 60, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, C.; Weber, G.; Cortinovis, F.; Calebiro, D.; Passoni, A.; Vigone, M.C.; Beck-Peccoz, P.; Chiumello, G.; Persani, L. A 7-year experience with low blood TSH cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (CH). Clin. Endocrinol. 2009, 71, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Mengreli, C.; Kanaka-Gantenbein, C.; Girginoudis, P.; Magiakou, M.A.; Christakopoulou, I.; Giannoulia-Karantana, A.; Chrousos, G.P.; Dacou-Voutetakis, C. Screening for congenital hypothyroidism: The significance of threshold limit in false-negative results. J. Clin. Endocrinol. Metab. 2010, 95, 4283–4290. [Google Scholar] [CrossRef] [PubMed]
- McGrath, N.; Hawkes, C.P.; Mayne, P.; Murphy, N.P. Permanent Decompensated Congenital Hypothyroidism in Newborns with Whole-Blood Thyroid-Stimulating Hormone Concentrations between 8 and 10 mU/L: The Case for Lowering the Threshold. Horm. Res. Paediatr. 2018, 89, 265–270. [Google Scholar] [CrossRef]
- Rose, S.R.; Wassner, A.J.; Wintergerst, K.A.; Yayah-Jones, N.H.; Hopkin, R.J.; Chuang, J.; Smith, J.R.; Abell, K.; LaFranchi, S.H. American Academy of Pediatrics Section on Endocrinology, AAP Council on Genetics, Pediatric Endocrine Society, American Thyroid Association. Technical report. Congenital hypothyroidism: Screening and management. Pediatrics 2023, 151, e2022060420. [Google Scholar] [CrossRef] [PubMed]
- Danner, E.; Niuro, L.; Huopio, H.; Niinikoski, H.; Viikari, L.; Kero, J.; Jääskeläinen, J. Incidence of primary congenital hypothyroidism over 24 years in Finland. Pediatr. Res. 2022, 93, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Gunnerbeck, A.; Lundholm, C.; von Döbeln, U.; Zetterström, R.H.; Almqvist, C.; Nordenström, A. Neonatal screening for congenital hypothyroidism in Sweden 1980–2013: Effects of lowering the thyroid-stimulating hormone threshold. Eur. J. Endocrinol. 2023, 188, 536–546. [Google Scholar] [CrossRef]
- Mehran, L.; Khalili, D.; Yarahmadi, S.; Amouzegar, A.; Mojarrad, M.; Ajang, N.; Azizi, F. Worldwide recall rate in newborn screening programs for congenital hypothyroidism. Int. J. Endocrinol. Metab. 2017, 15, e55451. [Google Scholar] [CrossRef] [PubMed]
- Anne, R.P.; Rahiman, E.A. Congenital hypothyroidism in India: A systematic review and meta-analysis of prevalence, screen positivity rates, and etiology. Lancet Reg. Health Southeast Asia 2022, 5, 100040. [Google Scholar] [CrossRef]
- Simonetti, S.; D’Amato, G.; Esposito, B.; Chiarito, M.; Dentico, D.; Lorè, T.; Cardinali, R.; Russo, S.; Laforgia, N.; Faienza, M.F. Congenital hypothyroidism after newborn screening program reorganization in the Apulia region. Ital. J. Pediatr. 2022, 48, 131. [Google Scholar] [CrossRef]
- Yu, A.; Alder, N.; Lain, S.J.; Wiley, V.; Nassar, N.; Jack, M. Outcomes of lowered newborn screening thresholds for congenital hypothyroidism. J. Paediatr. Child. Health 2023, 59, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Adad, F.C.; Mendes-dos-Santos, C.T.; Goto, M.M.F.; Sewaybricker, L.E.; D’Souza-Li, L.F.R.; Guerra-Junior, G.; Morcillo, A.M.; Lemos-Marini, S.H.V. Neonatal screening: 9% of children with filter paper thyroid-stimulating hormone levels between 5 and 10 μIU/mL have congenital hypothyroidism. J. Pediatr. 2017, 93, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, B.; Brockow, I.; Hanauer, M.; Lüders, A.; Nennstiel, U. Is Our Newborn Screening Working Well? A Literature Review of Quality Requirements for Newborn Blood Spot Screening (NBS) Infrastructure and Procedures. Int. J. Neonatal Screen. 2023, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Dorreh, F.; Chaijan, P.Y.; Javaheri, J.; Zeinalzadeh, A.H. Epidemiology of congenital hypothyroidism in Markazi Province, Iran. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 105–110. [Google Scholar] [CrossRef]
- Caiulo, S.; Corbetta, C.; Di Frenna, M.; Medda, E.; De Angelis, S.; Rotondi, D.; Vincenzi, G.; de Filippis, T.; Patricelli, M.G.; Persani, L.; et al. Newborn screening for congenital hypothyroidism: The benefit of using differential TSH cutoffs in a two-screen program. J. Clin. Endocrinol. Metab. 2021, 106, e338–e349. [Google Scholar] [CrossRef]
- Krude, H.; Blankenstein, O. Treating patients not numbers: The benefit and burden of lowering TSH newborn screening cut-offs. Arch. Dis. Child. 2011, 96, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Lain, S.; Trumpff, C.; Grosse, S.D.; Olivieri, A.; Van Vliet, G. Are lower TSH cutoffs in neonatal screening for congenital hypothyroidism warranted? Eur. J. Endocrinol. 2017, 177, D1–D12. [Google Scholar] [CrossRef]
- Trumpff, C.; se Schepper, J.; Vanderfaeillie, J.; Vercruysse, N.; Tafforeau, J.; Van Oyen, H.; Vandevijvere, S. No association between elevated thyroid-stimulating hormone at birth and parent-reported problem behavior at preschool age. Front. Endocrinol. 2016, 7, 161. [Google Scholar] [CrossRef]
- Trumpff, C.; De Schepper, J.; Vanderfaeillie, J.; Vercruysse, N.; Van Oyen, H.; Moreno-Reyes, R.; Tafforeau, J.; Vandevijvere, S. Neonatal thyroid-stimulating hormone concentration and psychomotor development at preschool age. Arch. Dis. Child. 2016, 101, 1100–1106. [Google Scholar] [CrossRef]
- West, R.; Hong, J.; Derraik, J.G.B.; Webster, D.; Heather, N.L.; Hofman, P.L. Newborn Screening TSH Values Less Than 15 mIU/L Are Not Associated with Long-term Hypothyroidism or Cognitive Impairment. J. Clin. Endocrinol. Metab. 2020, 105, dgaa415. [Google Scholar] [CrossRef]
- Freire, C.; Ramos, R.; Amaya, E.; Fernández, M.F.; Santiago-Fernández, P.; Lopez-Espinosa, M.J.; Arrebola, J.P.; Olea, N. Newborn TSH concentration and its association with cognitive development in healthy boys. Eur. J. Endocrinol. 2010, 163, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Lain, S.J.; Wiley, V.; Jack, M.; Martin, A.J.; Wilcken, B.; Nassar, N. Association of elevated neonatal thyroid-stimulating hormone levels with school performance and stimulant prescription for attention deficit hyperactivity disorder in childhood. Eur. J. Pediatr. 2021, 180, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, J.E.; Dias, V.M.A.; Jardim de Paula, J.; Silva, I.N. Socioeconomic aspects are crucial to better intellectual outcome in early-treated adolescents with congenital hypothyroidism. Child. Neuropsychol. 2021, 27, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, J.M.; Rojas-Ramos, J.C.R.; Domingos, M.T.; de Lima, M.R.; Kraemer, G.C.; Cardoso-Demartini, A.A.; Pereira, R.M.; Lacerda, L.D.; Nesi-França, S. Cognitive outcome of 458 children over 25 years of neonatal screening for congenital hypothyroidism. J. Pediatr. 2023, 99, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Bongers-Schokking, J.J.; Resing, W.C.M.; Oostdijk, W.; de Rijke, Y.B.; Keizer-Schramaa, S.M.P.d.M. Relation between Early Over- and Undertreatment and Behavioural Problems in Preadolescent Children with Congenital Hypothyroidism. Horm. Res. Paediatr. 2019, 90, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Villanger, G.D.; Ystrom, E.; Engel, S.M.; Longnecker, M.P.; Pettersen, R.; Rowe, A.D.; Reichborn-Kjennerud, T.; Aase, H. Neonatal thyroid-stimulating hormone and association with attention-deficit/hyperactivity disorder. Paediatr. Perinat. Epidemiol. 2020, 34, 590–596. [Google Scholar] [CrossRef]
- Bongers-Schokking, J.J.; Resing, W.C.M.; Oostdijk, W.; De Rijke, Y.B.; De Muinck Keizer-Schrama, S.M.P.F. Individualized treatment to optimize eventual cognitive outcome in congenital hypothyroidism. Pediatr. Res. 2016, 80, 816–823. [Google Scholar] [CrossRef]

| b-TSH Cut-Off (mIU/L) for the 1st Sample (n = 159,659) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Recall Rate for 2nd Sample |
|---|---|---|---|---|---|
| 6 | 100% (100–100) | 99.50% (99.46–99.53) | 11.87% (11.71–12.03) | 100.00% (100–100) | 0.57% |
| 7 | 85.30% (85.15–85.49) | 99.70% (99.67–99.73) | 16.88% (16.69–17.06) | 99.99% (99.99–99.99) | 0.35% |
| 8 | 78.90% (78.7–79.1) | 99.80% (99.82–99.86) | 24.93% (24.72–25.14) | 99.99% (99.98–99.99) | 0.22% |
| 9 | 70.60% (70.42–70.87) | 99.90% (99.89–99.92) | 34.69% (34.45–34.92) | 99.98% (99.97–99.99) | 0.14% |
| 10 | 64.20% (63.99–64.46) | 99.90% (99.93–99.95) | 41.42% (41.18–41.66) | 99.98% (99.97–99.98) | 0.11% |
| b-TSH cut-off (mIU/L) for the 2nd sample (n = 793) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Recall Rate for Appointment |
| 6 | 98.20% (97.33–99.16) | 98.10% (97.15–99.05) | 80.00% (77.22–82.78) | 99.86% (99.60–100) | 0.08% |
| 7 | 80.70% (77.95–83.45) | 98.10% (97.15–99.05) | 76.67% (73.72–79.61) | 98.50% (97.65–99.35) | 0.07% |
| 8 | 73.70% (68.80–75.06) | 98.40% (97.49–99.25) | 77.36% (74.45–80.27) | 97.84% (96.83–98.85) | 0.07% |
| 9 | 64.90% (61.59–68.23) | 98.90% (98.19–99.63) | 82.22% (79.56–84.88) | 97.33% (96.20–98.45) | 0.06% |
| 10 | 59.60% (56.23–63.06) | 99.20% (98.56–99.81) | 85.00% (82.51–87.49) | 96.95% (95.75–98.14) | 0.06% |
| Serum TSH | Free T4 | |||
|---|---|---|---|---|
| Group 1 (b-TSH 6–9.9 mIU/L) | Group 2 (b-TSH >= 10 mIU/L) | Group 1 (b-TSH 6–9.9 mIU/L) | Group 2 (b-TSH >= 10 mIU/L) | |
| Median | 14.52 * | 177.7 * | 1.14 * | 0.56 * |
| Minimum | 10.08 | 10.4 | 0.40 | 0.10 |
| Maximum | 193.65 | 1370.0 | 2.54 | 1.68 |
| 25th percentile | 11.72 | 47.81 | 0.93 | 0.27 |
| 75th percentile | 27.62 | 394.55 | 1.37 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira Palla Braga, N.; Vilela Antunes, J.M.; Colosimo, E.A.; Alves Dias, V.M.; Januário, J.N.; Novato Silva, I. Impact of Lowering TSH Cut-Off on Neonatal Screening for Congenital Hypothyroidism in Minas Gerais, Brazil. Int. J. Neonatal Screen. 2024, 10, 52. https://doi.org/10.3390/ijns10030052
Teixeira Palla Braga N, Vilela Antunes JM, Colosimo EA, Alves Dias VM, Januário JN, Novato Silva I. Impact of Lowering TSH Cut-Off on Neonatal Screening for Congenital Hypothyroidism in Minas Gerais, Brazil. International Journal of Neonatal Screening. 2024; 10(3):52. https://doi.org/10.3390/ijns10030052
Chicago/Turabian StyleTeixeira Palla Braga, Nathalia, Jáderson Mateus Vilela Antunes, Enrico Antônio Colosimo, Vera Maria Alves Dias, José Nélio Januário, and Ivani Novato Silva. 2024. "Impact of Lowering TSH Cut-Off on Neonatal Screening for Congenital Hypothyroidism in Minas Gerais, Brazil" International Journal of Neonatal Screening 10, no. 3: 52. https://doi.org/10.3390/ijns10030052
APA StyleTeixeira Palla Braga, N., Vilela Antunes, J. M., Colosimo, E. A., Alves Dias, V. M., Januário, J. N., & Novato Silva, I. (2024). Impact of Lowering TSH Cut-Off on Neonatal Screening for Congenital Hypothyroidism in Minas Gerais, Brazil. International Journal of Neonatal Screening, 10(3), 52. https://doi.org/10.3390/ijns10030052









