Evaluation of the First Three Years of Treatment of Children with Congenital Hypothyroidism Identified through the Alberta Newborn Screening Program
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Timeliness of Diagnosis
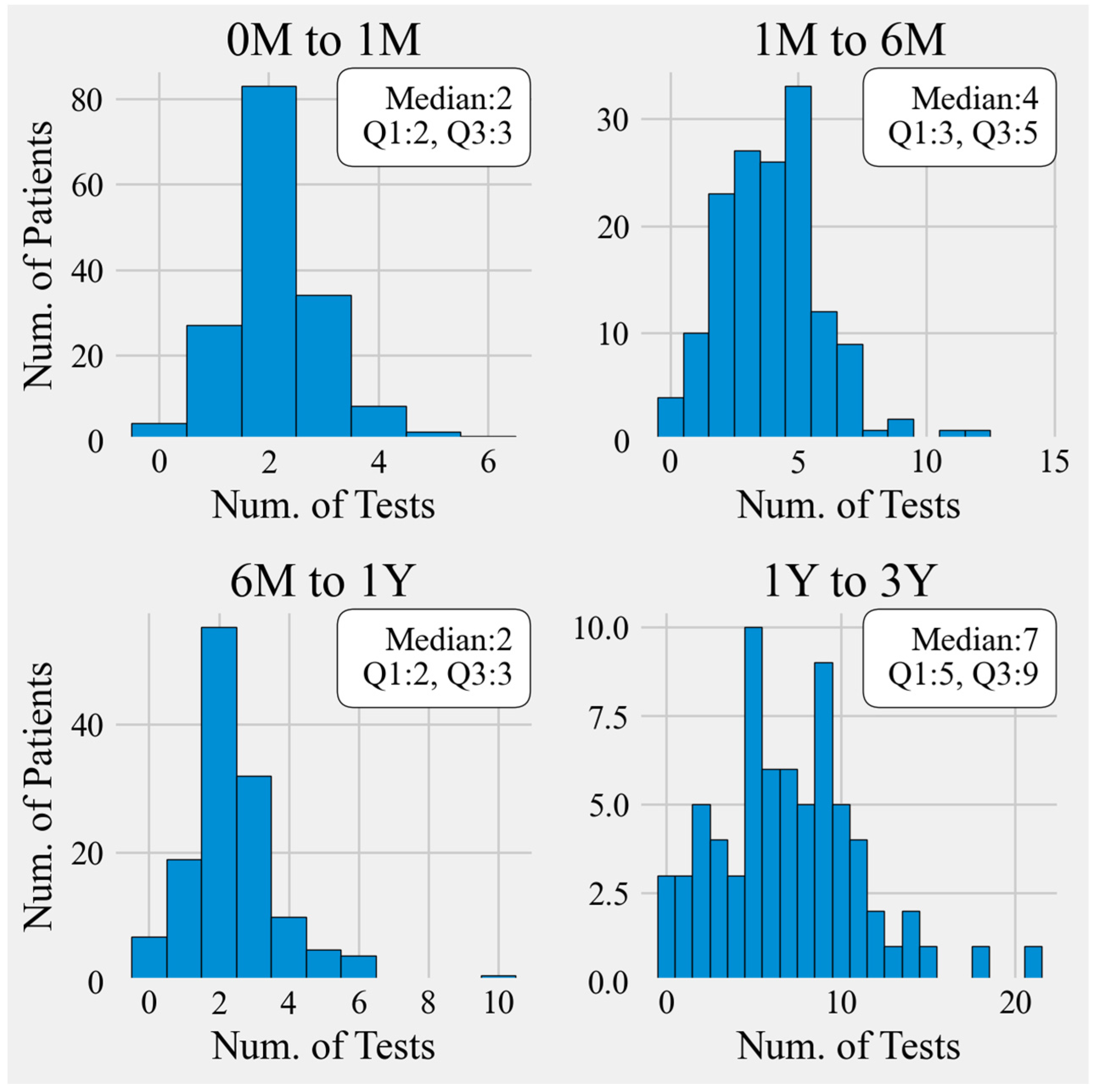
3.2. Timeliness of Monitoring
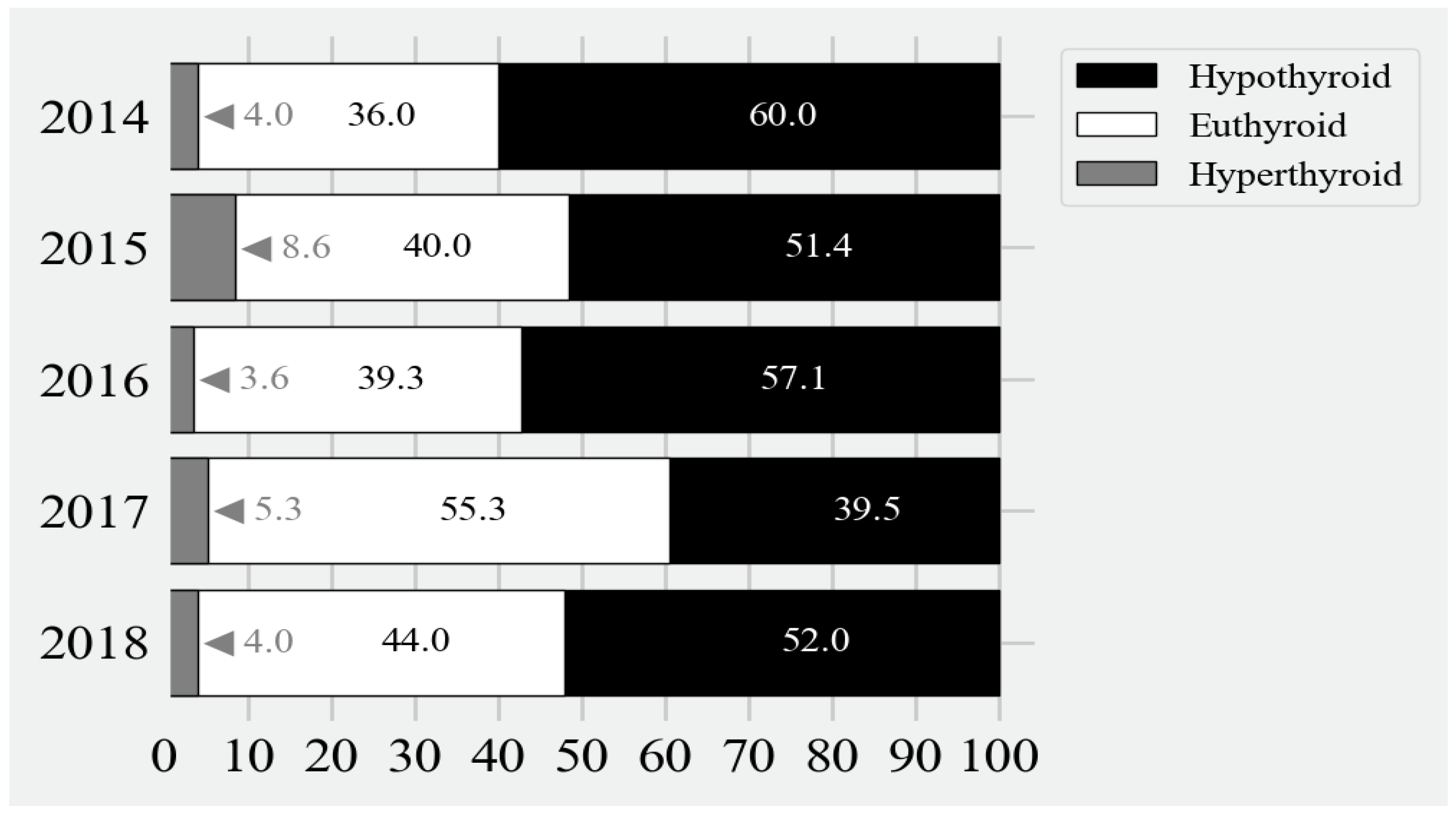
3.3. Appropriateness of Treatment
- In 2014, there was one infant who experienced prolonged hypothyroidism. This infant’s initial venous TSH was >500. This infant’s TSH level did not fall below 10 mU/L until day 437. Thereafter, this infant became euthyroid, which persisted until the last record at 1041 days.
- One infant born in 2015 did not become euthyroid until 89 days of age. Afterwards, this infant had fluctuating levels of hypothyroidism and euthyroidism on day 120 (TSH 24), became euthyroid on days 616 (TSH 126) to 734 (TSH 129), and then was euthyroid on days 778 (TSH 24) to 810 (TSH 23 mU/L).
- In 2016, an infant with a free T4 of 6.9 pmol/L was started on levothyroxine for CH. At 360 days of age, the TSH level was >150; and at 505 days of age, the TSH was 91 mU/L. There were no other thyroid function indices in between.
| Year | 2014 n = 27 | 2015 n = 35 | 2016 n = 30 | 2017 n = 39 | 2018 n = 23 |
|---|---|---|---|---|---|
| Hypothyroidism after euthyroidism (n; %) | 17 (63%) | 21 (60%) | 20 (67%) | 24 (62%) | 11 (48%) |
| TSH range (mU/L) | 8.4 to 59.1 | 7.1 to 129.4 | 7.6 to >150 | 8.2 to 191.4 | 7.7 to 78.2 |
| Hyperthyroidism after euthyroidism (n; %) | 13 (45%) | 22 (56%) | 13 (37%) | 16 (36%) | 6 (21%) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Van Vliet, G.; Deladoey, J. Diagnosis, treatment and outcome of congenital hypothyroidism. Endocr. Dev. 2014, 26, 50–59. [Google Scholar]
- Grosse, S.D.; van Vliet, G. Prevention of intellectual disability through screening for congenital hypothyroidism: How much and at what level? Arch. Dis. Child. 2011, 96, 374–379. [Google Scholar] [CrossRef]
- Dussault, J.H. The anecdotal history of screening for congenital hypothyroidism. J. Clin. Endocrinol. Metab. 1999, 84, 4332–4334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Souza, A.; Wolan, V.; Battochio, A.; Christian, S.; Hume, S.; Johner, G.; Lilley, M.; Ridsdale, R.; Schnabl, K.; Tran, C.; et al. Newborn Screening: Current Status in Alberta, Canada. Int. J. Neonatal Screen. 2019, 5, 37. [Google Scholar] [CrossRef]
- Alberta Health and Wellness. Newborn Metabolic Screening in Alberta 2002–2005; Alberta Health and Wellness, Public Health Division: Edmonton, AB, Canada, 2006.
- American Academy of Pediatrics; Rose, S.R.; Section on Endocrinology and Committee on Genetics, American Thyroid Association; Brown, R.S.; Public Health Committee, Lawson Wilkins Pediatric Endocrine Society; Foley, T.; Kaplowitz, B.P.; Kaye, C.I.; Sundararajan, S.; Varma, S.K. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006, 117, 2290–2303. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Screening for congenital hypothyroidism: US Preventive Services Task Force reaffirmation recommendation. Ann. Fam. Med. 2008, 6, 166. [Google Scholar]
- Toublanc, J.E. Guidelines for neonatal screening programs for congenital hypothyroidism. Working Group for Neonatal Screening in Paediatric Endocrinology of the European Society for Paediatric Endocrinology. Acta Paediatr. Suppl. 1999, 88, 13–14. [Google Scholar] [CrossRef]
- Leger, J.; Olivieri, A.; Donaldson, M.; Torresani, T.; Krude, H.; van Vliet, G.; Polak, M.; Butler, G.; on behalf of ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE, and the Congenital Hypothyroidism Consensus Conference Group. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm. Res. Paediatr. 2014, 81, 80–103. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Lipman Diaz, E.G. Hypothyroidism. Pediatr. Rev. 2014, 35, 336–347, quiz 348–349. [Google Scholar] [CrossRef]
- Bongers-Schokking, J.J.; Resing, W.C.; de Rijke, Y.B.; de Ridder, M.A.; de Muinck Keizer-Schrama, S.M. Cognitive development in congenital hypothyroidism: Is overtreatment a greater threat than undertreatment? J. Clin. Endocrinol. Metab. 2013, 98, 4499–4506. [Google Scholar] [CrossRef]
- Daneman, D.; Howard, N.J. Neonatal thyrotoxicosis: Intellectual impairment and craniosynostosis in later years. J. Pediatr. 1980, 97, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Dawrant, J.M.; Pacaud, D.; Wade, A.; Archer, S.; Bamforth, F.J. Informatics of newborn screening for congenital hypothyroidism in Alberta 2005–2008, flow of information from birth to treatment. Can. J. Public Health 2011, 102, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Van Trotsenburg, P.; Stoupa, A.; Leger, J.; Rohrer, T.; Peters, C.; Fugazzola, L.; Cassio, A.; Heinrichs, C.; Beauloye, V.; Pohlenz, J.; et al. Congenital Hypothyroidism: A 2020–2021 Consensus Guidelines Update—An ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021, 31, 387–419. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the treatment of hypothyroidism: Prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef] [PubMed]
- Alberta Health Services, ANSP, CH Algorithm V2.1. Clinical Algorithm for Congenital Hypothyroidism Abnormal Screen Result © March 2023. Available online: https://www.albertahealthservices.ca/assets/info/hp/nms/if-hp-nms-ch-algorithm.pdf (accessed on 1 October 2023).
- Available online: https://www.albertahealthservices.ca/assets/wf/lab/wf-lab-bulletin-thyroid-hormone-testing-changes.pdf (accessed on 1 October 2023).
- Fisher, D.A.; Klein, A.H. Thyroid development and disorders of thyroid function in the newborn. N. Engl. J. Med. 1981, 304, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.R.; Wassner, A.J.; Wintergerst, K.A.; Yayah-Jones, N.H.; Hopkin, R.J.; Chuang, J.; Smith, J.R.; Abell, K.; LaFranchi, S.H.; Section on Endocrinology Executive Committee; et al. Congenital Hypothyroidism: Screening and Management. Pediatrics 2023, 151, e2022060419. [Google Scholar] [CrossRef] [PubMed]
- Balhara, B.; Misra, M.; Levitsky, L.L. Clinical monitoring guidelines for congenital hypothyroidism: Laboratory outcome data in the first year of life. J. Pediatr. 2011, 158, 532–537. [Google Scholar] [CrossRef]
- Barry, Y.; Bonaldi, C.; Goulet, V.; Coutant, R.; Leger, J.; Paty, A.C.; Delmas, D.; Cheillan, D.; Roussey, M. Increased incidence of congenital hypothyroidism in France from 1982 to 2012, a nationwide multicenter analysis. Ann. Epidemiol. 2016, 26, 100–105.e4. [Google Scholar] [CrossRef] [PubMed]
- Deladoey, J.; Ruel, J.; Giguere, Y.; Van Vliet, G. Is the incidence of congenital hypothyroidism really increasing? A 20-year retrospective population-based study in Quebec. J. Clin. Endocrinol. Metab. 2011, 96, 2422–2429. [Google Scholar] [CrossRef]
- Lipska, E.; Lecka-Ambroziak, A.; Witkowski, D.; Szamotulska, K.; Mierzejewska, E.; Oltarzewski, M. Primary Congenital Hypothyroidism in Children Below 3 Years Old–Etiology and Treatment with Overtreatment and Undertreatment Risks, a 5-Year Single Centre Experience. Front. Endocrinol. 2022, 13, 895507. [Google Scholar] [CrossRef]
- Bongers-Schokking, J.J.; Resing, W.C.M.; Oostdijk, W.; de Rijke, Y.B.; de Muinck Keizer-Schrama, S. Relation between Early Over- and Undertreatment and Behavioural Problems in Preadolescent Children with Congenital Hypothyroidism. Horm. Res. Paediatr. 2018, 90, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Ouyang, L.; Grosse, S.D. Discontinuation of thyroid hormone treatment among children in the United States with congenital hypothyroidism: Findings from health insurance claims data. BMC Pediatr. 2010, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Sontag, M.K.; Sarkar, D.; Comeau, A.M.; Hassell, K.; Botto, L.D.; Parad, R.; Rose, S.R.; Wintergerst, K.A.; Smith-Whitley, K.; Singh, S.; et al. Case Definitions for Conditions Identified by Newborn Screening Public Health Surveillance. Int. J. Neonatal Screen. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, K.A.; Eugster, E.; Andruszewski, K.; Kleyn, M.; Vanderburg, N.; Sockalosky, J.; Menon, R.; Linard, S.; Kingery, S.; Rose, S.R.; et al. Congenital Hypothyroidism 3-Year Follow-Up Project: Region 4 Midwest Genetics Collaborative Results. Int. J. Neonatal Screen. 2018, 4, 18. [Google Scholar] [CrossRef]
- Rosenthal, N.A.; Bezar, E.; Mann, S.; Bachrach, L.K.; Banerjee, S.; Geffner, M.E.; Gottschalk, M.; Shapira, S.K.; Hasegawa, L.; Feuchtbaum, L. Primary Care Provider Management of Congenital Hypothyroidism Identified Through Newborn Screening. Ann. Thyroid Res. 2017, 3, 95–101. [Google Scholar]
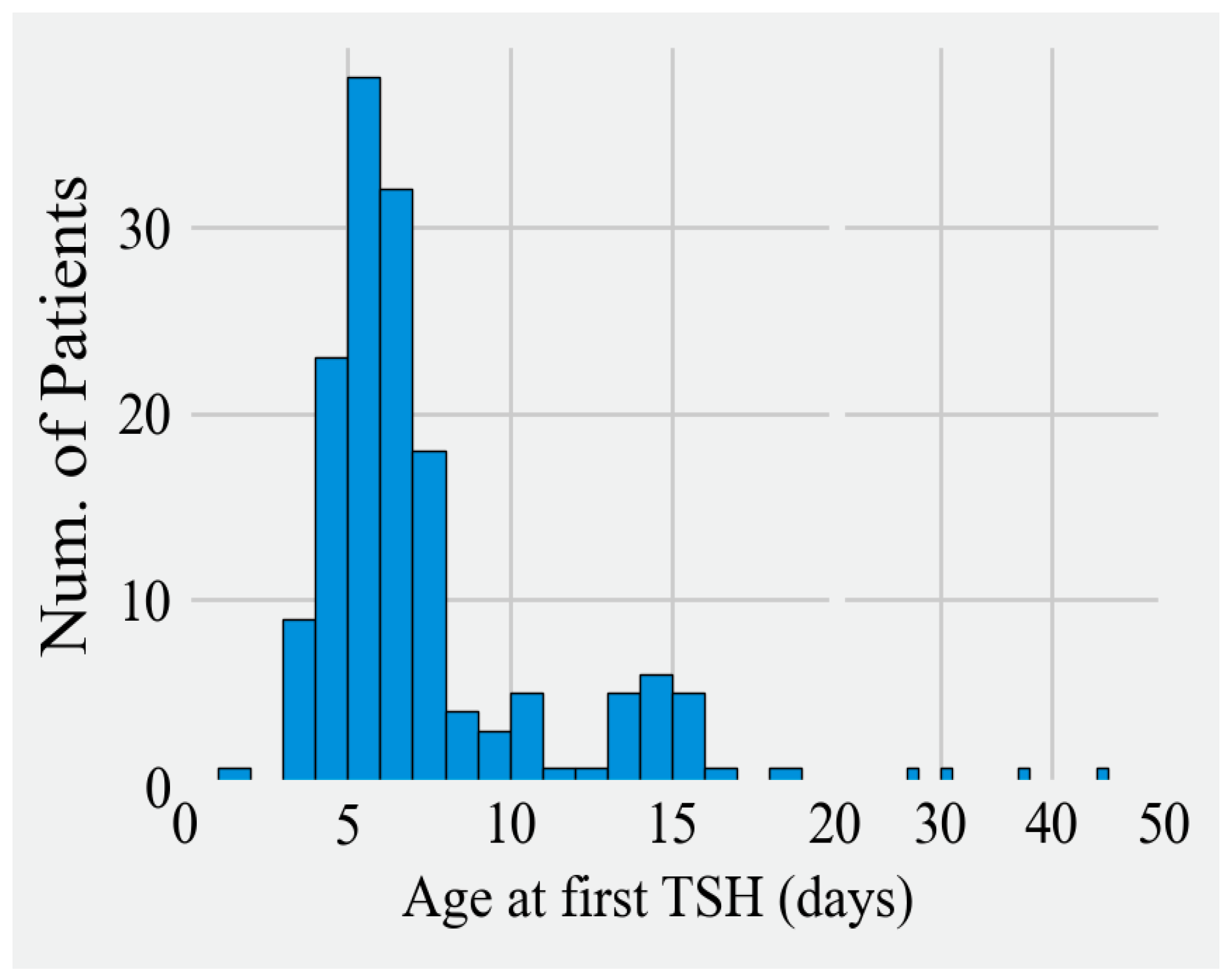

| Year | 2014 (n = 27) | 2015 (n = 35) | 2016 (n = 30) | 2017 (n = 39) | 2018 (n = 25) | 2019 (n = 4) |
|---|---|---|---|---|---|---|
| Time to 1st TSH (Days) | ||||||
| Range | 5 to 16 | 2 to 28 | 4 to 45 | 4 to 38 | 4 to 28 | 7 to 11 |
| Mode | 6 | 8 | 6 | 5 | 6 | N/A |
| Median (Q1, Q3) | 7 (6, 8) | 7 (6, 9) | 6 (6, 7) | 7 (5, 8) | 6 (6, 8) | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosova, I.; Archibald, A.; Rosolowsky, E.W.; Rathwell, S.; Christian, S.; Rosolowsky, E.T. Evaluation of the First Three Years of Treatment of Children with Congenital Hypothyroidism Identified through the Alberta Newborn Screening Program. Int. J. Neonatal Screen. 2024, 10, 35. https://doi.org/10.3390/ijns10020035
Sosova I, Archibald A, Rosolowsky EW, Rathwell S, Christian S, Rosolowsky ET. Evaluation of the First Three Years of Treatment of Children with Congenital Hypothyroidism Identified through the Alberta Newborn Screening Program. International Journal of Neonatal Screening. 2024; 10(2):35. https://doi.org/10.3390/ijns10020035
Chicago/Turabian StyleSosova, Iveta, Alyssa Archibald, Erik W. Rosolowsky, Sarah Rathwell, Susan Christian, and Elizabeth T. Rosolowsky. 2024. "Evaluation of the First Three Years of Treatment of Children with Congenital Hypothyroidism Identified through the Alberta Newborn Screening Program" International Journal of Neonatal Screening 10, no. 2: 35. https://doi.org/10.3390/ijns10020035
APA StyleSosova, I., Archibald, A., Rosolowsky, E. W., Rathwell, S., Christian, S., & Rosolowsky, E. T. (2024). Evaluation of the First Three Years of Treatment of Children with Congenital Hypothyroidism Identified through the Alberta Newborn Screening Program. International Journal of Neonatal Screening, 10(2), 35. https://doi.org/10.3390/ijns10020035








