Effect of Androgen Deprivation Therapy on the Results of PET/CT with 18F-Fluciclovine in Patients with Metastatic Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. 18F-Fluciclovine PET/CT
2.2. 18F-Fluciclovine PET/CT Interpretation
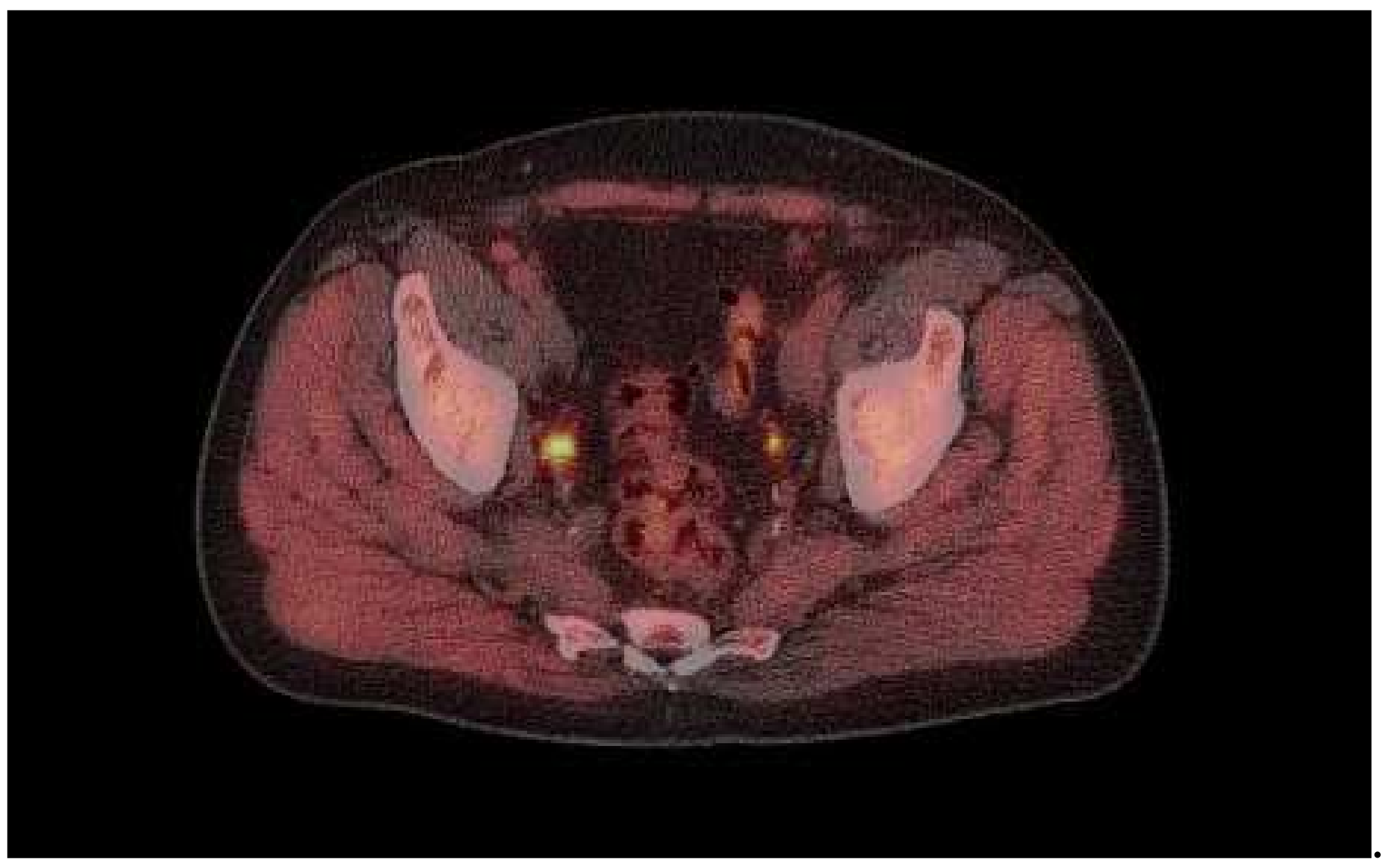
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bach-Gansmo, T.; Nanni, C.; Nieh, P.T.; Zanoni, L.; Bogsrud, T.V.; Sletten, H.; Korsan, K.A.; Kieboom, J.; Tade, F.I.; Odewole, O.; et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine (18F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J. Urol. 2017, 197, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Okudaira, H.; Shikano, N.; Nishii, R.; Miyagi, T.; Yoshimoto, M.; Kobayashi, M.; Ohe, K.; Nakanishi, T.; Tamai, I.; Namiki, M.; et al. Putative transport mechanism and intracellular fate of trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid in human prostate cancer. J. Nucl. Med. 2011, 52, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Okudaira, H.; Nakanishi, T.; Oka, S.; Kobayashi, M.; Tamagami, H.; Schuster, D.; Goodman, M.M.; Shirakami, Y.; Tamai, I.; Kawai, K. Kinetic analyses of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid transport in Xenopus laevis oocytes expressing human ASCT2 and SNAT2. Nucl. Med. Biol. 2013, 40, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Okudaira, H.; Yoshida, Y.; Schuster, D.M.; Goodman, M.M.; Shirakami, Y. Transport mechanisms of trans-1-amino-3-fluoro[1-14)C]cyclobutanecarboxylic acid in prostate cancer cells. Nucl. Med. Biol. 2012, 39, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.M.; Nanni, C.; Fanti, S.; Oka, S.; Okudaira, H.; Inoue, Y.; Sörensen, J.; Owenius, R.; Choyke, P.; Turkbey, B.; et al. Anti-1-Amino-3-18F-Fluorocyclobutane-1-Carboxylic Acid: Physiologic Uptake Patterns, Incidental Findings, and Variants That May Simulate Disease. J. Nucl. Med. 2014, 55, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Bode, B.P. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Semin. Cancer Biol. 2005, 15, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tiffen, J.; Bailey, C.G.; Lehman, M.L.; Ritchie, W.; Fazli, L.; Metierre, C.; Feng, Y.; Li, E.; Gleave, M.; et al. Targeting amino acid transport in metastatic castration-resistant prostate cancer: Effects on cell cycle, cell growth, and tumor development. J. Natl. Cancer Inst. 2013, 105, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Fuccio, C.; Schiavina, R.; Castellucci, P.; Rubello, D.; Martorana, G.; Celli, M.; Malizia, C.; Profitos, M.B.; Marzola, M.C.; Pettinato, C.; et al. Androgen deprivation therapy influences the uptake of 11C-choline in patients with recurrent prostate cancer: The preliminary results of a sequential PET/CT study. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- De Grado, T.R.; Coleman, R.E.; Wang, S.; Baldwin, S.W.; Orr, M.D.; Robertson, C.N.; Polascik, T.J.; Price, D.T. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: Initial findings in prostate cancer. Cancer Res. 2001, 61, 110–117. [Google Scholar]
- Giovacchini, G. Do we have to withdraw antiandrogenic therapy in prostate cancer patients before PET/CT with [11C]choline? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1964–1966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nanni, C.; Zanoni, L.; Bach-Gansmo, T.; Minn, H.; Willoch, F.; Bogsrud, T.V.; Edward, E.P.; Savir-Baruch, B.; Teoh, E.; Ingram, F.; et al. 18F-Fluciclovine PET/CT: Joint EANM and SNMMI procedure guidelines for prostate cancer imaging- version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bellmunt, J.; Briers, E.; den Broeck, T.V.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. The EAU Prostate Cancer Guidelines. 2019. Available online: http://uroweb.org/guideline/prostate-cancer (accessed on 8 January 2020).
- Schuster, D.M.; Nieh, P.T.; Jani, A.B.; Amzat, R.; Bowman, F.D.; Halkar, R.K.; Master, V.A.; Nye, J.A.; Odewolem, O.A.; Osunkoya, A.O.; et al. Anti-3-[18F]FACBC positron emission tomography-computerized tomography and 111In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: Results of a prospective clinical trial. J. Urol. 2014, 191, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Nanni, C.; Zanoni, L.; Pultrone, C.; Schiavina, R.; Brunocilla, E.; Lodi, F.; Malizia, C.; Ferrari, M.; Rigatti, P.; Fonti, C.; et al. (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: Results of a prospective trial. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.L.; Kostakoglu, L.; Chau, A.; Duan, F.; Mahmood, U.; Mankoff, D.A.; Schuster, D.M.; Siegel, B.A.; LOCATE study group. The Impact of Positron Emission Tomography with 18F-Fluciclovine on the Management of Patients with Biochemical Recurrence of Prostate Cancer: Results from the LOCATE Trial. J. Urol. 2019, 201, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Scarsbrook, A.F.; Bottomley, D.; Teoh, E.J.; Bradley, K.M.; Payne, H.; Afaq, A.; Bomanji, J.; van As, N.; Chua, S.; Hoskin, P.; et al. Effect of 18F-Fluciclovine Positron Emission Tomography on the Management of Patients with Recurrence of Prostate Cancer: Results From the FALCON Trial. Int. J. Radiat. Oncol. 2020, 107, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Fendler, W.P.; Rowe, S.P.; Calais, J.; Hofman, M.S.; Maurer, T.; Schwarzenboeck, S.M.; Kratowchil, C.; Herrmann, K.; Giesel, F.L. Prostate-Specific Membrane Antigen Ligands for Imaging and Therapy. J. Nucl. Med. 2017, 58, 67S–76S. [Google Scholar] [CrossRef] [PubMed]
- Vaz, S.; Hadaschik, B.; Gabriel, M.; Herrmann, K.; Eiber, M.; Costa, D. Influence of androgen deprivation therapy on PSMA expression and PSMA-ligand PET imaging of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.; Schiavina, R.; Castellucci, P.; Brunocilla, E.; Fuccio, C.; Colletti, P.M.; Ferretti, A.; Chondrogiannis, S.; Rubello, D.; Romagnoli, D.; et al. 11C-choline PET/CT scan in patients with prostate cancer treated with intermittent ADT: A sequential PET/CT study. Clin. Nucl. Med. 2013, 38, e279–e282. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Number of Patients (%) |
|---|---|
| Primary therapy | |
| Radical prostatectomy External beam radiation therapy - Alone - With cryotherapy Primary hormonal treatment Missing data | 29 (40%) 30 (41%) 28 (38%) 2 (2.7%) 9 (12%) 5 (7%) |
| Last recorded PSA (ng/mL) before PET/CT | |
| <0.5 | 5 (6.8%) |
| 0.5–2 | 17 (23%) |
| >2–6 | 12 (16%) |
| >6–20 | 15 (21%) |
| >20 | 19 (26%) |
| Missing data | 5 (7%) |
| Gleason Score | n (%) |
|---|---|
| 5–6 | 2 (2.7%) |
| 7 | 26 (36%) |
| 8 | 16 (22%) |
| 9 | 19 (26%) |
| 10 | 2 (2.7%) |
| Missing data | 8 (11%) |
| Scanner | Siemens Biograph mCT40 |
|---|---|
| Acquisition mode | 3D |
| CT for attenuation correction and anatomic correlation | 50 mA, 120 kVp |
| Target administered activity | 370 MBq |
| CT contrast | No |
| Position | Supine |
| Direction | Pelvis to head |
| Arm position | Above head |
| Scan start position | Just below inguinal regions |
| Scan end position | Vertex |
| Minutes per bed position | See text |
| Number of bed position | 6–8 |
| Time on ADT | Number of Patients (n) | Positive Scan/n (%) |
|---|---|---|
| >3 months–<1 year | 9 | 8/9 (89%) |
| 1–<2 years | 8 | 5/8 (63%) |
| 2–4 years | 24 | 20/24 (83%) |
| >4–10 years | 23 | 18/23 (78%) |
| >10 years | 6 | 4/6 (67%) |
| Unclear from records, but >3 months | 3 | 1/3 (33%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bach-Gansmo, T.; Korsan, K.; Bogsrud, T.V. Effect of Androgen Deprivation Therapy on the Results of PET/CT with 18F-Fluciclovine in Patients with Metastatic Prostate Cancer. Tomography 2022, 8, 1477-1484. https://doi.org/10.3390/tomography8030120
Bach-Gansmo T, Korsan K, Bogsrud TV. Effect of Androgen Deprivation Therapy on the Results of PET/CT with 18F-Fluciclovine in Patients with Metastatic Prostate Cancer. Tomography. 2022; 8(3):1477-1484. https://doi.org/10.3390/tomography8030120
Chicago/Turabian StyleBach-Gansmo, Tore, Katrine Korsan, and Trond Velde Bogsrud. 2022. "Effect of Androgen Deprivation Therapy on the Results of PET/CT with 18F-Fluciclovine in Patients with Metastatic Prostate Cancer" Tomography 8, no. 3: 1477-1484. https://doi.org/10.3390/tomography8030120
APA StyleBach-Gansmo, T., Korsan, K., & Bogsrud, T. V. (2022). Effect of Androgen Deprivation Therapy on the Results of PET/CT with 18F-Fluciclovine in Patients with Metastatic Prostate Cancer. Tomography, 8(3), 1477-1484. https://doi.org/10.3390/tomography8030120






