Application of Hydrogel Spacer SpaceOAR Vue for Prostate Radiotherapy
Abstract
1. Introduction
2. Background
3. Prostate Radiation Treatment
4. Spacers
5. Clinical Data for Hydrogels
5.1. IMRT
5.2. SBRT
5.3. Brachytherapy
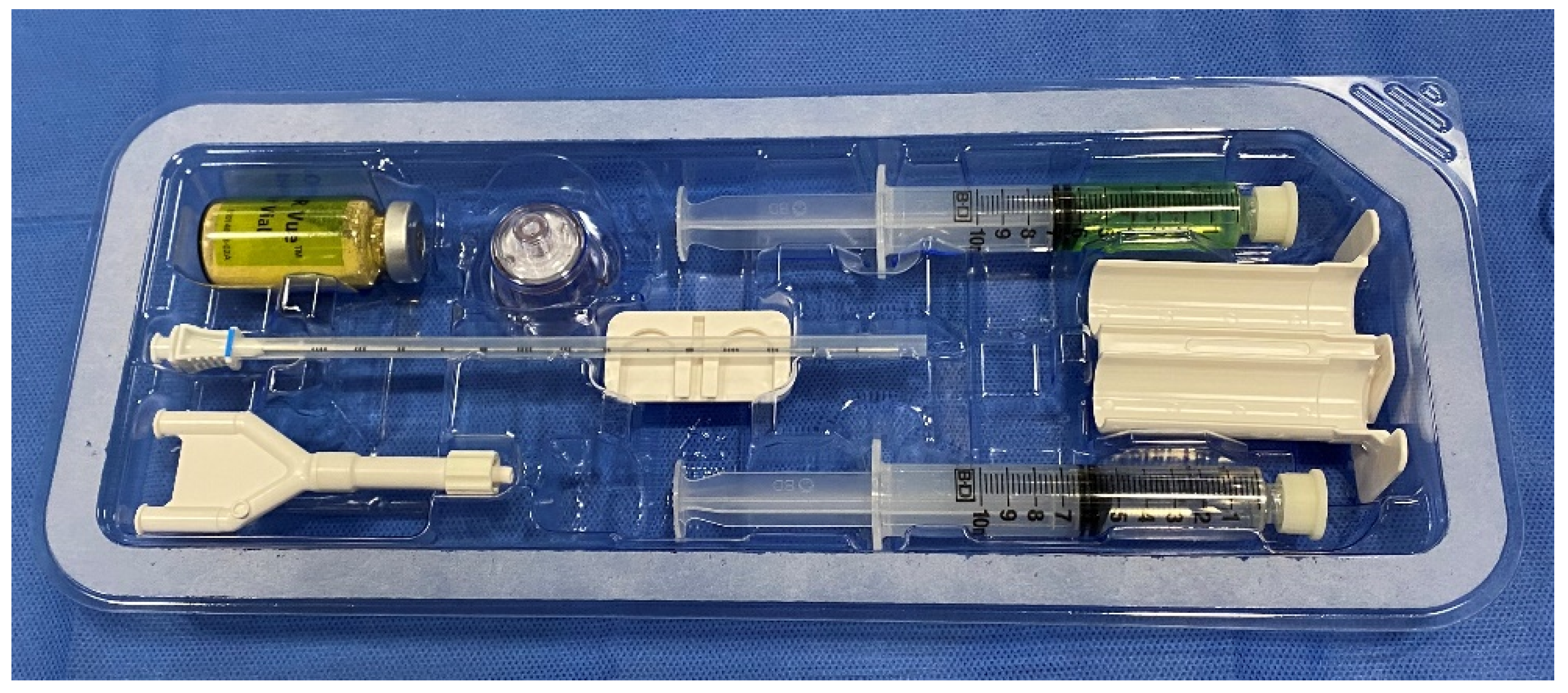
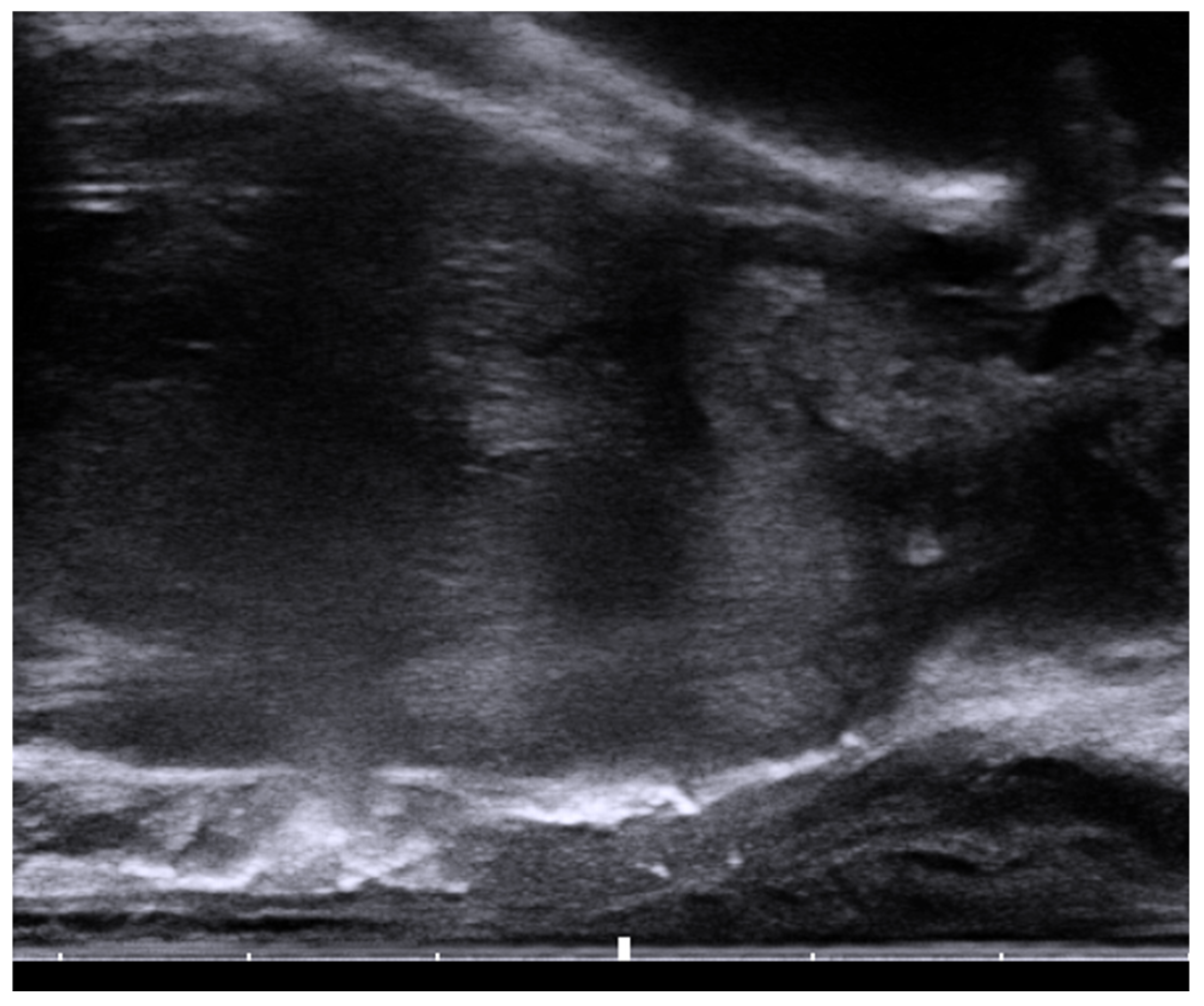
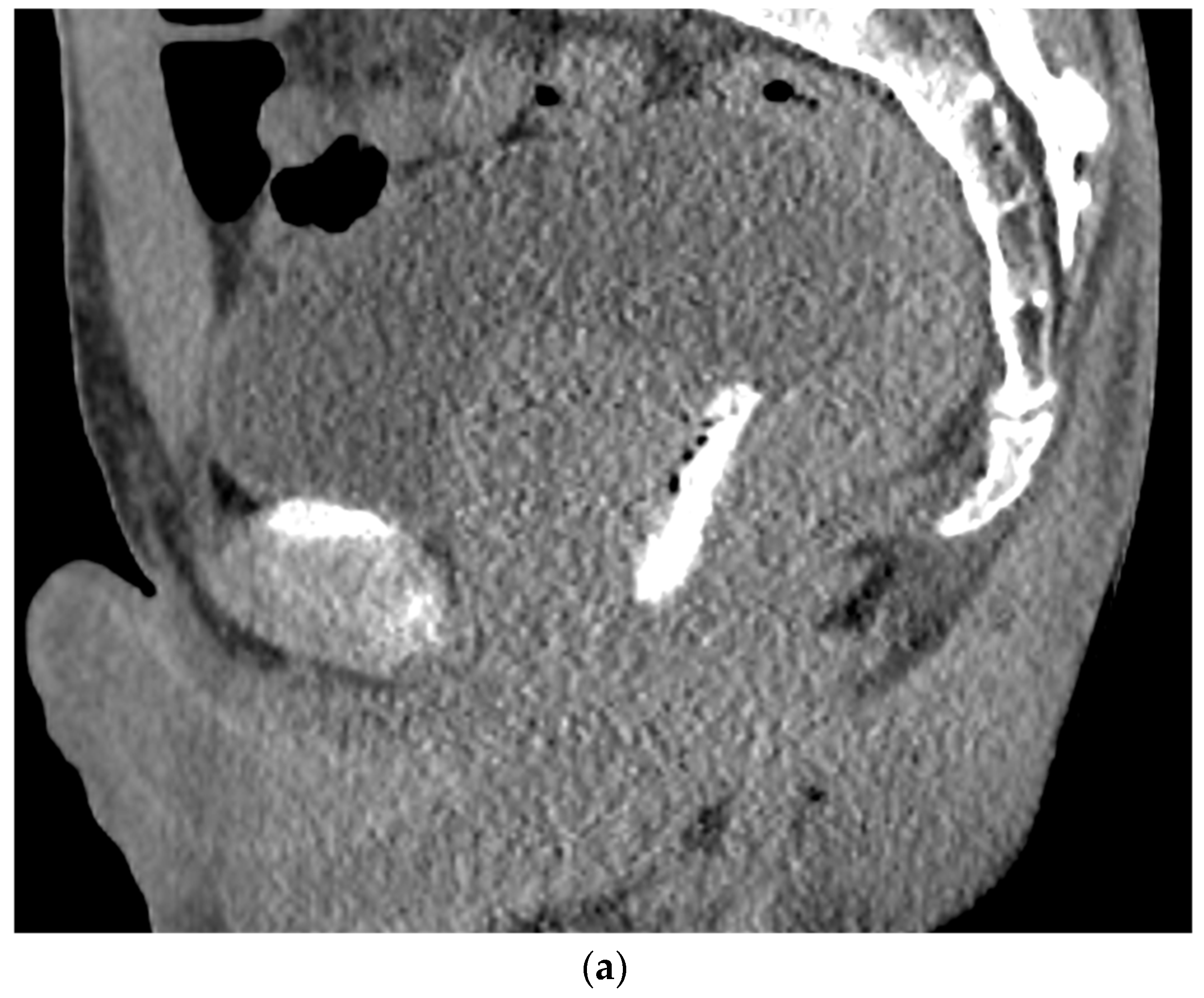
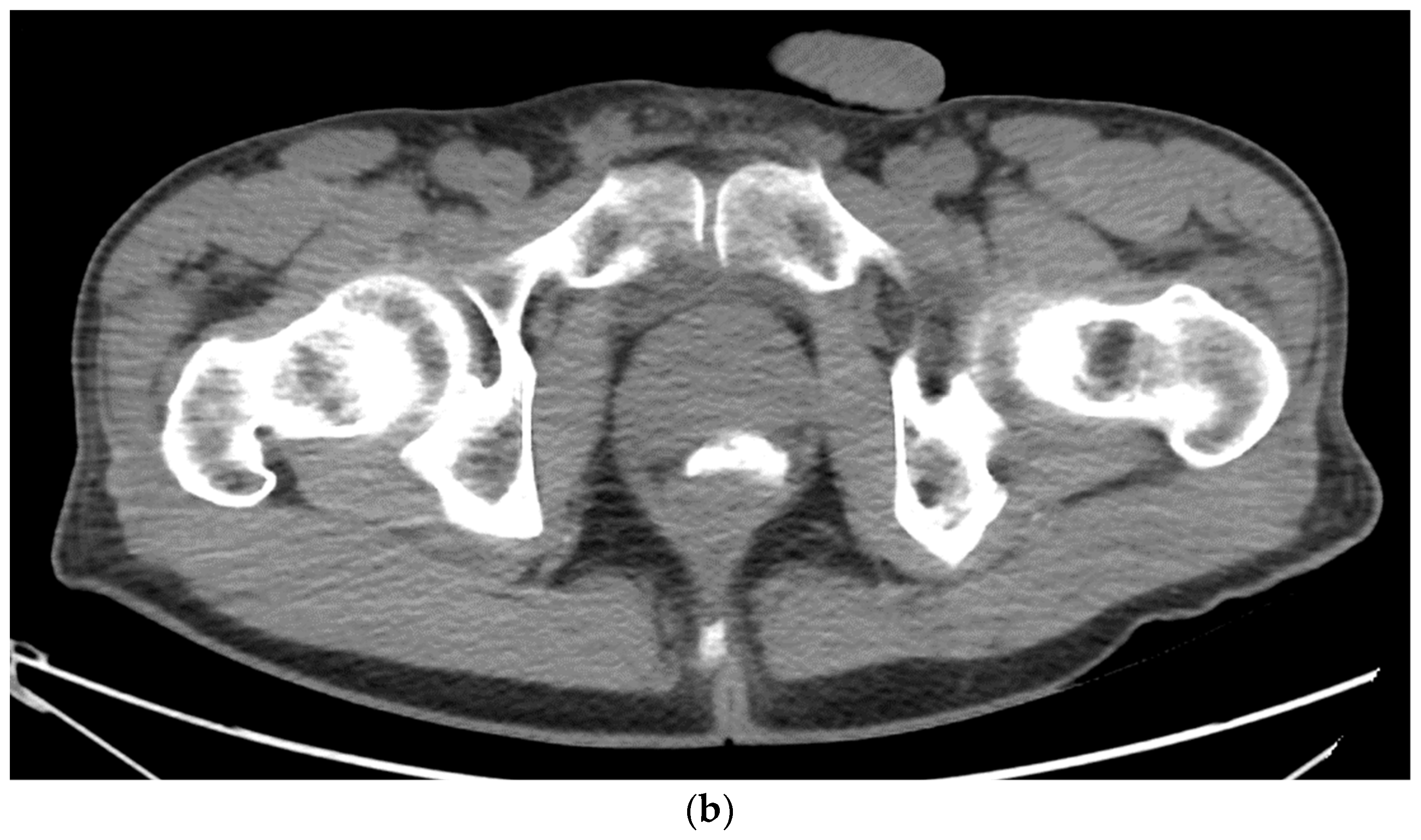
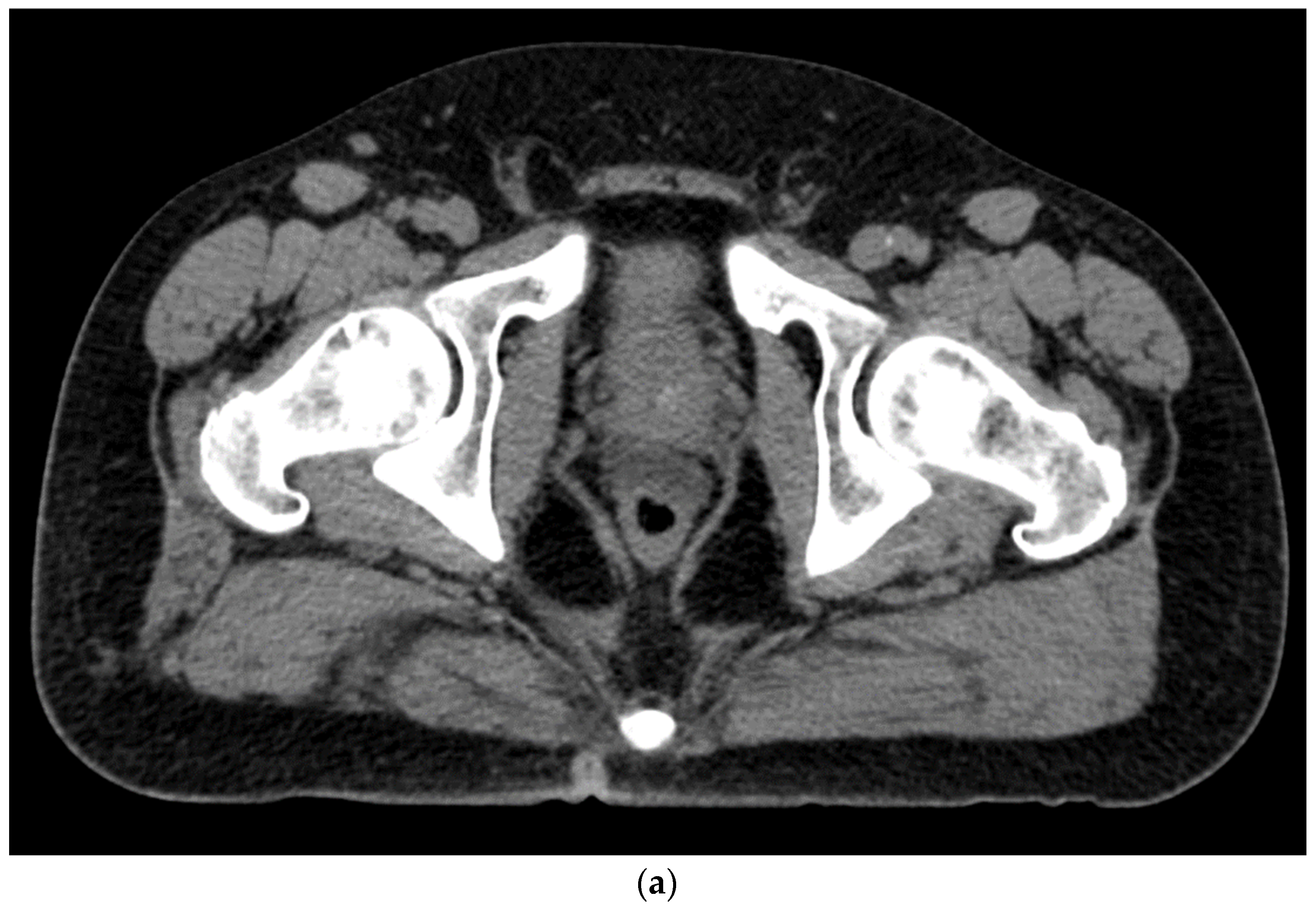
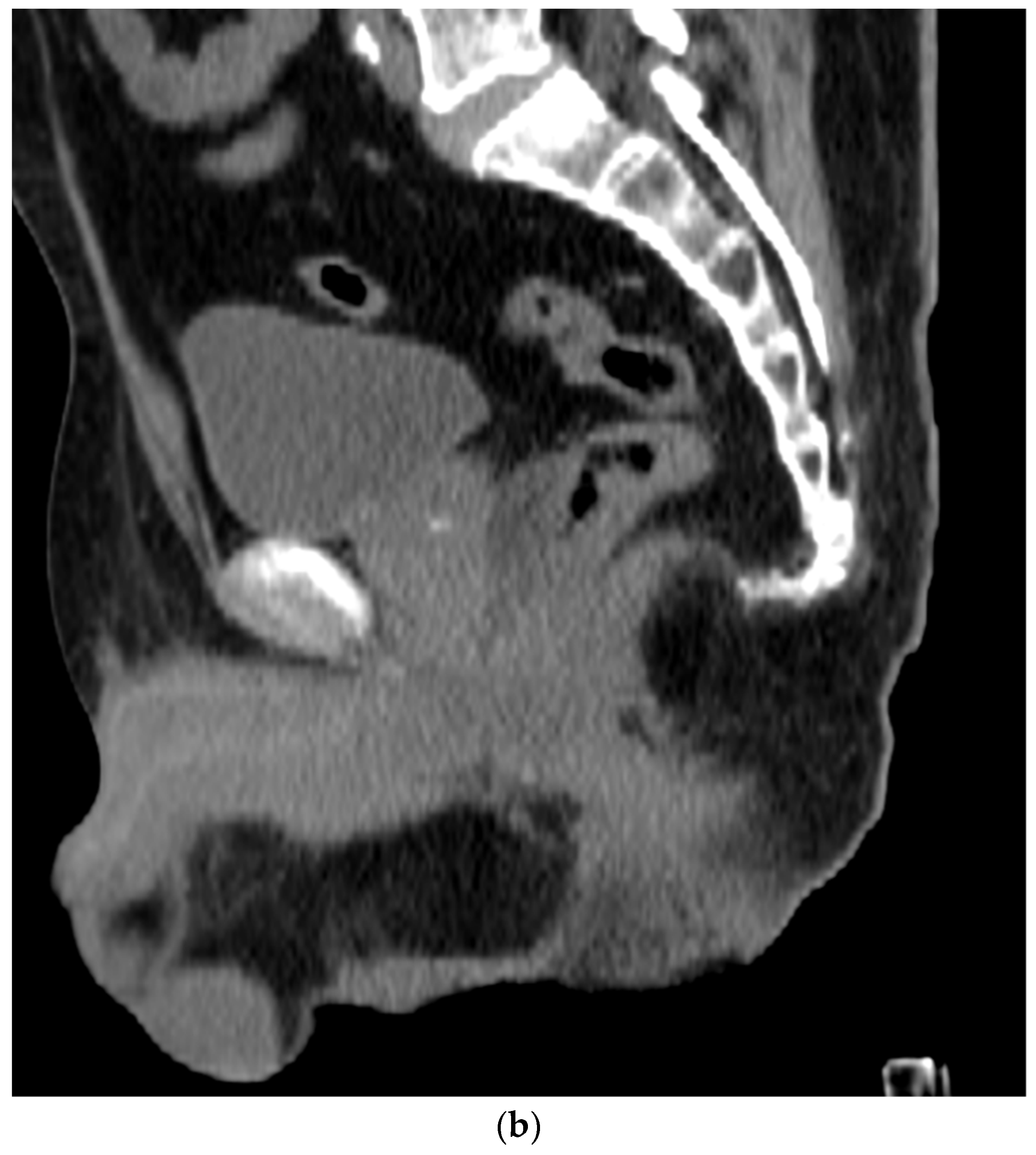
6. Placement
7. Contraindications to Hydrogel Placement
8. Adverse Effects of Hydrogels
9. Cost Effectiveness
10. Other Available Spacers
11. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H. Long-term survival rates of cancer patients achieved by the end of the 20th century: A period analysis. Lancet 2002, 360, 1131–1135. [Google Scholar] [CrossRef]
- Do, N.L.; Nagle, D.; Poylin, V.Y. Radiation proctitis: Current strategies in management. Gastroenterol. Res. Pract. 2011, 2011, 917941. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Kim, H.S.; Cho, M.S.; Kim, N.K. Three-dimensional anatomy of the Denonvilliers’ fascia after micro-CT reconstruction. Sci. Rep. 2021, 11, 21759. [Google Scholar] [CrossRef]
- Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; Kos, M.; et al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Laczko, I.; Hudson, D.L.; Freeman, A.; Feneley, M.R.; Masters, J.R. Comparison of the zones of the human prostate with the seminal vesicle: Morphology, immunohistochemistry, and cell kinetics. Prostate 2005, 62, 260–266. [Google Scholar] [CrossRef]
- Baxter, N.N.; Tepper, J.E.; Durham, S.B.; Rothenberger, D.A.; Virnig, B.A. Increased risk of rectal cancer after prostate radiation: A population-based study. Gastroenterology 2005, 128, 819–824. [Google Scholar] [CrossRef]
- Meyer, J. MRT, IGRT, SBRT: Advances in the Treatment Planning and Delivery of Radiotherapy. 2nd, rev. and extended ed. In Proceedings of the San Francisco Radiation Oncology Conference, San Francisco, CA, USA, 17–19 April 2009; Karger: Basel, Switzerland; London, UK, 2011. [Google Scholar]
- Vanneste, B.G.; Van Limbergen, E.J.; van Lin, E.N.; van Roermund, J.G.; Lambin, P. Prostate Cancer Radiation Therapy: What Do Clinicians Have to Know? Biomed. Res. Int. 2016, 2016, 6829875. [Google Scholar] [CrossRef]
- Shikama, N.; Kumazaki, Y.; Miyazawa, K.; Nihei, K.; Hashimoto, S.; Tsukamoto, N. Rectal Toxicity After Extremely Hypofractionated Radiotherapy Using a Non-Isocentric Robotic Radiosurgery System for Early Stage Prostate Cancer. World J. Oncol. 2016, 7, 98–103. [Google Scholar] [CrossRef]
- Pieczonka, C.M.; Mariados, N.; Sylvester, J.E.; Karsh, L.I.; Hudes, R.S.; Beyer, D.C.; Kurtzman, S.M.; Bogart, J.A. Hydrogel Spacer Application Technique, Patient Tolerance and Impact on Prostate Intensity Modulated Radiation Therapy: Results from a Prospective, Multicenter, Pivotal Randomized Controlled Trial. Urol. Pract. 2016, 3, 141–146. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [PubMed]
- Augmenix Announces FDA Clearance of SpaceOAR® System. Business Wire 2015. Available online: https://www.businesswire.com/news/home/20150402005778/en/Augmenix-Announces-FDA-Clearance-of-SpaceOAR%C2%AE-System (accessed on 2 August 2022).
- Folkert, M.R.; Zelefsky, M.J.; Hannan, R.; Desai, N.B.; Lotan, Y.; Laine, A.M.; Kim, D.W.N.; Neufeld, S.H.; Hornberger, B.; Kollmeier, M.A.; et al. A Multi-Institutional Phase 2 Trial of High-Dose SAbR for Prostate Cancer Using Rectal Spacer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Steenbakkers, R.J.; Deurloo, K.E.; Nowak, P.J.; Lebesque, J.V.; van Herk, M.; Rasch, C.R. Reduction of dose delivered to the rectum and bulb of the penis using MRI delineation for radiotherapy of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 1269–1279. [Google Scholar] [CrossRef]
- Ali, A.N.; Rossi, P.J.; Godette, K.D.; Martin, D.; Liauw, S.; Vijayakumar, S.; Cooper, S.; Jani, A.B. Impact of magnetic resonance imaging on computed tomography-based treatment planning and acute toxicity for prostate cancer patients treated with intensity modulated radiation therapy. Pract. Radiat. Oncol. 2013, 3, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Schiffner, D.C.; Gottschalk, A.R.; Lometti, M.; Aubin, M.; Pouliot, J.; Speight, J.; Hsu, I.C.; Shinohara, K.; Roach, M., III. Daily electronic portal imaging of implanted gold seed fiducials in patients undergoing radiotherapy after radical prostatectomy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 610–619. [Google Scholar] [CrossRef]
- Xie, Y.; Djajaputra, D.; King, C.R.; Hossain, S.; Ma, L.; Xing, L. Intrafractional motion of the prostate during hypofractionated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 236–246. [Google Scholar] [CrossRef]
- Cuccia, F.; Mazzola, R.; Nicosia, L.; Figlia, V.; Giaj-Levra, N.; Ricchetti, F.; Rigo, M.; Vitale, C.; Mantoan, B.; De Simone, A.; et al. Impact of hydrogel peri-rectal spacer insertion on prostate gland intra-fraction motion during 1.5 T MR-guided stereotactic body radiotherapy. Radiat. Oncol. 2020, 15, 178. [Google Scholar] [CrossRef]
- Kamran, S.C.; McClatchy, D.M., 3rd; Pursley, J.; Trofimov, A.V.; Remillard, K.; Saraf, A.; Ghosh, A.; Thabet, A.; Sutphin, P.; Miyamoto, D.T.; et al. Characterization of an Iodinated Rectal Spacer for Prostate Photon and Proton Radiation Therapy. Pract. Radiat. Oncol. 2022, 12, 135–144. [Google Scholar] [CrossRef]
- SpaceOAR™ Vue System. 2022. Available online: https://www.bostonscientific.com/content/dam/bostonscientific/spaceoar/vue/URO-855204-AA%20SpaceOAR%20VUE_Brief%20Summary.pdf (accessed on 9 August 2022).
- SpaceOAR Vue™ Hydrogel. 2020. Available online: https://www.bostonscientific.com/content/dam/bostonscientific/spaceoar/vue/SpaceOAR-Vue-Hydrogel-Brochure.pdf (accessed on 9 August 2022).
- Brenneman, R.J.; Andruska, N.; Roy, A.; Waters, M.R.; Fischer-Valuck, B.W.; Schiff, J.P.; Goddu, S.M.; Henke, L.E.; Gay, H.A.; Baumann, B.C.; et al. Characterization of a Novel Radiopaque Perirectal Hydrogel Spacer for Prostate Cancer Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, e536. [Google Scholar] [CrossRef]
- Hamstra, D.A.; Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; et al. Continued Benefit to Rectal Separation for Prostate Radiation Therapy: Final Results of a Phase III Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 976–985. [Google Scholar] [CrossRef]
- Hamstra, D.A.; Mariados, N.; Sylvester, J.; Shah, D.; Gross, E.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: Secondary analysis of a phase 3 trial. Pract. Radiat. Oncol. 2018, 8, e7–e15. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.J.; Daignault-Newton, S.; Bosch, W.; Mariados, N.; Sylvester, J.; Shah, D.; Gross, E.; Hudes, R.; Beyer, D.; Kurtzman, S.; et al. Who Benefits From a Prostate Rectal Spacer? Secondary Analysis of a Phase III Trial. Pract. Radiat. Oncol. 2020, 10, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.U.; Dang, A.; Katz, A.J.; Mantz, C.A.; Collins, S.P.; Aghdam, N.; Chu, F.I.; Kaplan, I.D.; Appelbaum, L.; Fuller, D.B.; et al. Long-term Outcomes of Stereotactic Body Radiotherapy for Low-Risk and Intermediate-Risk Prostate Cancer. JAMA Netw. Open 2019, 2, e188006. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Pinitpatcharalert, A.; Kollmeier, M.; Goldman, D.A.; McBride, S.; Gorovets, D.; Zhang, Z.; Varghese, M.; Happersett, L.; Tyagi, N.; et al. Early Tolerance and Tumor Control Outcomes with High-dose Ultrahypofractionated Radiation Therapy for Prostate Cancer. Eur. Urol. Oncol. 2020, 3, 748–755. [Google Scholar] [CrossRef]
- Kim, D.W.; Cho, L.C.; Straka, C.; Christie, A.; Lotan, Y.; Pistenmaa, D.; Kavanagh, B.D.; Nanda, A.; Kueplian, P.; Brindle, J.; et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 509–517. [Google Scholar] [CrossRef]
- Payne, H.A.; Pinkawa, M.; Peedell, C.; Bhattacharyya, S.K.; Woodward, E.; Miller, L.E. SpaceOAR hydrogel spacer injection prior to stereotactic body radiation therapy for men with localized prostate cancer: A systematic review. Medicine 2021, 100, e28111. [Google Scholar] [CrossRef]
- Ruggieri, R.; Naccarato, S.; Stavrev, P.; Stavreva, N.; Fersino, S.; Giaj Levra, N.; Mazzola, R.; Mancosu, P.; Scorsetti, M.; Alongi, F. Volumetric-modulated arc stereotactic body radiotherapy for prostate cancer: Dosimetric impact of an increased near-maximum target dose and of a rectal spacer. Br. J. Radiol. 2015, 88, 20140736. [Google Scholar] [CrossRef]
- Paydar, I.; Kim, B.S.; Cyr, R.A.; Rashid, H.; Anjum, A.; Yung, T.M.; Lei, S.; Collins, B.T.; Suy, S.; Dritschilo, A.; et al. Urethrogram-Directed Stereotactic Body Radiation Therapy for Clinically Localized Prostate Cancer in Patients with Contraindications to Magnetic Resonance Imaging. Front. Oncol. 2015, 5, 194. [Google Scholar] [CrossRef]
- Conroy, D.; Becht, K.; Forsthoefel, M.; Pepin, A.N.; Lei, S.; Rashid, A.; Collins, B.T.; Lischalk, J.W.; Suy, S.; Aghdam, N.; et al. Utilization of Iodinated SpaceOAR Vue During Robotic Prostate Stereotactic Body Radiation Therapy (SBRT) to Identify the Rectal-Prostate Interface and Spare the Rectum: A Case Report. Front. Oncol. 2020, 10, 607698. [Google Scholar] [CrossRef]
- Spratt, D.E.; Soni, P.D.; McLaughlin, P.W.; Merrick, G.S.; Stock, R.G.; Blasko, J.C.; Zelefsky, M.J. American Brachytherapy Society Task Group Report: Combination of brachytherapy and external beam radiation for high-risk prostate cancer. Brachytherapy 2017, 16, 1–12. [Google Scholar] [CrossRef]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Giberti, C.; Gallo, F.; Schenone, M.; Gastaldi, E.; Cortese, P.; Ninotta, G.; Becco, D. Robotic prostatectomy versus brachytherapy for the treatment of low risk prostate cancer. Can. J. Urol. 2017, 24, 8728–8733. [Google Scholar] [PubMed]
- Giberti, C.; Chiono, L.; Gallo, F.; Schenone, M.; Gastaldi, E. Radical retropubic prostatectomy versus brachytherapy for low-risk prostatic cancer: A prospective study. World J. Urol. 2009, 27, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Chou, R.H.; Dodge, R.K.; Clough, R.W.; Kang, H.S.; Hahn, C.A.; Whitehurst, A.W.; Buckley, N.J.; Kim, J.H.; Joyner, R.E.; et al. Gastrointestinal toxicity of transperineal interstitial prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 99–103. [Google Scholar] [CrossRef]
- Taggar, A.S.; Charas, T.; Cohen, G.N.; Boonyawan, K.; Kollmeier, M.; McBride, S.; Mathur, N.; Damato, A.L.; Zelefsky, M.J. Placement of an absorbable rectal hydrogel spacer in patients undergoing low-dose-rate brachytherapy with palladium-103. Brachytherapy 2018, 17, 251–258. [Google Scholar] [CrossRef]
- Gross, A.; Yuan, J.; Spratt, D.; Fredman, E. Case Report: Role of an Iodinated Rectal Hydrogel Spacer, SpaceOAR Vue, in the Context of Low-Dose-Rate Prostate Brachytherapy, for Enhanced Post-Operative Contouring to Aid in Accurate Implant Evaluation and Dosimetry. Front. Oncol. 2021, 11, 810955. [Google Scholar] [CrossRef]
- Basourakos, S.P.; Alshak, M.N.; Lewicki, P.J.; Cheng, E.; Tzeng, M.; DeRosa, A.P.; Allaway, M.J.; Ross, A.E.; Schaeffer, E.M.; Patel, H.D.; et al. Role of Prophylactic Antibiotics in Transperineal Prostate Biopsy: A Systematic Review and Meta-analysis. Eur. Urol. Open Sci. 2022, 37, 53–63. [Google Scholar] [CrossRef]
- Fagundes, M.; Rodrigues, M.A.; Olszewski, S.; Khan, F.; McKenzie, C.; Gutierrez, A.; Chuong, M.; Mehta, M. Expanding the Utilization of Rectal Spacer Hydrogel for Larger Prostate Glands (>80 cc): Feasibility and Dosimetric Outcomes. Adv. Radiat. Oncol. 2021, 6, 100651. [Google Scholar] [CrossRef]
- Repka, M.C.; Creswell, M.; Lischalk, J.W.; Carrasquilla, M.; Forsthoefel, M.; Lee, J.; Lei, S.; Aghdam, N.; Kataria, S.; Obayomi-Davies, O.; et al. Rationale for Utilization of Hydrogel Rectal Spacers in Dose Escalated SBRT for the Treatment of Unfavorable Risk Prostate Cancer. Front. Oncol. 2022, 12, 860848. [Google Scholar] [CrossRef]
- Mahal, B.A.; Ziehr, D.R.; Hyatt, A.S.; Neubauer-Sugar, E.H.; O’Farrell, D.A.; O’Leary, M.P.; Steele, G.S.; Niedermayr, T.R.; Beard, C.J.; Martin, N.E.; et al. Use of a rectal spacer with low-dose-rate brachytherapy for treatment of prostate cancer in previously irradiated patients: Initial experience and short-term results. Brachytherapy 2014, 13, 442–449. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Fang, J.; Zeng, Z.; Wei, B.; Chen, T.; Wei, H. Identification of the surgical indication line for the Denonvilliers’ fascia and its anatomy in patients with rectal cancer. Cancer Commun. (Lond.) 2020, 40, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.C.; Mischinger, J.; Klotz, T.; Gagel, B.; Habl, G.; Hatiboglu, G.; Pinkawa, M. Interdisciplinary consensus statement on indication and application of a hydrogel spacer for prostate radiotherapy based on experience in more than 250 patients. Radiol. Oncol. 2016, 50, 329–336. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.F.; Folkert, M.R.; Timmerman, R.D.; Hannan, R.; Garant, A.; Hudak, S.J.; Costa, D.N.; Desai, N.B. Hydrogel Spacer Rectal Wall Infiltration Associated With Severe Rectal Injury and Related Complications After Dose Intensified Prostate Cancer Stereotactic Ablative Radiation Therapy. Adv. Radiat. Oncol. 2021, 6, 100713. [Google Scholar] [CrossRef] [PubMed]
- Aminsharifi, A.; Kotamarti, S.; Silver, D.; Schulman, A. Major Complications and Adverse Events Related to the Injection of the SpaceOAR Hydrogel System Before Radiotherapy for Prostate Cancer: Review of the Manufacturer and User Facility Device Experience Database. J. Endourol. 2019, 33, 868–871. [Google Scholar] [CrossRef]
- Levy, J.F.; Khairnar, R.; Louie, A.V.; Showalter, T.N.; Mullins, C.D.; Mishra, M.V. Evaluating the Cost-Effectiveness of Hydrogel Rectal Spacer in Prostate Cancer Radiation Therapy. Pract. Radiat. Oncol. 2019, 9, e172–e179. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, B.G.; Pijls-Johannesma, M.; Van De Voorde, L.; van Lin, E.N.; van de Beek, K.; van Loon, J.; Ramaekers, B.L.; Lambin, P. Spacers in radiotherapy treatment of prostate cancer: Is reduction of toxicity cost-effective? Radiother. Oncol. 2015, 114, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.S.; MacDougall, D.; de Nanassy, A. Hydrogel Spacers for Patients with Prostate Cancer: A Review of Clinical Effectiveness and Cost-Effectiveness. In CADTH Rapid Response Reports; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- Hutchinson, R.C.; Sundaram, V.; Folkert, M.; Lotan, Y. Decision analysis model evaluating the cost of a temporary hydrogel rectal spacer before prostate radiation therapy to reduce the incidence of rectal complications. Urol. Oncol. 2016, 34, 291 e219–e226. [Google Scholar] [CrossRef]
- 510(k) Summary for Barrigel® Injectable Gel. 2022. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf22/K220641.pdf (accessed on 9 August 2022).
- First FDA-Reviewed Randomized Controlled Study of Rectal Spacing Using Hypofractionated Radiotherapy (RT). Available online: https://www.barrigel.com/hcp/efficacy-and-safety/ (accessed on 30 August 2022).
- Latorzeff, I.; Bruguiere, E.; Bogart, E.; Le Deley, M.C.; Lartigau, E.; Marre, D.; Pasquier, D. Use of a Biodegradable, Contrast-Filled Rectal Spacer Balloon in Intensity-Modulated Radiotherapy for Intermediate-Risk Prostate Cancer Patients: Dosimetric Gains in the BioPro-RCMI-1505 Study. Front. Oncol. 2021, 11, 701998. [Google Scholar] [CrossRef]
- Pasquier, D.; Bogart, E.; Bonodeau, F.; Lacornerie, T.; Lartigau, E.; Latorzeff, I. BioPro-RCMI-1505 trial: Multicenter study evaluating the use of a biodegradable balloon for the treatment of intermediate risk prostate cancer by intensity modulated radiotherapy; study protocol. BMC Cancer 2018, 18, 566. [Google Scholar] [CrossRef]
- Gez, E.; Cytron, S.; Ben Yosef, R.; London, D.; Corn, B.W.; Alani, S.; Scarzello, G.; Dal Moro, F.; Sotti, G.; Zattoni, F.; et al. Application of an interstitial and biodegradable balloon system for prostate-rectum separation during prostate cancer radiotherapy: A prospective multi-center study. Radiat. Oncol. 2013, 8, 96. [Google Scholar] [CrossRef]
- Noyes, W.R.; Hosford, C.C.; Schultz, S.E. Human collagen injections to reduce rectal dose during radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1918–1922. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadigal, S.R.; Gupta, A.K. Application of Hydrogel Spacer SpaceOAR Vue for Prostate Radiotherapy. Tomography 2022, 8, 2648-2661. https://doi.org/10.3390/tomography8060221
Hadigal SR, Gupta AK. Application of Hydrogel Spacer SpaceOAR Vue for Prostate Radiotherapy. Tomography. 2022; 8(6):2648-2661. https://doi.org/10.3390/tomography8060221
Chicago/Turabian StyleHadigal, Satvik R., and Atul K. Gupta. 2022. "Application of Hydrogel Spacer SpaceOAR Vue for Prostate Radiotherapy" Tomography 8, no. 6: 2648-2661. https://doi.org/10.3390/tomography8060221
APA StyleHadigal, S. R., & Gupta, A. K. (2022). Application of Hydrogel Spacer SpaceOAR Vue for Prostate Radiotherapy. Tomography, 8(6), 2648-2661. https://doi.org/10.3390/tomography8060221





