Initial Experience on Hyperpolarized [1-13C]Pyruvate MRI Multicenter Reproducibility—Are Multicenter Trials Feasible?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Volunteers
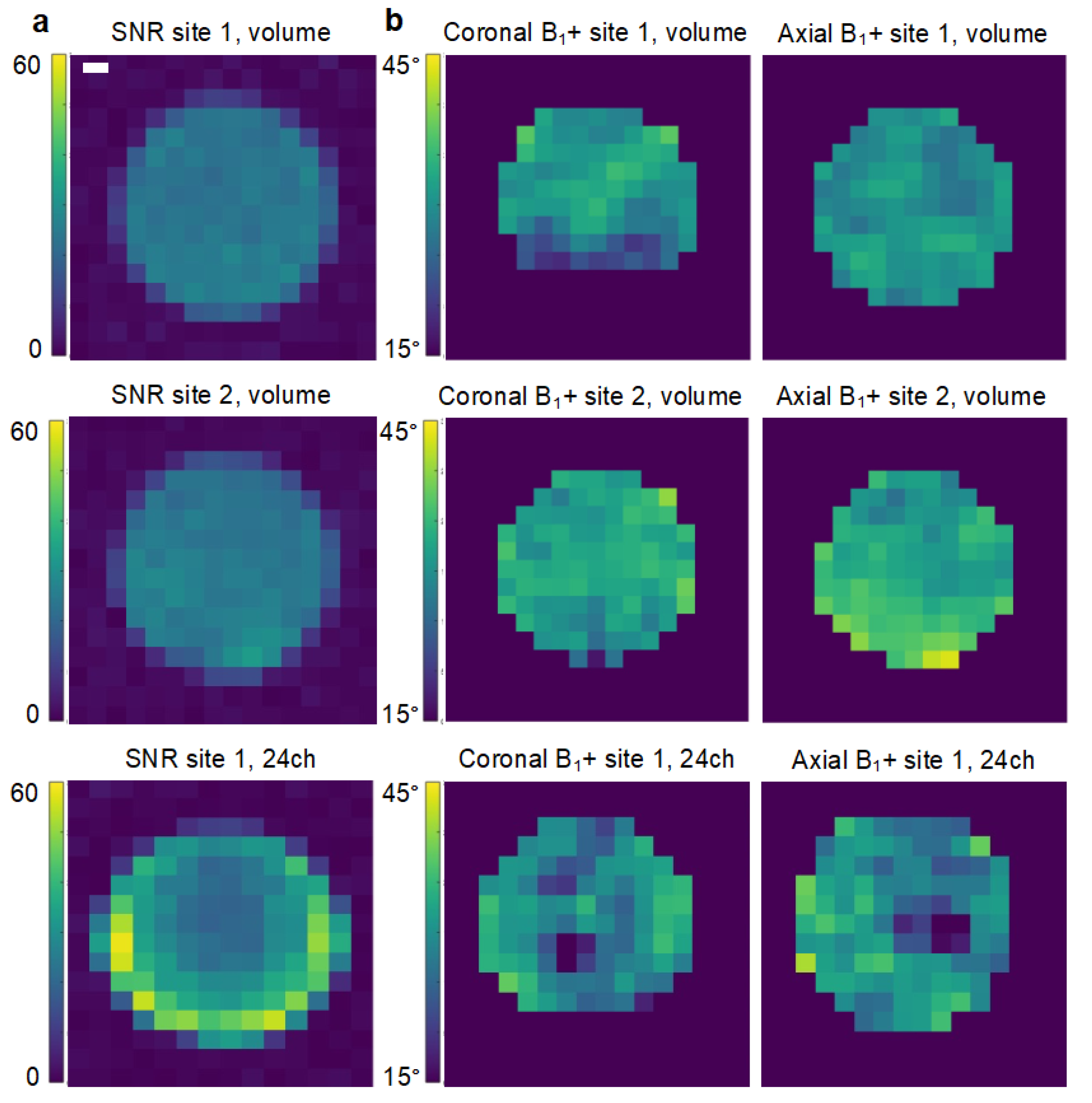
2.2. Coil Comparison
2.3. Hyperpolarized MRI
2.4. Data Reconstruction and Analysis
2.5. Statistics
3. Results
3.1. Comparability of Coils
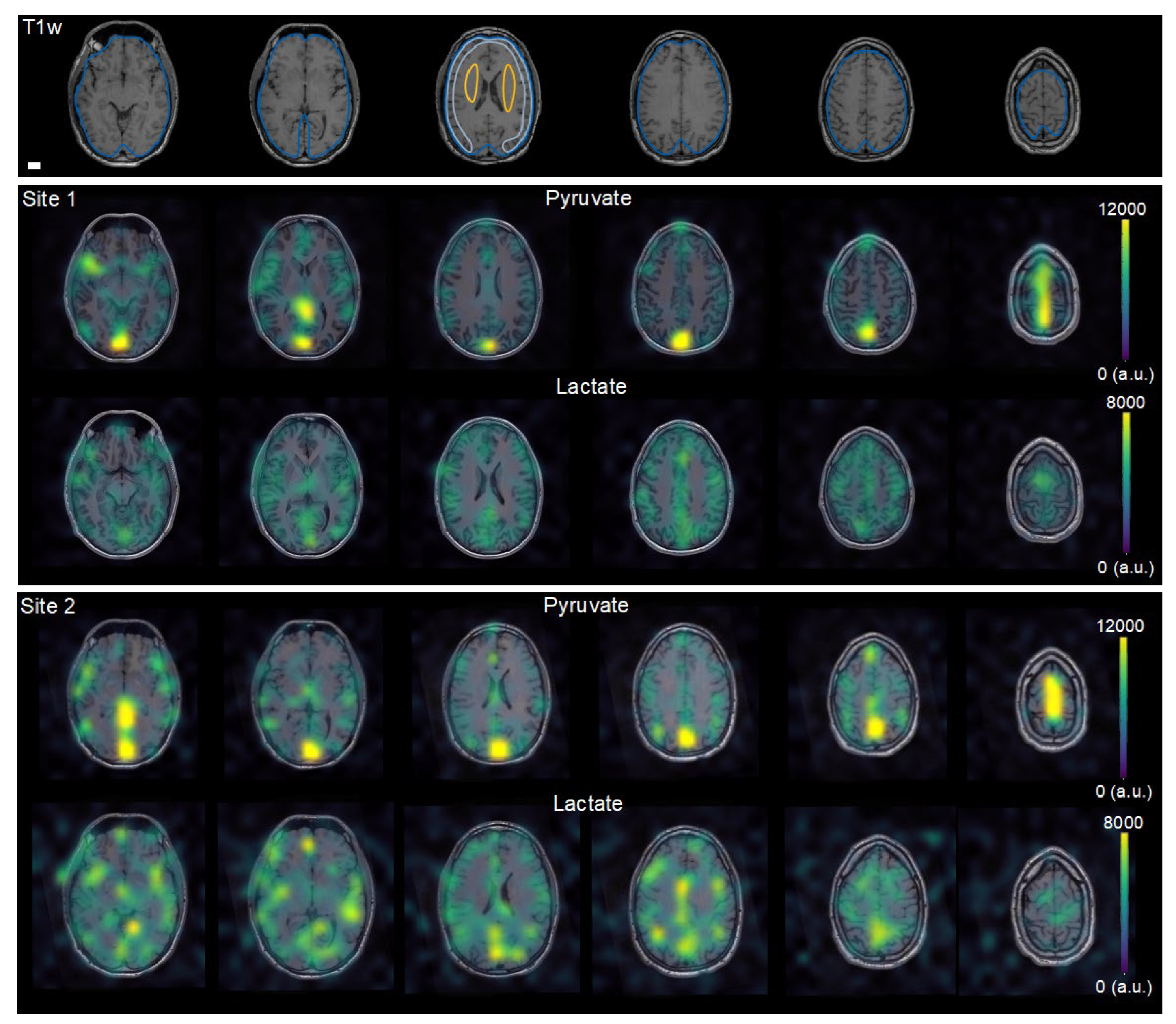
3.2. Metadata of Human Examinations
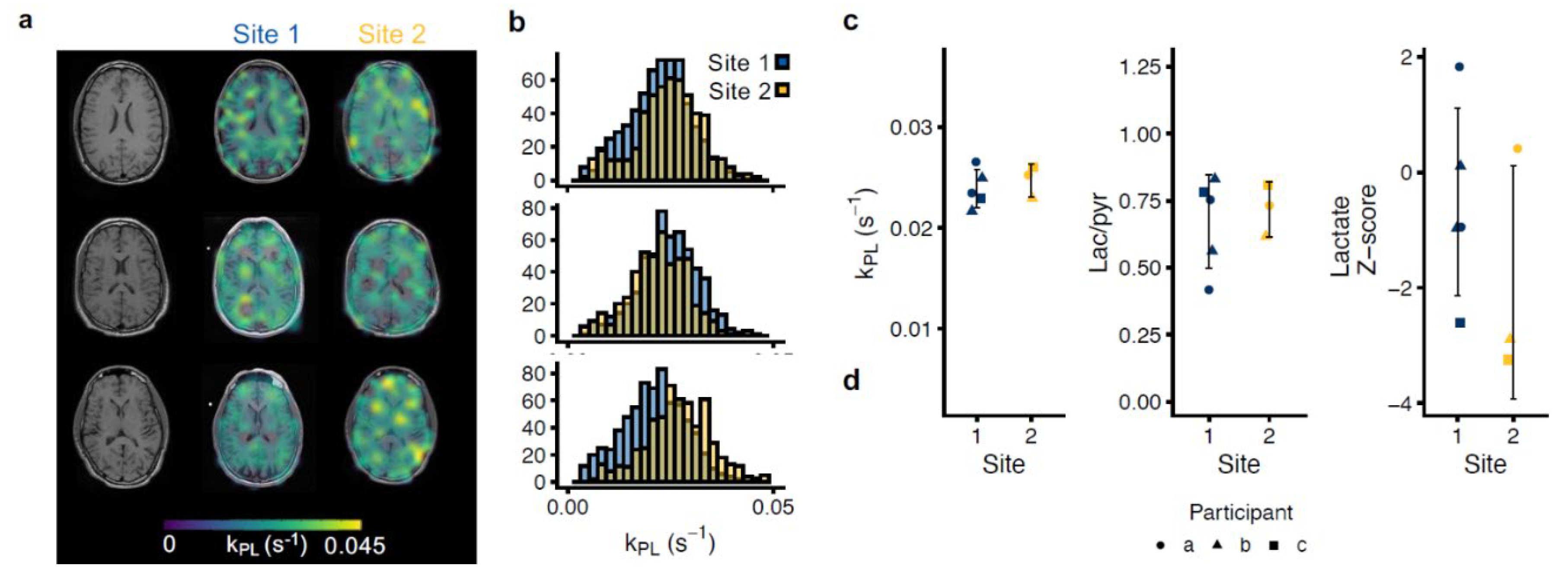
3.3. Multisite Agreement of Hyperpolarized MRI
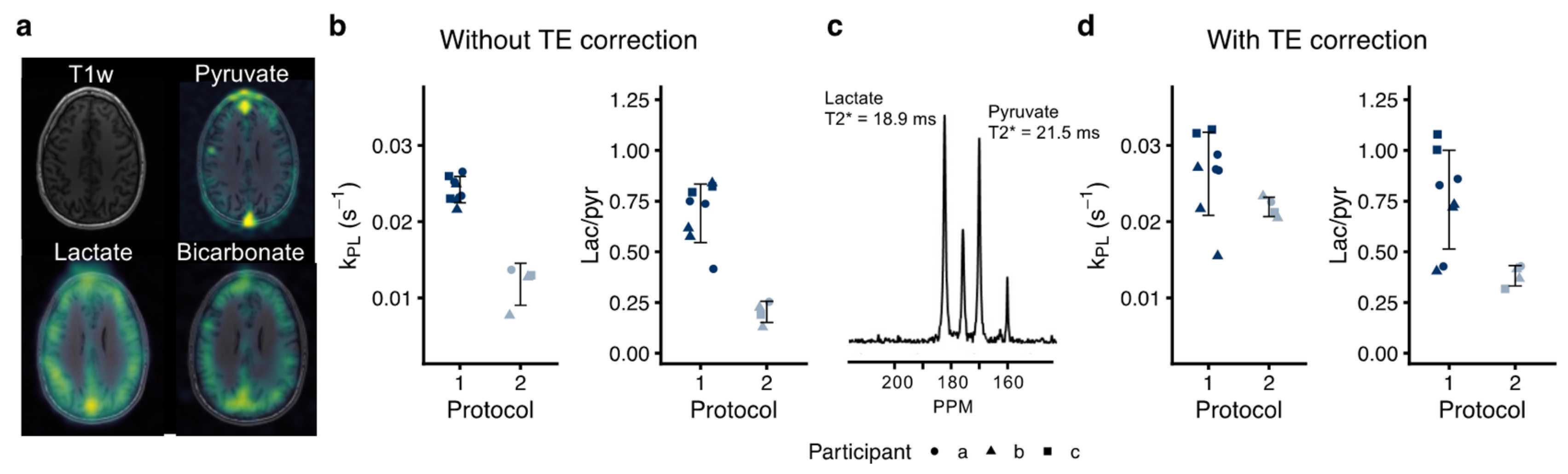
3.4. Inter-Protocol Variation of Hyperpolarized [1-13C]pyruvate MRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in Signal-to-Noise Ratio of > 10,000 Times in Liquid-State NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.J.; Ohliger, M.A.; Larson, P.E.Z.; Gordon, J.W.; Bok, R.A.; Slater, J.; Villanueva-Meyer, J.E.; Hess, C.P.; Kurhanewicz, J.; Vigneron, D.B. Hyperpolarized 13C MRI: State of the Art and Future Directions. Radiology 2019, 291, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Woitek, R.; McLean, M.A.; Ursprung, S.; Rueda, O.M.; Garcia, R.M.; Locke, M.J.; Beer, L.; Baxter, G.; Rundo, L.; Provenzano, E.; et al. Hyperpolarized Carbon-13 MRI for Early Response Assessment of Neoadjuvant Chemotherapy in Breast Cancer Patients. Cancer Res. 2021, 81, 6004–6017. [Google Scholar] [CrossRef]
- Grist, J.T.; McLean, M.A.; Riemer, F.; Schulte, R.F.; Deen, S.S.; Zaccagna, F.; Woitek, R.; Daniels, C.J.; Kaggie, J.D.; Matys, T.; et al. Quantifying Normal Human Brain Metabolism Using Hyperpolarized [1–13C]Pyruvate and Magnetic Resonance Imaging. Neuroimage 2019, 189, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Soliman, H.; Geraghty, B.J.; Chen, A.P.; Connelly, K.A.; Endre, R.; Perks, W.J.; Heyn, C.; Black, S.E.; Cunningham, C.H. Lactate Topography of the Human Brain Using Hyperpolarized 13C-MRI. Neuroimage 2020, 204, 116202. [Google Scholar] [CrossRef]
- Miloushev, V.Z.; Granlund, K.L.; Boltyanskiy, R.; Lyashchenko, S.K.; DeAngelis, L.M.; Mellinghoff, I.K.; Brennan, C.W.; Tabar, V.; Yang, T.J.; Holodny, A.I.; et al. Metabolic Imaging of the Human Brain with Hyperpolarized 13C Pyruvate Demonstrates 13C Lactate Production in Brain Tumor Patients. Cancer Res. 2018, 78, 3755–3760. [Google Scholar] [CrossRef] [Green Version]
- Park, I.; Larson, P.E.Z.; Gordon, J.W.; Carvajal, L.; Chen, H.Y.; Bok, R.; Van Criekinge, M.; Ferrone, M.; Slater, J.B.; Xu, D.; et al. Development of Methods and Feasibility of Using Hyperpolarized Carbon-13 Imaging Data for Evaluating Brain Metabolism in Patient Studies. Magn. Reson. Med. 2018, 80, 864–873. [Google Scholar] [CrossRef] [Green Version]
- Autry, A.W.; Gordon, J.W.; Chen, H.-Y.; LaFontaine, M.; Bok, R.; Van Criekinge, M.; Slater, J.B.; Carvajal, L.; Villanueva-Meyer, J.E.; Chang, S.M.; et al. Characterization of Serial Hyperpolarized 13C Metabolic Imaging in Patients with Glioma. NeuroImage Clin. 2020, 27, 102323. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Soliman, H.; Bragagnolo, N.D.; Sahgal, A.; Geraghty, B.J.; Chen, A.P.; Endre, R.; Perks, W.J.; Detsky, J.S.; Leung, E.; et al. Predicting Response to Radiotherapy of Intracranial Metastases with Hyperpolarized 13C MRI. J. Neurooncol. 2021, 152, 551–557. [Google Scholar] [CrossRef]
- Guglielmetti, C.; Chou, A.; Krukowski, K.; Najac, C.; Feng, X.; Riparip, L.K.; Rosi, S.; Chaumeil, M.M. In Vivo Metabolic Imaging of Traumatic Brain Injury. Sci. Rep. 2017, 7, 17525. [Google Scholar] [CrossRef] [Green Version]
- Guglielmetti, C.; Najac, C.; Didonna, A.; Van der Linden, A.; Ronen, S.M.; Chaumeil, M.M. Hyperpolarized 13C MR Metabolic Imaging Can Detect Neuroinflammation in Vivo in a Multiple Sclerosis Murine Model. Proc. Natl. Acad. Sci. USA 2017, 114, E6982–E6991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bøgh, N.; Olin, R.B.; Hansen, E.S.; Gordon, J.W.; Bech, S.K.; Bertelsen, L.B.; Sánchez-Heredia, J.D.; Blicher, J.U.; Østergaard, L.; Ardenkjær-Larsen, J.H.; et al. Metabolic MRI with Hyperpolarized [1-13C]Pyruvate Separates Benign Oligemia from Infarcting Penumbra in Porcine Stroke. J. Cereb. Blood Flow Metab. 2021, 41, 2916–2920. [Google Scholar] [CrossRef]
- Grist, J.T.; Miller, J.J.; Zaccagna, F.; McLean, M.A.; Riemer, F.; Matys, T.; Tyler, D.J.; Laustsen, C.; Coles, A.J.; Gallagher, F.A. Hyperpolarized 13C MRI: A Novel Approach for Probing Cerebral Metabolism in Health and Neurological Disease. J. Cereb. Blood Flow Metab. 2020, 40, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Kranzusch, R.; aus dem Siepen, F.; Wiesemann, S.; Zange, L.; Jeuthe, S.; Ferreira da Silva, T.; Kuehne, T.; Pieske, B.; Tillmanns, C.; Friedrich, M.G.; et al. Z-Score Mapping for Standardized Analysis and Reporting of Cardiovascular Magnetic Resonance Modified Look-Locker Inversion Recovery (MOLLI) T1 Data: Normal Behavior and Validation in Patients with Amyloidosis. J. Cardiovasc. Magn. Reson. 2020, 22, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grech-Sollars, M.; Hales, P.W.; Miyazaki, K.; Raschke, F.; Rodriguez, D.; Wilson, M.; Gill, S.K.; Banks, T.; Saunders, D.E.; Clayden, J.D.; et al. Multi-Centre Reproducibility of Diffusion MRI Parameters for Clinical Sequences in the Brain. NMR Biomed. 2015, 28, 468–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. [Google Scholar] [CrossRef]
- Gordon, J.W.; Chen, H.Y.; Autry, A.; Park, I.; Van Criekinge, M.; Mammoli, D.; Milshteyn, E.; Bok, R.; Xu, D.; Li, Y.; et al. Translation of Carbon-13 EPI for Hyperpolarized MR Molecular Imaging of Prostate and Brain Cancer Patients. Magn. Reson. Med. 2019, 81, 2702–2709. [Google Scholar] [CrossRef]
- Gordon, J.W.; Autry, A.W.; Tang, S.; Graham, J.Y.; Bok, R.A.; Zhu, X.; Villanueva-Meyer, J.E.; Li, Y.; Ohilger, M.A.; Abraham, M.R.; et al. A Variable Resolution Approach for Improved Acquisition of Hyperpolarized 13C Metabolic MRI. Magn. Reson. Med. 2020, 84, 2943–2952. [Google Scholar] [CrossRef]
- Grist, J.T.; Hansen, E.S.S.; Sánchez-Heredia, J.D.; McLean, M.A.; Tougaard, R.; Riemer, F.; Schulte, R.F.; Kaggie, J.D.; Ardenkjaer-Larsen, J.H.; Laustsen, C.; et al. Creating a Clinical Platform for Carbon-13 Studies Using the Sodium-23 and Proton Resonances. Magn. Reson. Med. 2020, 84, 1817–1827. [Google Scholar] [CrossRef] [Green Version]
- Qin, Q. Point Spread Functions of the T2 Decay in K-Space Trajectories with Long Echo Train. Magn. Reson. Imaging 2012, 30, 1134–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Zhu, X.; Ohliger, M.A.; Tang, S.; Cao, P.; Carvajal, L.; Autry, A.W.; Li, Y.; Kurhanewicz, J.; Chang, S.; et al. Coil Combination Methods for Multi-Channel Hyperpolarized 13C Imaging Data from Human Studies. J. Magn. Reson. 2019, 301, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Mammoli, D.; Gordon, J.; Autry, A.; Larson, P.E.Z.; Li, Y.; Chen, H.Y.; Chung, B.; Shin, P.; Van Criekinge, M.; Carvajal, L.; et al. Kinetic Modeling of Hyperpolarized Carbon-13 Pyruvate Metabolism in the Human Brain. IEEE Trans. Med. Imaging 2019, 39, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granlund, K.L.; Tee, S.S.; Vargas, H.A.; Lyashchenko, S.K.; Reznik, E.; Fine, S.; Laudone, V.; Eastham, J.A.; Touijer, K.A.; Reuter, V.E.; et al. Hyperpolarized MRI of Human Prostate Cancer Reveals Increased Lactate with Tumor Grade Driven by Monocarboxylate Transporter 1. Cell Metab. 2019, 31, 105–114. [Google Scholar] [CrossRef]
- Tang, S.; Meng, M.V.; Slater, J.B.; Gordon, J.W.; Vigneron, D.B.; Stohr, B.A.; Larson, P.E.Z.; Wang, Z.J. Metabolic Imaging with Hyperpolarized 13C Pyruvate Magnetic Resonance Imaging in Patients with Renal Tumors-Initial Experience. Cancer 2021, 127, 2693–2704. [Google Scholar] [CrossRef]
- Proesmans, S.; Raedt, R.; Germonpré, C.; Christiaen, E.; Descamps, B.; Boon, P.; De Herdt, V.; Vanhove, C. Voxel-Based Analysis of [18F]-FDG Brain PET in Rats Using Data-Driven Normalization. Front. Med. 2021, 8, 744157. [Google Scholar] [CrossRef]
- Fahey, F.H.; Kinahan, P.E.; Doot, R.K.; Kocak, M.; Thurston, H.; Poussaint, T.Y. Variability in PET Quantitation within a Multicenter Consortium. Med. Phys. 2010, 37, 3660–3666. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Gordon, J.W.; Bok, R.A.; Cao, P.; von Morze, C.; van Criekinge, M.; Milshteyn, E.; Carvajal, L.; Hurd, R.E.; Kurhanewicz, J.; et al. Pulse Sequence Considerations for Quantification of Pyruvate-to-Lactate Conversion KPL in Hyperpolarized 13C Imaging. NMR Biomed. 2019, 32, e4052. [Google Scholar] [CrossRef]
- Ma, J.; Chen, J.; Reed, G.D.; Hackett, E.P.; Harrison, C.E.; Ratnakar, J.; Schulte, R.F.; Zaha, V.G.; Malloy, C.R.; Park, J.M. Cardiac Measurement of Hyperpolarized 13C Metabolites Using Metabolite-Selective Multi-Echo Spiral Imaging. Magn. Med. 2021, 86, 1494–1504. [Google Scholar] [CrossRef]
- Hill, D.K.; Orton, M.R.; Mariotti, E.; Boult, J.K.R.; Panek, R.; Jafar, M.; Parkes, H.G.; Jamin, Y.; Miniotis, M.F.; Al-Saffar, N.M.S.; et al. Model Free Approach to Kinetic Analysis of Real-Time Hyperpolarized 13C Magnetic Resonance Spectroscopy Data. PLoS ONE 2013, 8, e71996. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.B.; Sánchez-Heredia, J.D.; Bøgh, N.; Hansen, E.S.S.; Laustsen, C.; Hanson, L.G.; Ardenkjaer-Larsen, J.H. Coil Profile Estimation Strategies for Parallel Imaging with Hyperpolarized 13C MRI. Magn. Reson. Med. 2019, 82, 2104–2117. [Google Scholar] [CrossRef] [PubMed]
- Bonett, D.G. Sample Size Requirements for Estimating Intraclass Correlations with Desired Precision. Stat. Med. 2002, 21, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
| Protocol | Site | Excitation | Flip-Angles, Pyruvate/ Metabolites | Read Out | TR/TE (ms) | Voxel Size †, Pyruvate/ Metabolites (mm3) | Temporal Resolution (s) | Coil | N |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 + 2 | Spectral-spatial | 10°/40° * | EPI | 62.5/ 21.7 | 15 × 15 × 15/ 15 × 15 × 15 | 3 | Birdcage volume | 8 |
| 2 | 1 | Spectral-spatial | 20°/30° | EPI | 125/ 30.7 | 7.5 × 7.5 × 15/ 15 × 15 × 15 | 3 | Birdcage transmit + 24-ch receive | 4 |
| Protocol 1 | ||||||
|---|---|---|---|---|---|---|
| Site 1 | Site 2 | |||||
| kPL | Lactate/ Pyruvate | Lactate Z-Score | kPL | Lactate/ Pyruvate | Lactate Z-Score | |
| Whole brain | 0.024 ± 0.0018 | 0.67 ± 0.17 | - | 0.024 ± 0.0021 | 0.63 ± 0.2 | - |
| Cortex | 0.025 ± 0.0019 | 0.75 ± 0.16 | −0.5 ± 1.63 | 0.026 ± 0.0013 | 0.73 ± 0.32 | −1.66 ± 1.72 |
| Deep white matter | 0.029 ± 0.0033 | 0.96 ± 0.22 | 1.56 ± 1.5 | 0.028 ± 0.0016 | 0.96 ± 0.23 | 0.95 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bøgh, N.; Gordon, J.W.; Hansen, E.S.S.; Bok, R.A.; Blicher, J.U.; Hu, J.Y.; Larson, P.E.Z.; Vigneron, D.B.; Laustsen, C. Initial Experience on Hyperpolarized [1-13C]Pyruvate MRI Multicenter Reproducibility—Are Multicenter Trials Feasible? Tomography 2022, 8, 585-595. https://doi.org/10.3390/tomography8020048
Bøgh N, Gordon JW, Hansen ESS, Bok RA, Blicher JU, Hu JY, Larson PEZ, Vigneron DB, Laustsen C. Initial Experience on Hyperpolarized [1-13C]Pyruvate MRI Multicenter Reproducibility—Are Multicenter Trials Feasible? Tomography. 2022; 8(2):585-595. https://doi.org/10.3390/tomography8020048
Chicago/Turabian StyleBøgh, Nikolaj, Jeremy W. Gordon, Esben S. S. Hansen, Robert A. Bok, Jakob U. Blicher, Jasmine Y. Hu, Peder E. Z. Larson, Daniel B. Vigneron, and Christoffer Laustsen. 2022. "Initial Experience on Hyperpolarized [1-13C]Pyruvate MRI Multicenter Reproducibility—Are Multicenter Trials Feasible?" Tomography 8, no. 2: 585-595. https://doi.org/10.3390/tomography8020048
APA StyleBøgh, N., Gordon, J. W., Hansen, E. S. S., Bok, R. A., Blicher, J. U., Hu, J. Y., Larson, P. E. Z., Vigneron, D. B., & Laustsen, C. (2022). Initial Experience on Hyperpolarized [1-13C]Pyruvate MRI Multicenter Reproducibility—Are Multicenter Trials Feasible? Tomography, 8(2), 585-595. https://doi.org/10.3390/tomography8020048






