The Effect of Iron Oxide Insertion on the In Vitro Bioactivity, and Antibacterial Properties of the 45S5 Bioactive Glass
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Structural Characterization
2.3. Electrical Characterization
2.4. Bioactivity
2.5. Antibacterial Activity
3. Results and Discussion
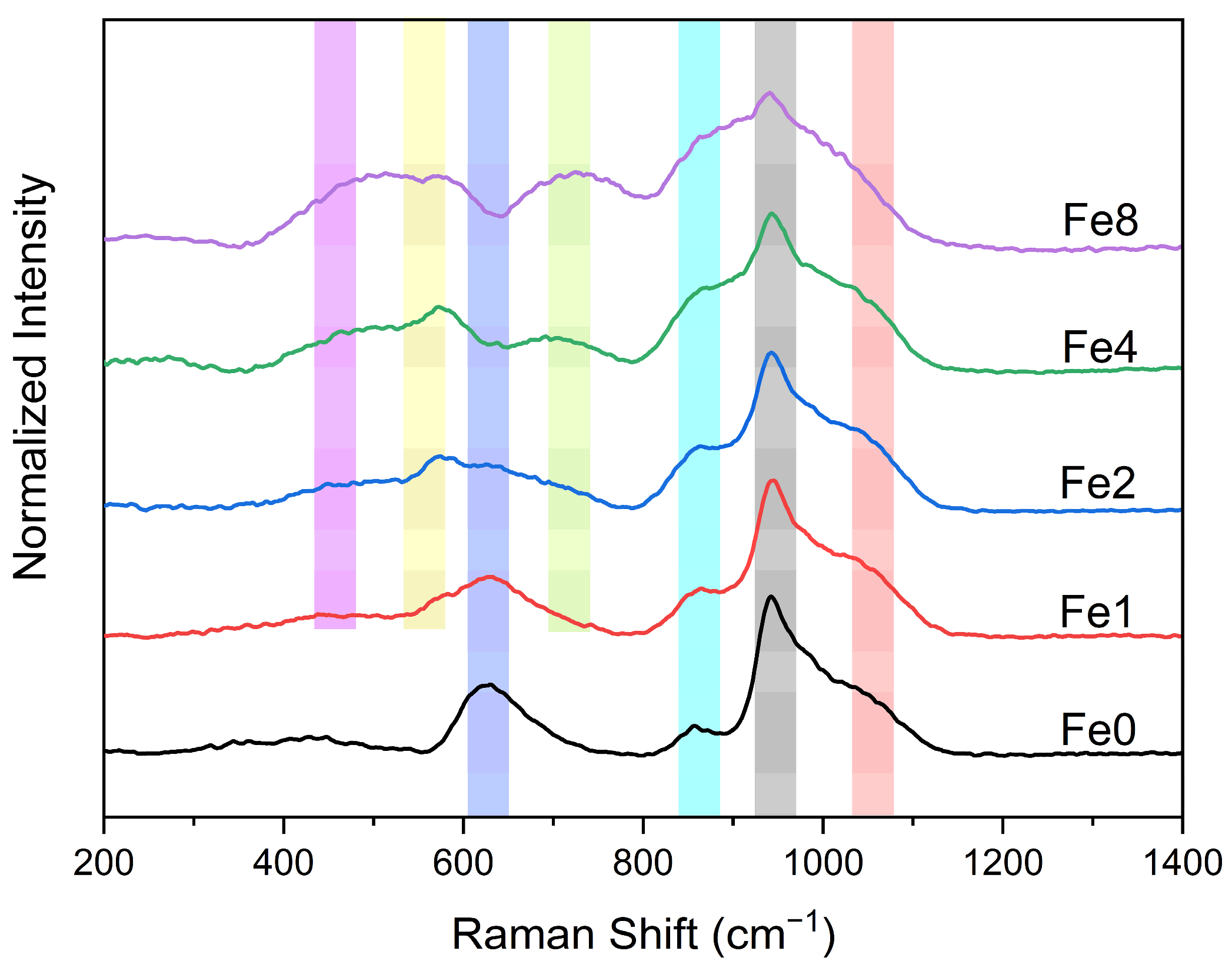
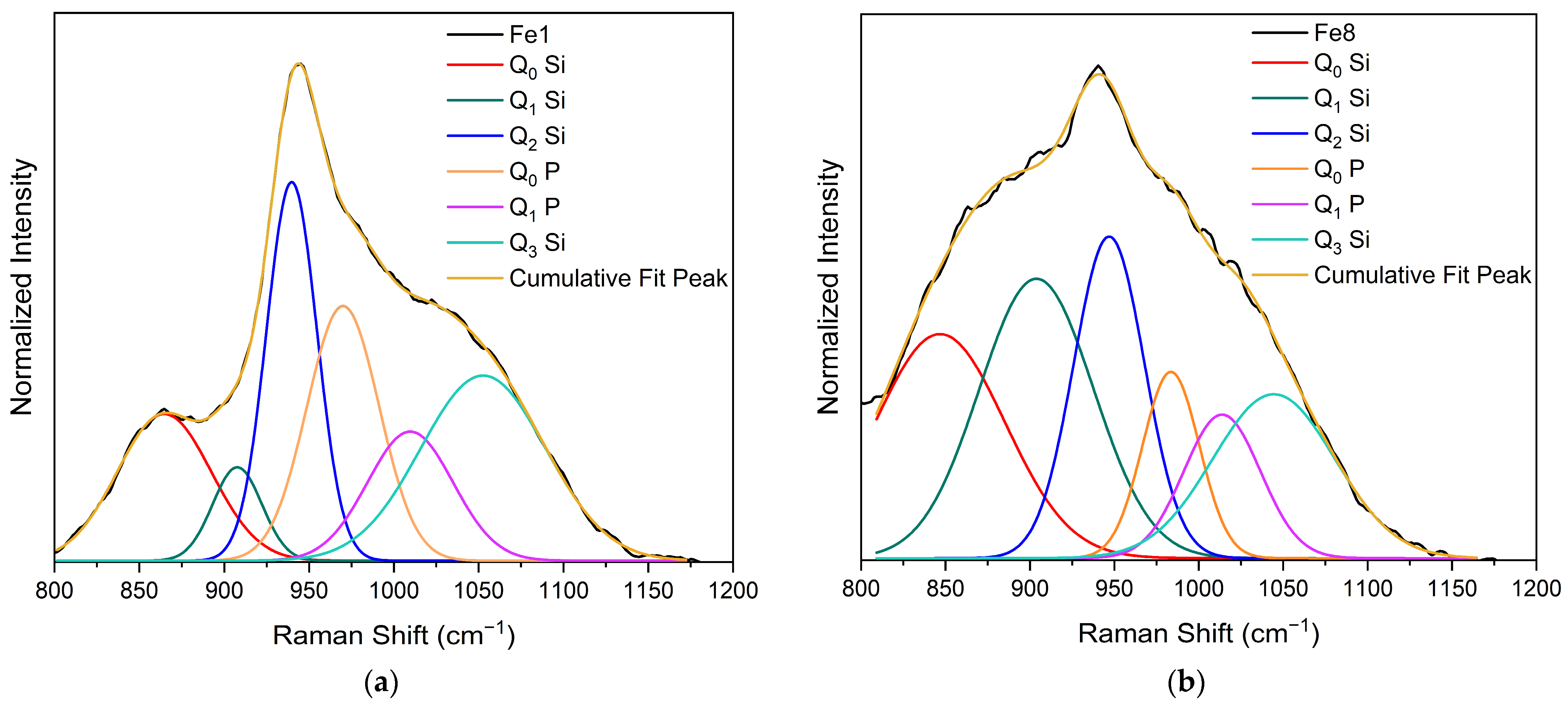
3.1. Structural Characterization
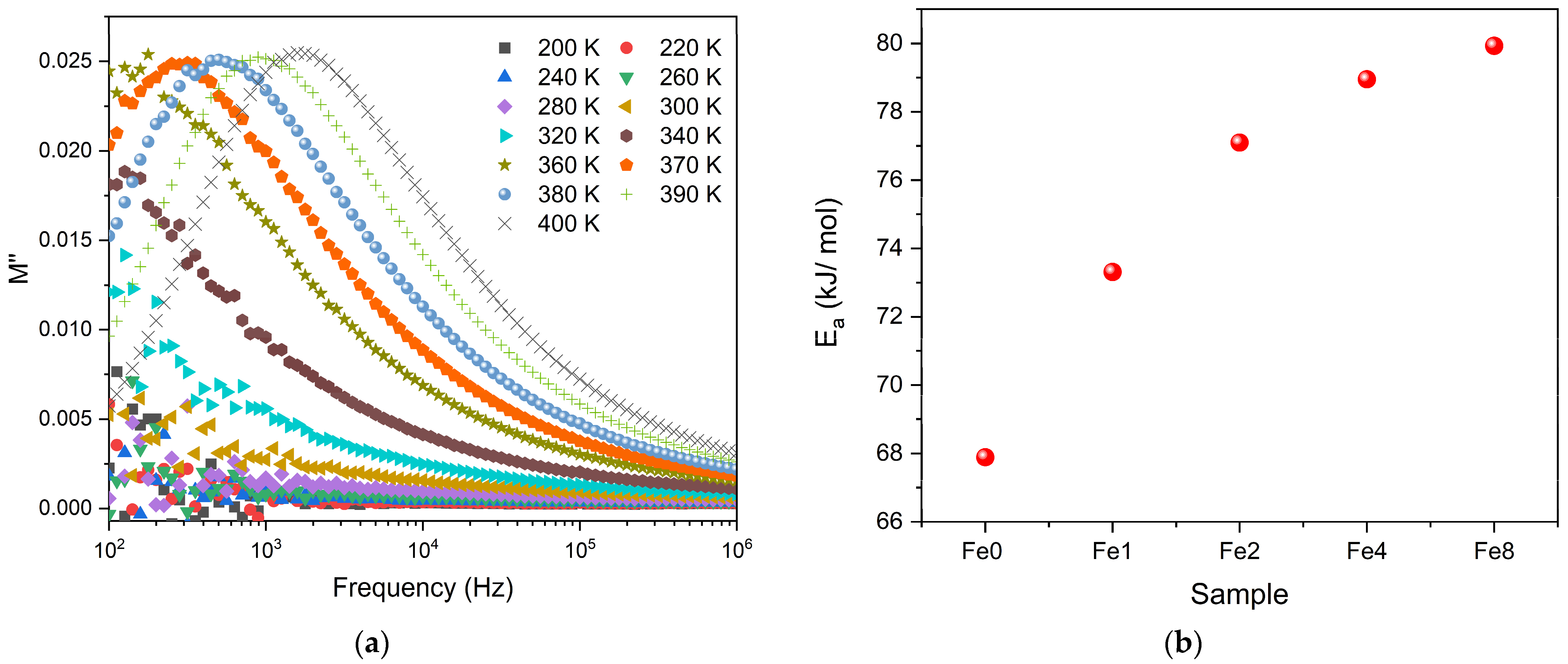
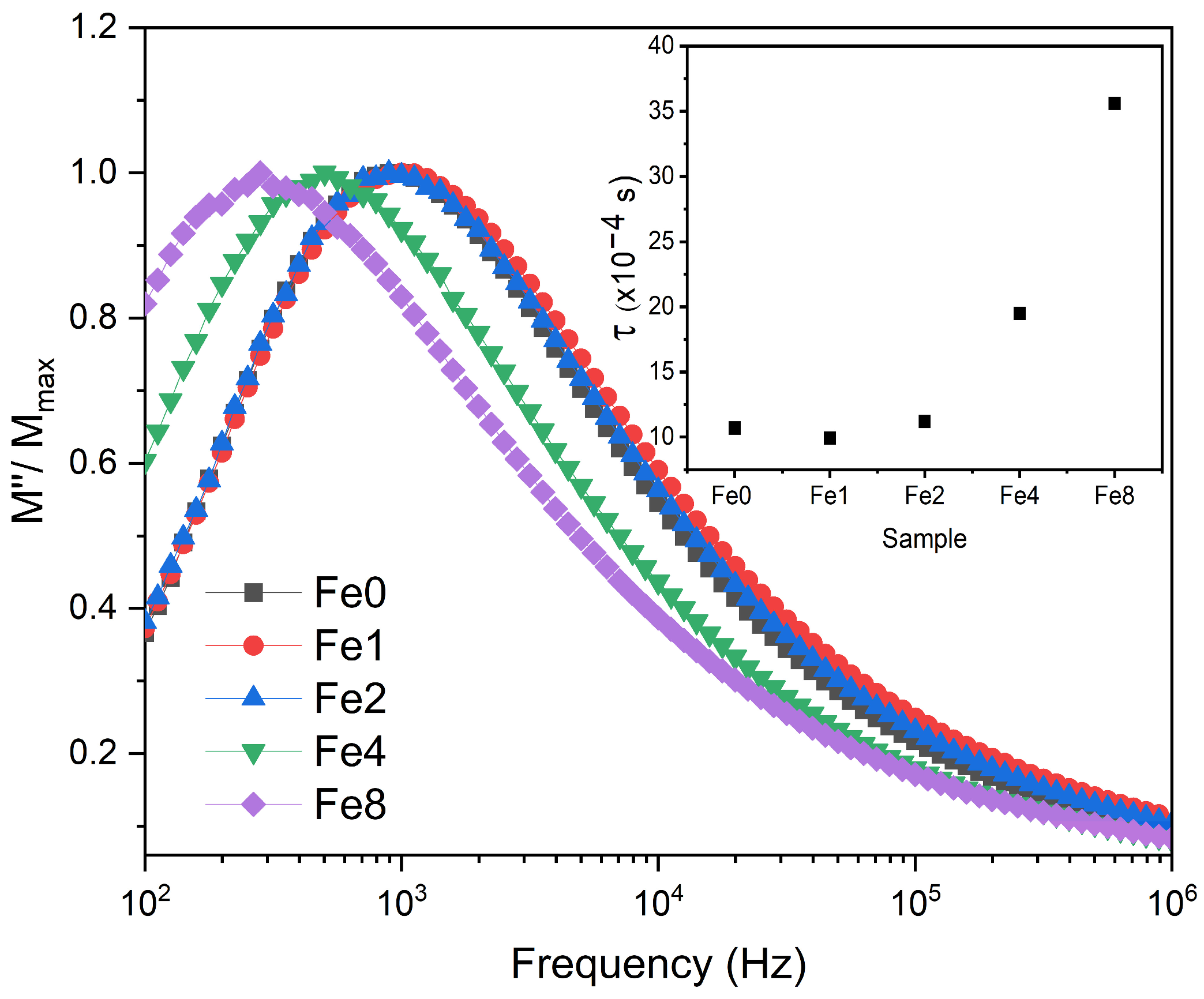
3.2. Electrical Characterization
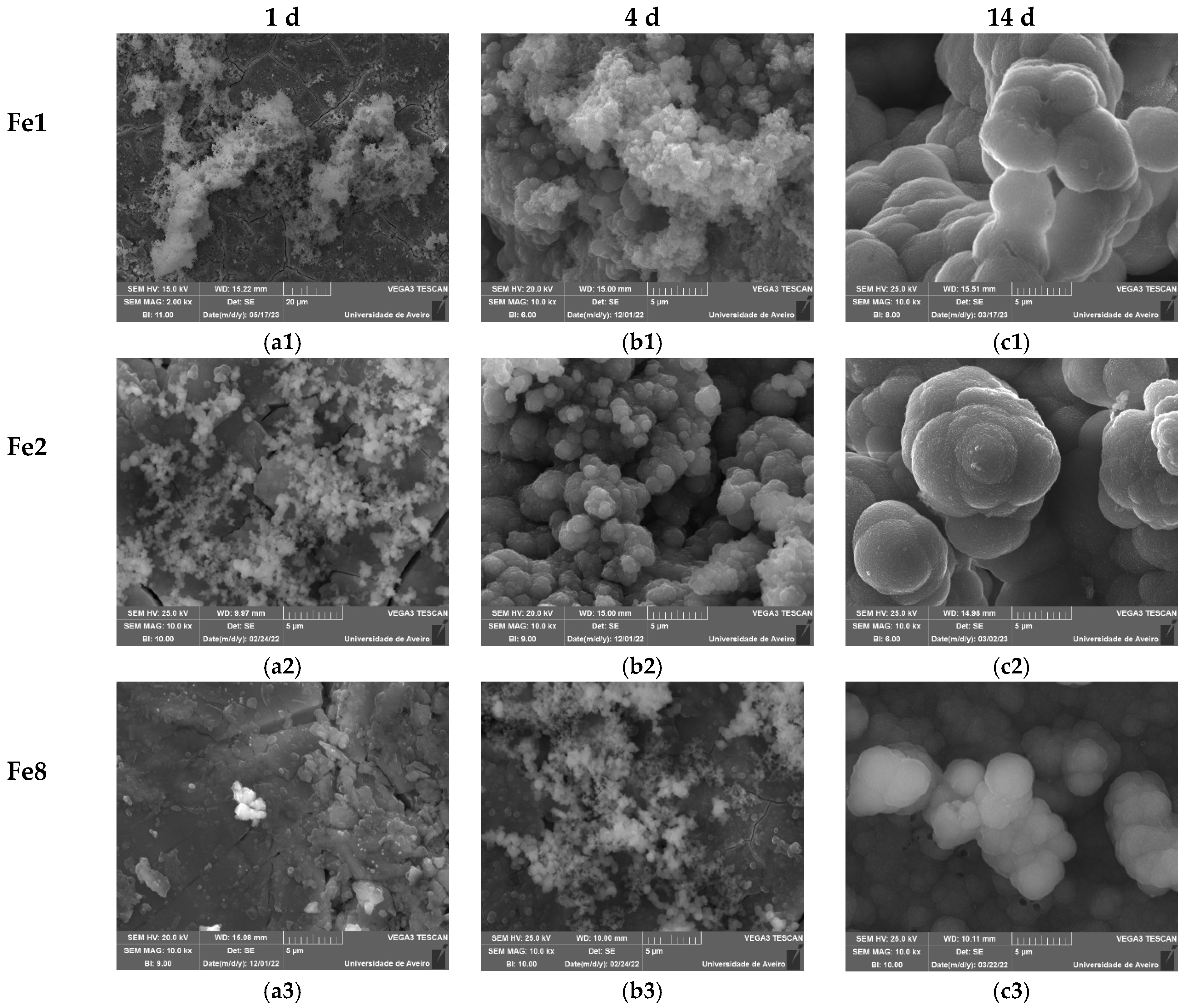
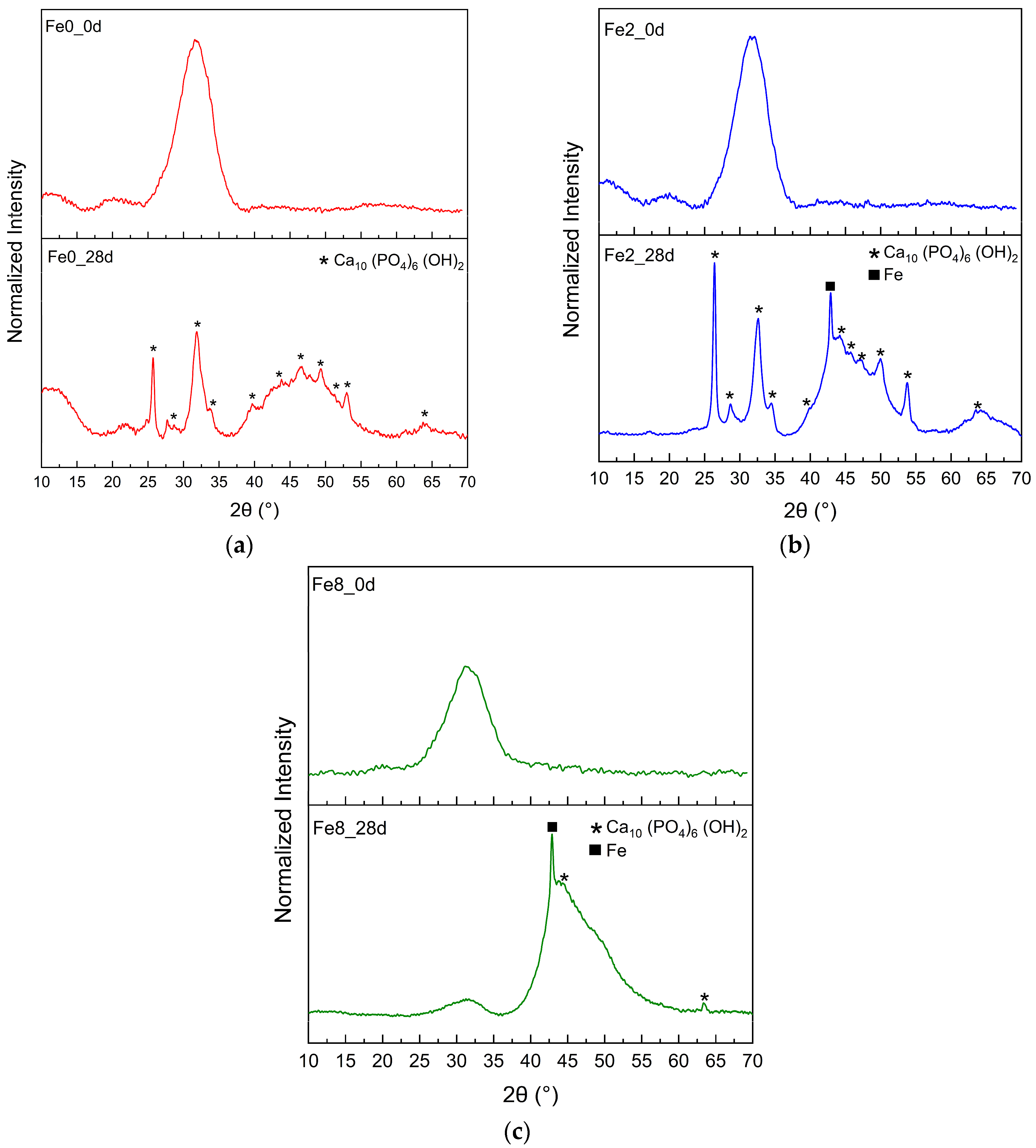
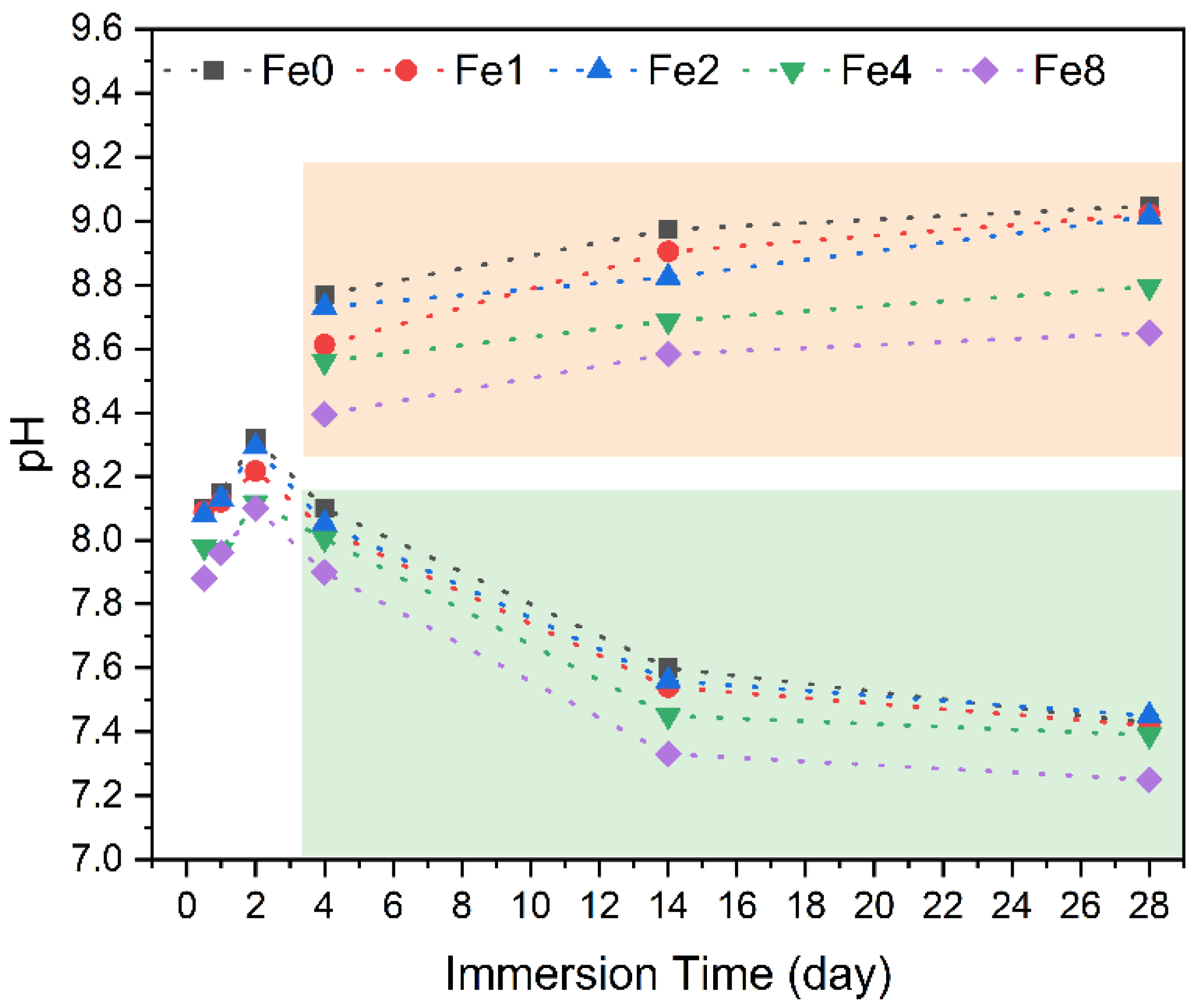
3.3. Bioactivity
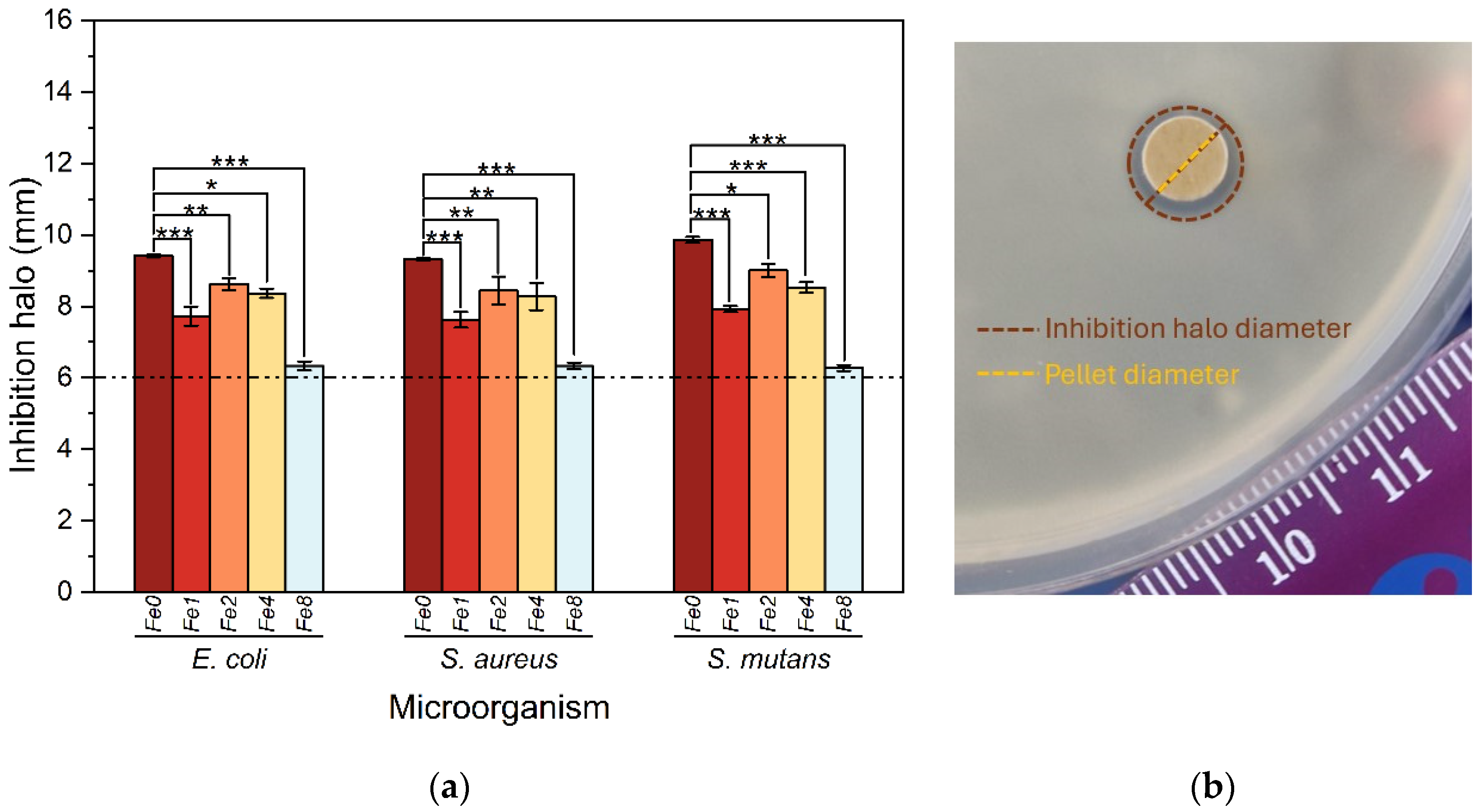
3.4. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; Chen, X.; Kong, L.; Liu, P. Mechanical and Biological Properties of Titanium and Its Alloys for Oral Implant with Preparation Techniques: A Review. Materials 2023, 16, 6860. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Showva, N.-N.; Ahmed, M.; Rashid, A.B.; Sadique, S.E.; El-Bialy, T.; Xu, H. Titanium and Titanium Alloys in Dentistry: Current Trends, Recent Developments, and Future Prospects. Heliyon 2022, 8, e11300. [Google Scholar] [CrossRef]
- Cruz, M.B.; Silva, N.; Marques, J.F.; Mata, A.; Silva, F.S.; Caramês, J. Biomimetic Implant Surfaces and Their Role in Biological Integration—A Concise Review. Biomimetics 2022, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Gavinho, S.R.; Pádua, A.S.; Lança, M.D.C.; Borges, J.P.; Silva, J.C.; Sá-Nogueira, I.; Jakka, S.K.; Graça, M.P.F. Extensive Investigation on the Effect of Niobium Insertion on the Physical and Biological Properties of 45S5 Bioactive Glass for Dental Implant. Int. J. Mol. Sci. 2023, 24, 5244. [Google Scholar] [CrossRef] [PubMed]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Pádua, A.S.; Gavinho, S.R.; Vieira, T.; Hammami, I.; Silva, J.C.; Borges, J.P.; Graça, M.P.F. In Vitro Characterization of Doped Bioglass 45S5/HAp Coatings Obtained by CoBlastTM Deposition. Coatings 2023, 13, 1775. [Google Scholar] [CrossRef]
- Kulkarni Aranya, A.; Pushalkar, S.; Zhao, M.; LeGeros, R.Z.; Zhang, Y.; Saxena, D. Antibacterial and Bioactive Coatings on Titanium Implant Surfaces. J Biomed. Mater. Res 2017, 105, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.-W.; Zhong, Y.; Wang, L. Multi-Scale Surface Treatments of Titanium Implants for Rapid Osseointegration: A Review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Joy-anne, N.O.; Su, Y.; Lu, X.; Kuo, P.-H.; Du, J.; Zhu, D. Bioactive Glass Coatings on Metallic Implants for Biomedical Applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar]
- Bargavi, P.; Chitra, S.; Durgalakshmi, D.; Radha, G.; Balakumar, S. Zirconia Reinforced Bio-Active Glass Coating by Spray Pyrolysis: Structure, Surface Topography, in-Vitro Biological Evaluation and Antibacterial Activities. Mater. Today Commun. 2020, 25, 101253. [Google Scholar] [CrossRef]
- Hench, L.L. The Story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Hench, L.L. An Introduction to Bioceramics, 2nd ed.; World Scientific Publishing: Singapore, 2013; pp. 1–600. [Google Scholar] [CrossRef]
- Hench, L.L.; Greenspan, D. Interactions between Bioactive Glass and Collagen: A Review and New Perspectives. J. Aust. Ceram. Soc. 2013, 49, 1–40. [Google Scholar]
- Mačković, M.; Hoppe, A.; Detsch, R.; Mohn, D.; Stark, W.J.; Spiecker, E.; Boccaccini, A.R. Bioactive Glass (Type 45S5) Nanoparticles: In Vitro Reactivity on Nanoscale and Biocompatibility. J. Nanopart. Res. 2012, 14, 966. [Google Scholar] [CrossRef]
- Kumar, A.; Murugavel, S.; Aditya, A.; Boccaccini, A.R. Mesoporous 45S5 Bioactive Glass: Synthesis, in Vitro Dissolution and Biomineralization Behavior. J. Mater. Chem. B 2017, 5, 8786–8798. [Google Scholar] [CrossRef] [PubMed]
- Silver, I.A.; Deas, J.; Erecińska, M. Interactions of Bioactive Glasses with Osteoblasts in Vitro: Effects of 45S5 Bioglass®, and 58S and 77S Bioactive Glasses on Metabolism, Intracellular Ion Concentrations and Cell Viability. Biomaterials 2001, 22, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Allan, I.; Newman, H.; Wilson, M. Antibacterial Activity of Particulate Bioglass® against Supra-and Subgingival Bacteria. Biomaterials 2001, 22, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chang, J.; Liu, M.; Ning, C. Study on Antibacterial Effect of 45S5 Bioglass®. J. Mater. Sci. Mater. Med. 2009, 20, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials 2018, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Gavinho, S.R.; Jakka, S.K.; Valente, M.A.; Graça, M.P.F.; Pádua, A.S.; Silva, J.C.; Sá-Nogueira, I.; Borges, J.P. Antibacterial Biomaterial Based on Bioglass Modified with Copper for Implants Coating. J. Funct. Biomater. 2023, 14, 369. [Google Scholar] [CrossRef] [PubMed]
- Baino, F. Copper-Doped Ordered Mesoporous Bioactive Glass: A Promising Multifunctional Platform for Bone Tissue Engineering. Bioengineering 2020, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, A.; Balossier, G.; Laurent-Maquin, D.; Pina, S.; Rebelo, A.H.S.; Faure, J.; Ferreira, J.M.F. An in Vitro Biological and Anti-Bacterial Study on a Sol-Gel Derived Silver-Incorporated Bioglass System. Dent. Mater. 2008, 24, 1343–1351. [Google Scholar] [CrossRef]
- Hammami, I.; Gavinho, S.R.; Pádua, A.S.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Valente, M.A.; Graça, M.P.F. Bioactive Glass Modified with Zirconium Incorporation for Dental Implant Applications: Fabrication, Structural, Electrical, and Biological Analysis. Int. J. Mol. Sci. 2023, 24, 10571. [Google Scholar] [CrossRef]
- Cacciotti, I. Bivalent Cationic Ions Doped Bioactive Glasses: The Influence of Magnesium, Zinc, Strontium and Copper on the Physical and Biological Properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Tabia, Z.; Mabrouk, K.E.; Bricha, M.; Nouneh, K. Mesoporous Bioactive Glass Nanoparticles Doped with Magnesium: Drug Delivery and Acellular in Vitro Bioactivity. RSC Adv. 2019, 9, 12232–12246. [Google Scholar] [CrossRef]
- Teerakanok, S.; Zhao, M.; Giordano, R.; Fan, Y. Interaction of Doped Magnesium, Zinc and Fluoride Ions on Hydroxyapatite Crystals Grown on Etched Human Enamel. J. Cryst. Growth 2021, 571, 126262. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Cai, M.; Fu, T.; Yin, D.; Peng, H.; Liao, S.; Du, Y.; Kong, J.; Ni, J.; Yin, X. Fe-Based Metal Organic Frameworks (Fe-MOFs) for Bio-Related Applications. Pharmaceutics 2023, 15, 1599. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.; Ahmad, R.; Tanweer, M.S.; Azam, I. Magnetic Iron Oxide Nanoparticles as a Tool for the Advancement of Biomedical and Environmental Application: A Review. Biomed. Mater. Devices 2023, 2, 139–157. [Google Scholar] [CrossRef]
- Nehra, P.; Chauhan, R.P.; Garg, N.; Verma, K. Antibacterial and Antifungal Activity of Chitosan Coated Iron Oxide Nanoparticles. Br. J. Biomed. Sci. 2017, 75, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Nakamura, S.; Moriyoshi, Y.; Yamashita, K. Electrical Polarization of Bioactive Glass and Assessment of Their in Vitro Apatite Deposition. J. Biomed. Mater. Res. Part A Biomater. 2003, 67, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Nakamura, S.; Yamashita, K. Interpretation of Electrical Polarization and Depolarization Mechanisms of Bioactive Glasses in Relation to Ionic Migration. Biomaterials 2004, 25, 5163–5169. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Nakamura, S.; Sekijima, Y.; Yamashita, K. Control of Surface Reactions of Bioactive Glass by Electrical Polarization. J. Ceram. Soc. Jpn. Suppl. 2004, 112, S822–S825. [Google Scholar] [CrossRef]
- Singh, A.; Singh, P.; Dubey, A.K. Effect of Incorporation of Piezoelectric Phases on Antibacterial and Cellular Response of Borate Bioactive Glass. Open Ceram. 2022, 9, 100234. [Google Scholar] [CrossRef]
- Graça, M.P.F.; da Silva, M.F.; Sombra, A.S.B.; Valente, M.A. Electric and Dielectric Properties of a SiO2–Na2O–Nb2O5 Glass Subject to a Controlled Heat-Treatment Process. Phys. B Condens. Matter 2007, 396, 62–69. [Google Scholar] [CrossRef]
- Hammami, I.; Sales, A.M.J.; Benhamou, K.; Arous, M.; Costa, L.C.; da Cruz, J.A.; Kaddami, H. Dielectric Response and Molecular Dynamics of Nanocomposites Based on TEMPO-Oxidized Cellulose Nanofibrils and Polyvinyl Acetate. Therm. Sci. Eng. Prog. 2022, 34, 101428. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- El-Mallawany, R.A. Tellurite Glasses Handbook: Physical Properties and Data; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Ashok, J.; Purnachand, N.; Suresh Kumar, J.; Srinivasa Reddy, M.; Suresh, B.; Graça, M.P.F.; Veeraiah, N. Studies on Dielectric Dispersion, Relaxation Kinetics and a.c. Conductivity of Na2OCuOSiO2 Glasses Mixed with Bi2O3-Influence of Redox Behavior of Copper Ions. J. Alloys Compd. 2017, 696, 1260–1268. [Google Scholar] [CrossRef]
- Hammami, I.; Gavinho, S.R.; Pádua, A.S.; Graça, M.P.F.; Silva, J.C. Synthesis and Characterization of Iron Containing Bioactive Glass for Implants. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022; pp. 1–4. [Google Scholar]
- Macdonald, J.R. Emphasizing Solid Materials and Systems. In Impedance Spectroscopy; John Wiley Sons Inc.: New York, NY, USA, 1987. [Google Scholar]
- Kumari, K.; Prasad, A.; Kumari, K.; Prasad, K. Dielectric, Impedance/Modulus and Conductivity Studies on [Bi0.5(Na1−xKx)0.5]0.94Ba0.06TiO3, (0.16 ≤ x ≤ 0.20) Lead-Free Ceramics. Am. J. Mater. Sci. 2016, 6, 1–18. [Google Scholar] [CrossRef]
- ISO 23317:2012; Implants for Surgery: In Vitro Evaluation for Apatite-Forming Ability of Implant Material. ISO Copyright Office: Geneva, Switzerland, 2012.
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural Study of Sol–Gel Silicate Glasses by IR and Raman Spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Aguiar, H.; Solla, E.L.; Serra, J.; González, P.; León, B.; Almeida, N.; Cachinho, S.; Davim, E.J.C.; Correia, R.; Oliveira, J.M. Orthophosphate Nanostructures in SiO2–P2O5–CaO–Na2O–MgO Bioactive Glasses. J. Non-Cryst. Solids 2008, 354, 4075–4080. [Google Scholar] [CrossRef]
- Lopes, J.H.; Magalhães, A.; Mazali, I.O.; Bertran, C.A. Effect of Niobium Oxide on the Structure and Properties of Melt-Derived Bioactive Glasses. J. Am. Ceram. Soc. 2014, 97, 3843–3852. [Google Scholar] [CrossRef]
- Li, Y.-S.; Church, J.S.; Woodhead, A.L. Infrared and Raman Spectroscopic Studies on Iron Oxide Magnetic Nano-Particles and Their Surface Modifications. J. Magn. Magn. Mater. 2012, 324, 1543–1550. [Google Scholar] [CrossRef]
- Nayak, M.T.; Desa, J.A.E. Roles of Iron and Lithium in Silicate Glasses by Raman Spectroscopy. J Raman Spectrosc. 2018, 49, 1507–1513. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, N.; Li, X.; Li, G.; Zhang, K.; Jing, A.; Li, J.; Tang, H. Porous Magnetic Fe3O4/Bioactive Glass–Ceramic (CaO-SiO2-P2O5-MgO) Scaffold with Enhanced Self-Heating Ability for Hyperthermia Treatment of Bone Tumor—An in Vitro Study. J. Aust. Ceram. Soc. 2022, 58, 1729–1745. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared- and Raman-Active Phonons of Magnetite, Maghemite, and Hematite: A Computer Simulation and Spectroscopic Study. J. Phys. Chem. B 2005, 109, 4965–4968. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Graça, M.P.F.; Gavinho, S.R.; Jakka, S.K.; Borges, J.P.; Silva, J.C.; Costa, L.C. Exploring the Impact of Copper Oxide Substitution on Structure, Morphology, Bioactivity, and Electrical Properties of 45S5 Bioglass®. Biomimetics 2024, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Zagrajczuk, B.; Jelen, P.; Olejniczak, Z.; Cholewa-Kowalska, K. Structural Variations of Bioactive Glasses Obtained by Different Synthesis Routes. Ceram. Int. 2016, 42, 14700–14709. [Google Scholar] [CrossRef]
- Berezicka, A.; Szumera, M.; Sułowska, J.; Jeleń, P.; Olejniczak, Z.; Stępień, J.; Zając, M.; Pollastri, S.; Olivi, L. Unraveling the Nature of Sulfur-Bearing Silicate-Phosphate Glasses: Insights from Multi-Spectroscopic (Raman, MIR, 29Si, 31P MAS-NMR, XAS, XANES) Investigation. Ceram. Int. 2022, 48, 4238–4254. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Liu, L.; Wang, X. FTIR, Raman and NMR Investigation of CaO–SiO2–P2O5 and CaO–SiO2–TiO2–P2O5 Glasses. J. Non-Cryst. Solids 2015, 420, 26–33. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Hammami, I.; Jakka, S.K.; Teixeira, S.S.; Silva, J.C.; Borges, J.P.; Graça, M.P.F. Influence of the Addition of Zinc, Strontium, or Magnesium Oxides to the Bioglass 45S5 Network on Electrical Behavior. Materials 2024, 17, 499. [Google Scholar] [CrossRef] [PubMed]
- Plewinski, M.; Schickle, K.; Lindner, M.; Kirsten, A.; Weber, M.; Fischer, H. The Effect of Crystallization of Bioactive Bioglass 45S5 on Apatite Formation and Degradation. Dent. Mater. 2013, 29, 1256–1264. [Google Scholar] [CrossRef]
- Maximov, M.; Maximov, O.-C.; Craciun, L.; Ficai, D.; Ficai, A.; Andronescu, E. Bioactive Glass—An Extensive Study of the Preparation and Coating Methods. Coatings 2021, 11, 1386. [Google Scholar] [CrossRef]
- Boukha, Z.; Yeste, M.P.; Cauqui, M.Á.; González-Velasco, J.R. Influence of Ca/P Ratio on the Catalytic Performance of Ni/Hydroxyapatite Samples in Dry Reforming of Methane. Appl. Catal. A Gen. 2019, 580, 34–45. [Google Scholar] [CrossRef]
- Miola, M.; Verné, E.; Ciraldo, F.E.; Cordero-Arias, L.; Boccaccini, A.R. Electrophoretic Deposition of Chitosan/45S5 Bioactive Glass Composite Coatings Doped with Zn and Sr. Front. Bioeng. Biotechnol. 2015, 3, 159. [Google Scholar] [CrossRef] [PubMed]
- Głąb, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Kordyka, A.; Godzierz, M.; Wróbel, P.S.; Tomala, A.; Tyliszczak, B.; Sobczak-Kupiec, A. Evaluation of the Impact of pH of the Reaction Mixture, Type of the Stirring, and the Reagents’ Concentration in the Wet Precipitation Method on Physicochemical Properties of Hydroxyapatite so as to Enhance Its Biomedical Application Potential. J. Biomed. Mater. Res. 2022, 110, 2649–2666. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Bolelli, G.; Cannillo, V.; Cattini, A.; Sola, A. In Situ Raman Spectroscopy Investigation of Bioactive Glass Reactivity: Simulated Body Fluid Solution vs TRIS-Buffered Solution. Mater. Charact. 2011, 62, 1021–1028. [Google Scholar] [CrossRef]
- Awonusi, A.; Morris, M.D.; Tecklenburg, M.M.J. Carbonate Assignment and Calibration in the Raman Spectrum of Apatite. Calcif. Tissue Int. 2007, 81, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Gavinho, S.R.; Pádua, A.S.; Holz, L.I.V.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Valente, M.A.; Graça, M.P.F. Bioactive Glasses Containing Strontium or Magnesium Ions to Enhance the Biological Response in Bone Regeneration. Nanomaterials 2023, 13, 2717. [Google Scholar] [CrossRef] [PubMed]


| Composition (mol%) | |||||
|---|---|---|---|---|---|
| Sample | SiO2 | Na2O | CaO | P2O5 | Fe3O4 |
| Fe0 | 46.10 | 24.40 | 26.90 | 2.60 | - |
| Fe1 | 45.64 | 24.16 | 26.63 | 2.57 | 1 |
| Fe2 | 45.18 | 23.91 | 26.36 | 2.55 | 2 |
| Fe4 | 44.26 | 23.42 | 25.82 | 2.50 | 4 |
| Fe8 | 42.41 | 22.45 | 24.75 | 2.39 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammami, I.; Jakka, S.K.; Sá-Nogueira, I.; Borges, J.P.; Graça, M.P.F. The Effect of Iron Oxide Insertion on the In Vitro Bioactivity, and Antibacterial Properties of the 45S5 Bioactive Glass. Biomimetics 2024, 9, 325. https://doi.org/10.3390/biomimetics9060325
Hammami I, Jakka SK, Sá-Nogueira I, Borges JP, Graça MPF. The Effect of Iron Oxide Insertion on the In Vitro Bioactivity, and Antibacterial Properties of the 45S5 Bioactive Glass. Biomimetics. 2024; 9(6):325. https://doi.org/10.3390/biomimetics9060325
Chicago/Turabian StyleHammami, Imen, Suresh Kumar Jakka, Isabel Sá-Nogueira, João Paulo Borges, and Manuel Pedro Fernandes Graça. 2024. "The Effect of Iron Oxide Insertion on the In Vitro Bioactivity, and Antibacterial Properties of the 45S5 Bioactive Glass" Biomimetics 9, no. 6: 325. https://doi.org/10.3390/biomimetics9060325
APA StyleHammami, I., Jakka, S. K., Sá-Nogueira, I., Borges, J. P., & Graça, M. P. F. (2024). The Effect of Iron Oxide Insertion on the In Vitro Bioactivity, and Antibacterial Properties of the 45S5 Bioactive Glass. Biomimetics, 9(6), 325. https://doi.org/10.3390/biomimetics9060325









