Bioactive Hydroxyapatite Aerogels with Piezoelectric Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Barium Titanate
2.2. Hydroxypatite-Based Aerogels
2.3. Materials Characterization
2.3.1. Structural, Chemical, and Morphologic Analysis
2.3.2. Biocompatibility Assays
2.3.3. Bioactivity Assessment
3. Results and Discussion
3.1. Barium Titanate
3.1.1. X-ray Diffraction Analysis
3.1.2. Fourier-Transform Infrared Spectroscopy Analysis
3.1.3. Raman Spectroscopy Analysis
3.2. Hydroxyapatite Nanowires
3.2.1. SEM Analysis
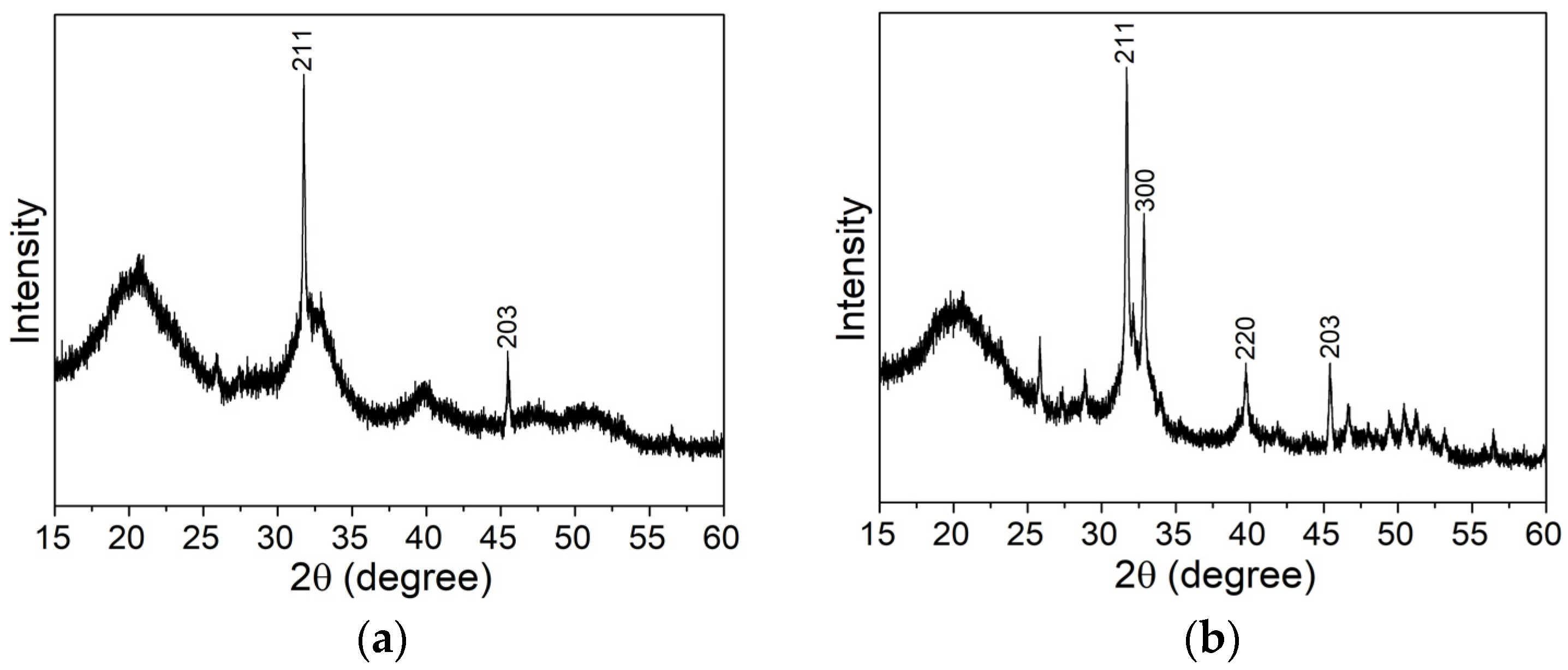
3.2.2. X-ray Diffraction Analysis
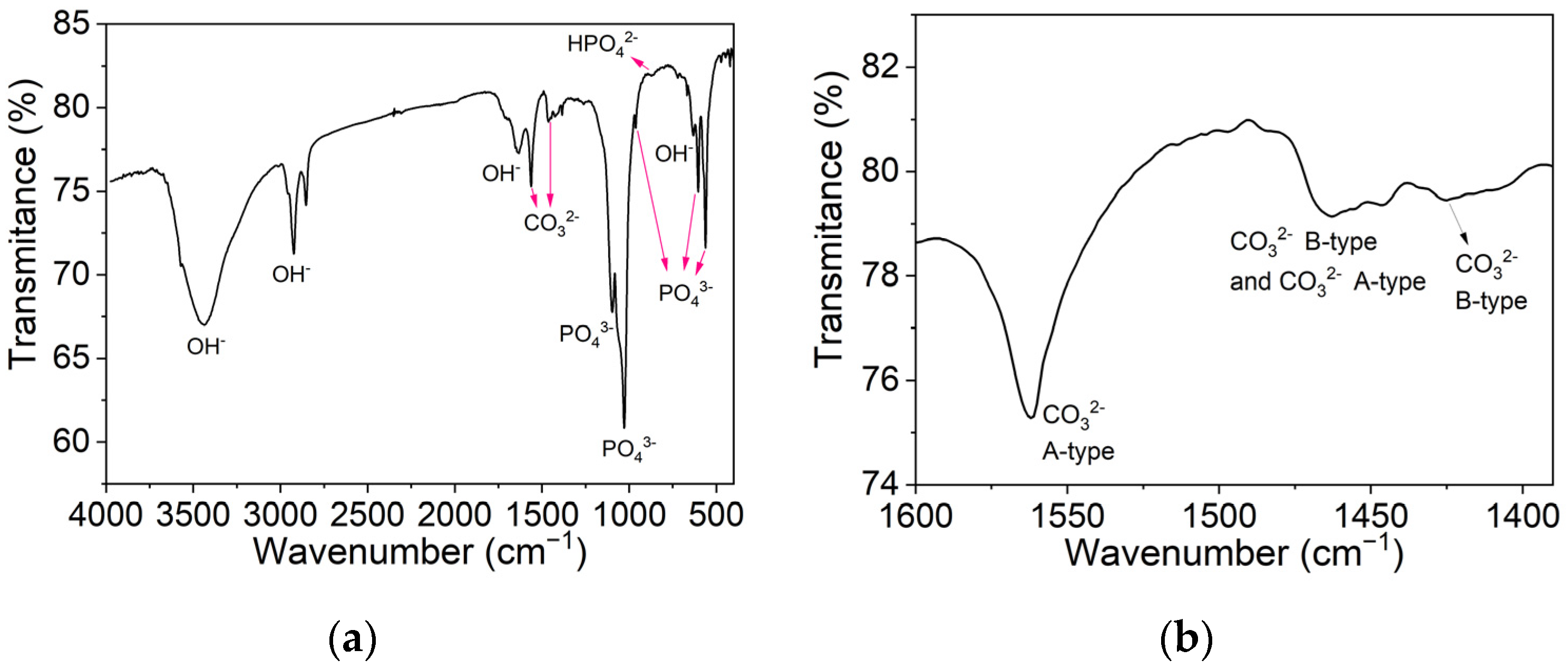
3.2.3. Fourier-Transform Infrared Spectroscopy Analysis
3.3. HAp and HAp/BT Aerogels
3.3.1. Porosity of the Aerogels
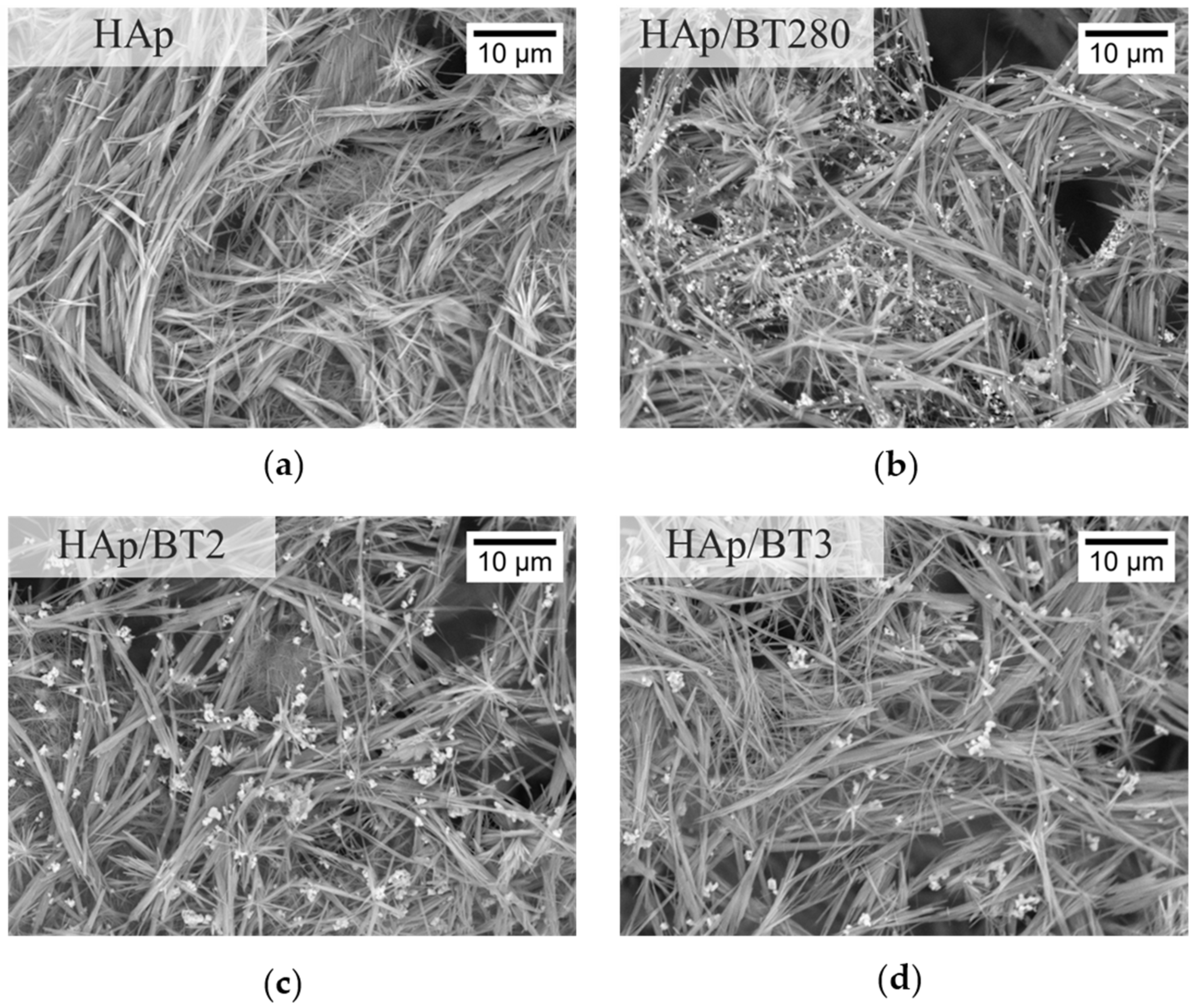
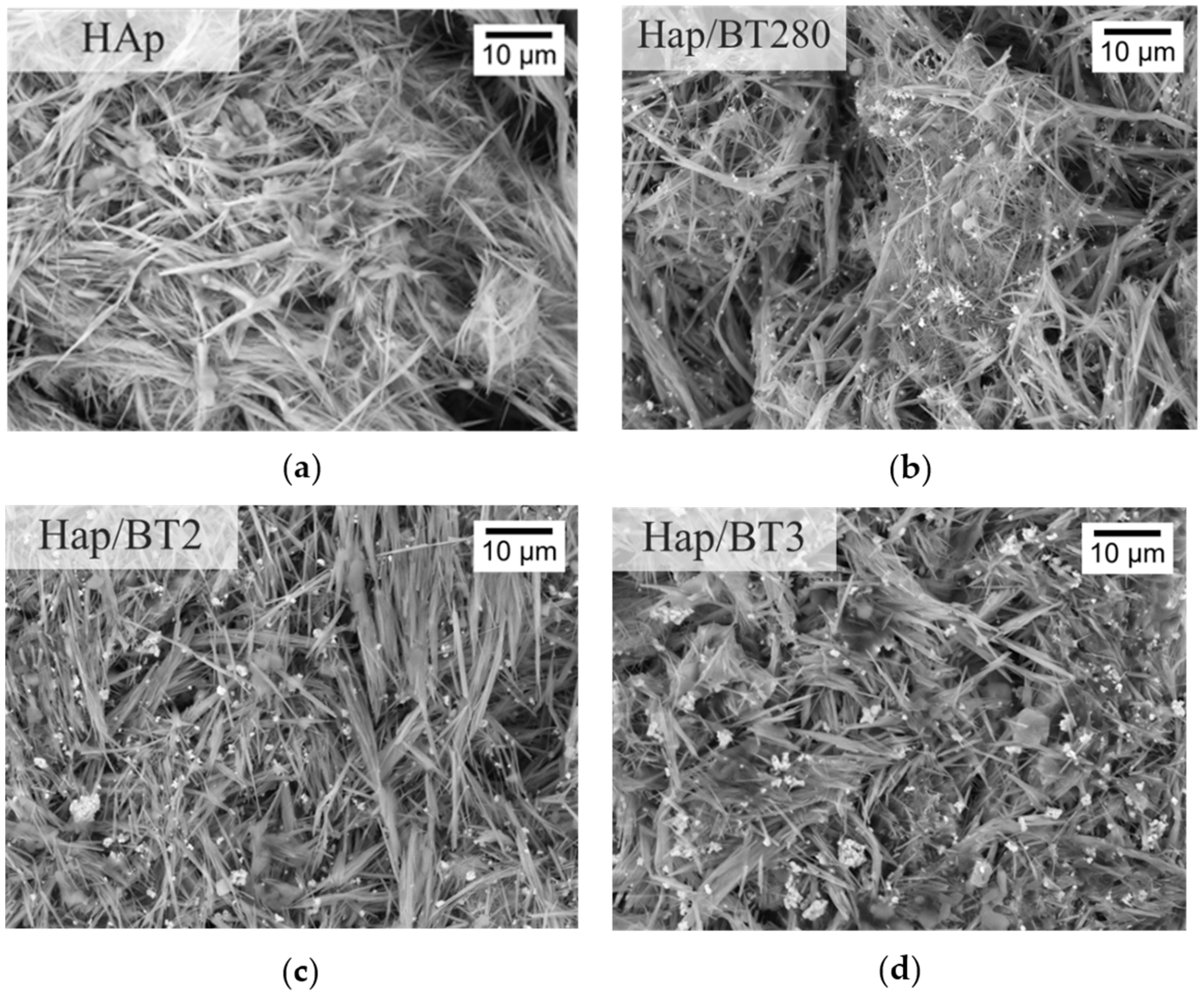
3.3.2. SEM Analysis
3.3.3. Cytotoxicity Assays
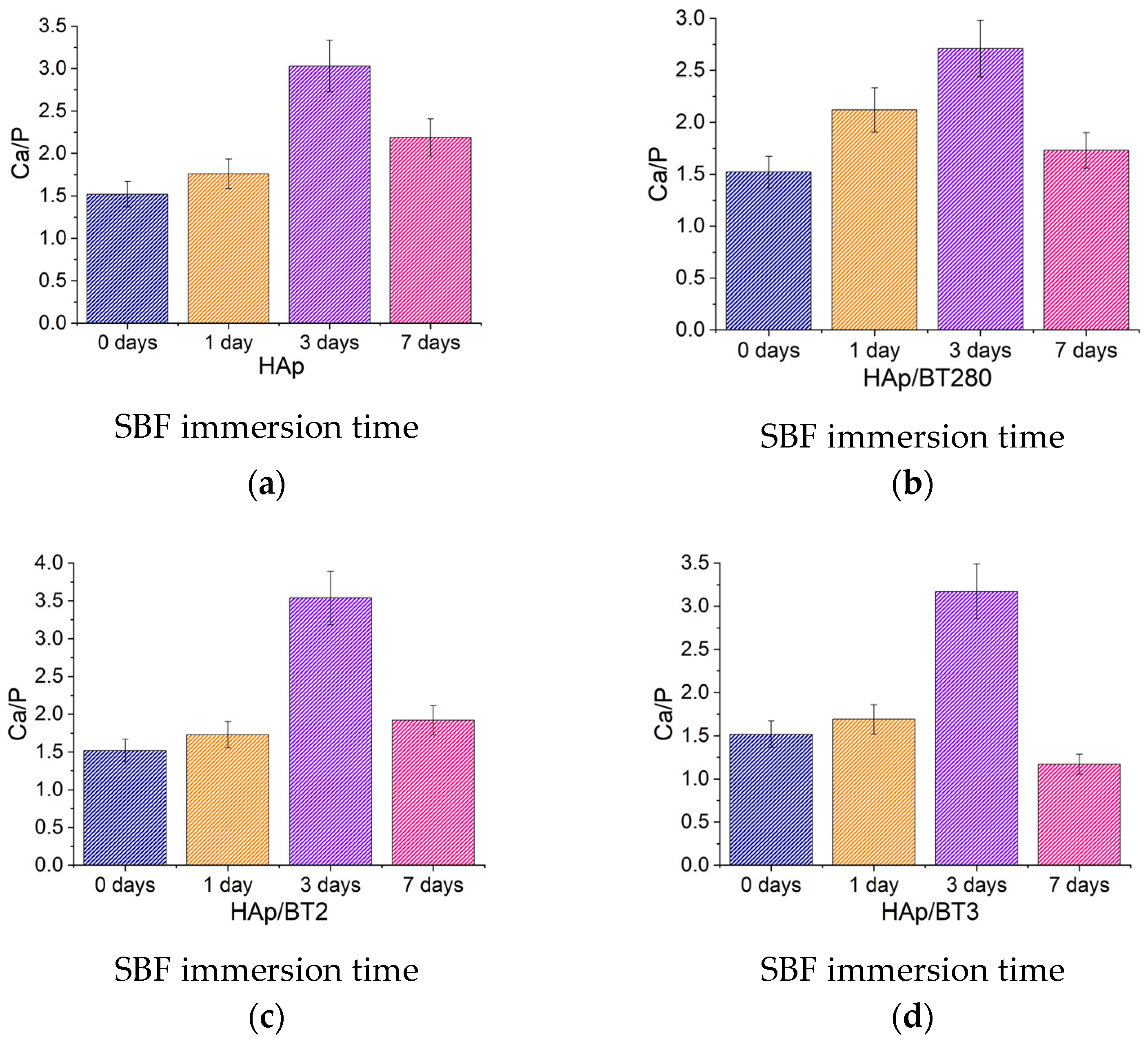
3.3.4. Bioactivity Assays in Simulated Body Fluid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, Y.; Sun, Y. Aging of the Bone. Adv. Exp. Med. Biol. 2018, 1086, 189–197. [Google Scholar] [CrossRef]
- Tresguerres, F.G.F.; Torres, J.; López-Quiles, J.; Hernández, G.; Vega, J.A.; Tresguerres, I.F. The Osteocyte: A Multifunctional Cell within the Bone. Ann. Anat. Anat. Anzeiger 2020, 227, 151422. [Google Scholar] [CrossRef]
- Orthopedic Biomaterials Market Size, Trends and Global Forecast to 2032. Available online: https://www.thebusinessresearchcompany.com/report/orthopedic-biomaterials-global-market-report (accessed on 4 October 2023).
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.S.; Santos, A.M.C.; Neves, G.A.; Menezes, R.R. A Brief Review on Hydroxyapatite Production and Use in Biomedicine. Ceramica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium Phosphate Ceramics in Bone Tissue Engineering: A Review of Properties and Their Influence on Cell Behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Sopyan, I.; Mel, M.; Ramesh, S.; Khalid, K.A. Porous Hydroxyapatite for Artificial Bone Applications. Sci. Technol. Adv. Mater. 2007, 8, 116–123. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Xiong, Z.C.; Yang, Z.Y.; Zhu, Y.J.; Chen, F.F.; Zhang, Y.G.; Yang, R.L. Ultralong Hydroxyapatite Nanowires-Based Paper Co-Loaded with Silver Nanoparticles and Antibiotic for Long-Term Antibacterial Benefit. ACS Appl. Mater. Interfaces 2017, 9, 22212–22222. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, B. Deformable Biomaterials Based on Ultralong Hydroxyapatite Nanowires. ACS Biomater. Sci. Eng. 2019, 5, 4951–4961. [Google Scholar] [CrossRef]
- Huang, G.-J.; Yu, H.-P.; Wang, X.-L.; Ning, B.-B.; Gao, J.; Shi, Y.-Q.; Zhu, Y.-J.; Duan, J.-L. Highly Porous and Elastic Aerogel Based on Ultralong Hydroxyapatite Nanowires for High-Performance Bone Regeneration and Neovascularization. J. Mater. Chem. B 2021, 9, 1277–1287. [Google Scholar] [CrossRef]
- Ball, J.P.; Mound, B.A.; Nino, J.C.; Allen, J.B. Biocompatible Evaluation of Barium Titanate Foamed Ceramic Structures for Orthopedic Applications. J. Biomed. Mater. Res. Part A 2014, 102, 2089–2095. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. Piezoelectric Effects in Collagen. Jpn. J. Appl. Phys. 1964, 3, 117–121. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric Smart Biomaterials for Bone and Cartilage Tissue Engineering. Inflamm. Regen. 2018, 38, 2. [Google Scholar] [CrossRef] [PubMed]
- Pienkowski, D.; Pollack, S.R. The Origin of Stress-generated Potentials in Fluid-saturated Bone. J. Orthop. Res. 1983, 1, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Metwally, S.; Stachewicz, U. Surface Potential and Charges Impact on Cell Responses on Biomaterials Interfaces for Medical Applications. Mater. Sci. Eng. C 2019, 104, 109883. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Geng, Q.; Li, C.; Wang, H.-C.; Ren, C.; Zhang, Y.-F.; Bai, J.-S.; Pan, H.-B.; Cui, X.; Yao, M.-X.; et al. Application of Piezoelectric Materials in the Field of Bone: A Bibliometric Analysis. Front. Bioeng. Biotechnol. 2023, 11, 1210637. [Google Scholar] [CrossRef] [PubMed]
- Diez-Escudero, A.; Espanol, M.; Ginebra, M.P. High-Aspect-Ratio Nanostructured Hydroxyapatite: Towards New Functionalities for a Classical Material. Chem. Sci. 2023, 15, 55–76. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, W.; Yang, Z.; Zhang, H.; Ma, J.; Li, T.; Niu, H.; Zhou, Y.; Yao, Q.; Chang, J.; et al. An Ultralong Hydroxyapatite Nanowire Aerogel for Rapid Hemostasis and Wound Healing. Chem. Eng. J. 2021, 430, 1385–8947. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, S.; Ying, Z.; Liu, J.; Zhou, Y.; Chen, F. Engineering of Aerogel-Based Biomaterials for Biomedical Applications. Int. J. Nanomed. 2020, 15, 2363. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A Unified in Vitro Evaluation for Apatite-Forming Ability of Bioactive Glasses and Their Variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef]
- Qian, H.; Zhu, G.; Xu, H.; Zhang, X.; Zhao, Y.; Yan, D.; Hong, X.; Han, Y.; Fu, Z.; Ta, S.; et al. Preparation of Tetragonal Barium Titanate Nanopowders by Microwave Solid-State Synthesis. Appl. Phys. A 2020, 126, 294. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Millán-Malo, B.M.; Rivera-Muñoz, E.M.; Rodriguez-García, M.E. Effect of the Nano Crystal Size on the X-Ray Diffraction Patterns of Biogenic Hydroxyapatite from Human, Bovine, and Porcine Bones. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Ingham, B.; Toney, M.F. X-ray Diffraction for Characterizing Metallic Films. In Metallic Films for Electronic, Optical and Magnetic Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–38. ISBN 9780857090577. [Google Scholar]
- Bhuiyan, M.R.A.; Alam, M.M.; Momin, M.A.; Uddin, M.J.; Islam, M. Synthesis and Characterization of Barium Amidoborane. Int. J. Mater. Mech. Eng. 2012, 1, 21–24. [Google Scholar]
- Singh, M.; Yadav, B.C.; Ranjan, A.; Kaur, M.; Gupta, S.K. Synthesis and Characterization of Perovskite Barium Titanate Thin Film and Its Application as LPG Sensor. Sens. Actuators B Chem. 2017, 241, 1170–1178. [Google Scholar] [CrossRef]
- Buscaglia, V.; Randall, C.A. Size and Scaling Effects in Barium Titanate. An Overview. J. Eur. Ceram. Soc. 2020, 40, 3744–3758. [Google Scholar] [CrossRef]
- Lazarević, Z.; Romčević, N.; Vijatović, M.; Paunović, N.; Romčević, M.; Stojanović, B.; Dohčević-Mitrović, Z. Characterization of Barium Titanate Ceramic Powders by Raman Spectroscopy, Proceedings of the Tenth Annual Conference of the Materials Research Society of Serbia, September 2008. ACTA Phys. Pol. A 2009, 115, 808–810. [Google Scholar] [CrossRef]
- Jiang, Y.-Y.; Zhu, Y.-J.; Chen, F.; Wu, J. Solvothermal Synthesis of Submillimeter Ultralong Hydroxyapatite Nanowires Using a Calcium Oleate Precursor in a Series of Monohydroxy Alcohols. Ceram. Int. 2015, 41, 6098–6102. [Google Scholar] [CrossRef]
- Borkiewicz, O.; Rakovan, J.; Cahill, C.L. Time-Resolved in Situ Studies of Apatite Formation in Aqueous Solutions. Am. Mineral. 2010, 95, 1224–1236. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate Substitution in the Mineral Component of Bone: Discriminating the Structural Changes, Simultaneously Imposed by Carbonate in A and B Sites of Apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-G.; Zhu, Y.-J.; Xiong, Z.-C.; Wu, J.; Chen, F. Bioinspired Ultralight Inorganic Aerogel for Highly Efficient Air Filtration and Oil-Water Separation. ACS Appl. Mater. Interfaces 2018, 10, 13019–13027. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Belcarz, A.; Pazik, R.; Sobierajska, P.; Han, T.; Wiglusz, R.J.; Ginalska, G. “False” Cytotoxicity of Ions-Adsorbing Hydroxyapatite—Corrected Method of Cytotoxicity Evaluation for Ceramics of High Specific Surface Area. Mater. Sci. Eng. C 2016, 65, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Ginebra, M.P.; Engel, E.; Planell, J. Ion Reactivity of Calcium-Deficient Hydroxyapatite in Standard Cell Culture Media. Acta Biomater. 2011, 7, 4242–4252. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Khare, D.; Basu, B.; Dubey, A.K. Electrical Stimulation and Piezoelectric Biomaterials for Bone Tissue Engineering Applications. Biomaterials 2020, 258, 120280. [Google Scholar] [CrossRef]
- Sood, A.; Desseigne, M.; Dev, A.; Maurizi, L.; Kumar, A.; Millot, N.; Han, S.S. A Comprehensive Review on Barium Titanate Nanoparticles as a Persuasive Piezoelectric Material for Biomedical Applications: Prospects and Challenges. Small 2023, 19, 2206401. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Zeng, J.; Zhou, K.; Zhang, D. Aligned Porous Barium Titanate/Hydroxyapatite Composites with High Piezoelectric Coefficients for Bone Tissue Engineering. Mater. Sci. Eng. C 2014, 39, 143–149. [Google Scholar] [CrossRef]
- Baxter, F.R.; Bowen, C.R.C.R.C.; Turner, I.G.I.G.; Dent, A.C.E.E. Electrically Active Bioceramics: A Review of Interfacial Responses. Ann. Biomed. 2010, 38, 2079–2092. [Google Scholar] [CrossRef]
- Park, J.B.; Kelly, B.J.; Kenner, G.H.; von Recum, A.F.; Grether, M.F.; Coffeen, W.W. Piezoelectric Ceramic Implants: In Vivo Results. J. Biomed. Mater. Res. 1981, 15, 103–110. [Google Scholar] [CrossRef]
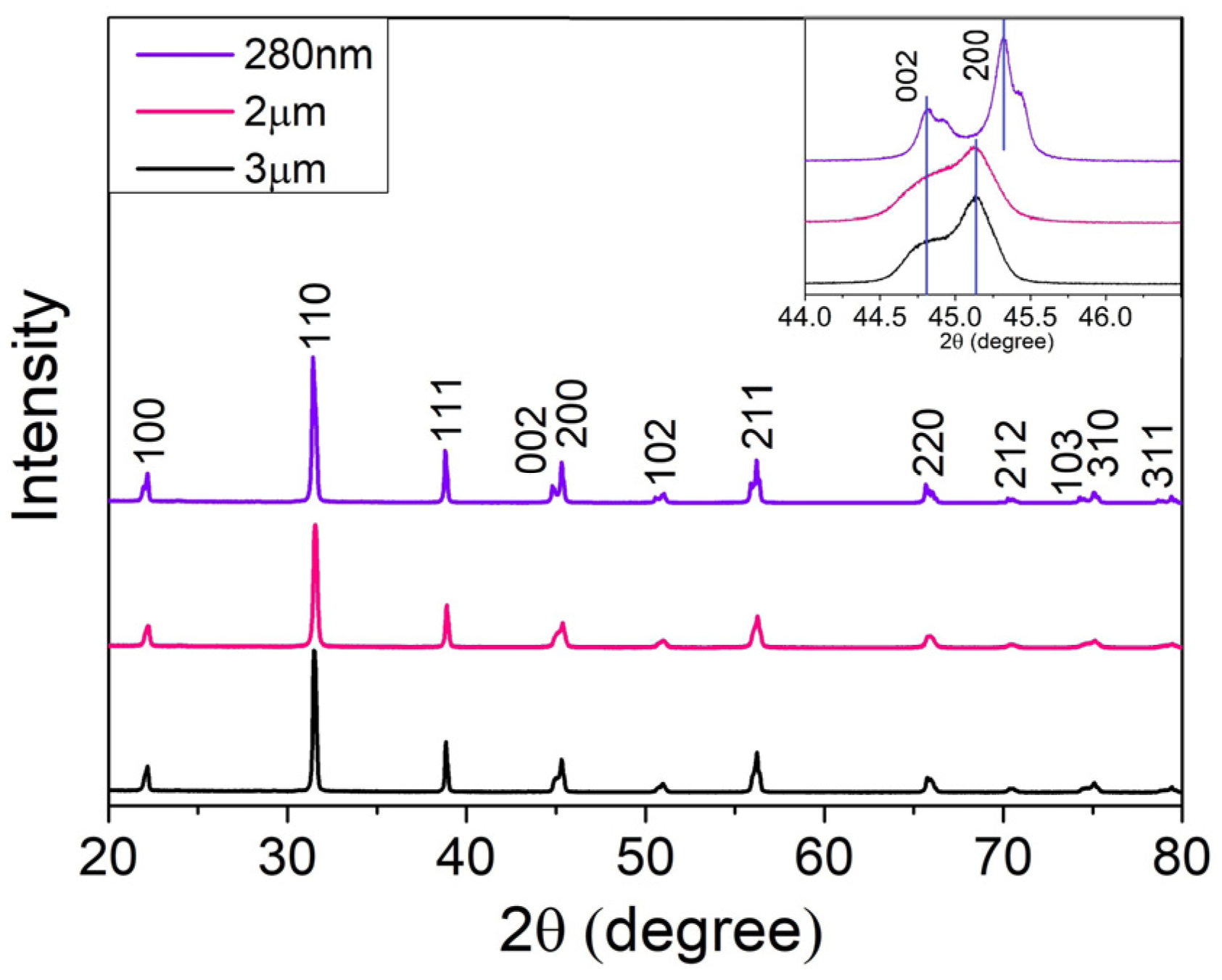






| Sample | Peak, 2θ (°) | FWHM, β (rad) | Crystallite Size, τ (nm) |
|---|---|---|---|
| 280 nm | 31.43 | 4.81 × 10−3 | 29.9 |
| 2 µm | 31.54 | 4.42 × 10−3 | 32.5 |
| 3 µm | 31.49 | 3.90 × 10−3 | 36.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, C.; Vieira, T.; Silva, J.C.; Borges, J.P.M.R.; Lança, M.C. Bioactive Hydroxyapatite Aerogels with Piezoelectric Particles. Biomimetics 2024, 9, 143. https://doi.org/10.3390/biomimetics9030143
Tavares C, Vieira T, Silva JC, Borges JPMR, Lança MC. Bioactive Hydroxyapatite Aerogels with Piezoelectric Particles. Biomimetics. 2024; 9(3):143. https://doi.org/10.3390/biomimetics9030143
Chicago/Turabian StyleTavares, Catarina, Tânia Vieira, Jorge C. Silva, João P. M. R. Borges, and M. Carmo Lança. 2024. "Bioactive Hydroxyapatite Aerogels with Piezoelectric Particles" Biomimetics 9, no. 3: 143. https://doi.org/10.3390/biomimetics9030143
APA StyleTavares, C., Vieira, T., Silva, J. C., Borges, J. P. M. R., & Lança, M. C. (2024). Bioactive Hydroxyapatite Aerogels with Piezoelectric Particles. Biomimetics, 9(3), 143. https://doi.org/10.3390/biomimetics9030143









