Review of Spider Silk Applications in Biomedical and Tissue Engineering
Abstract
1. Introduction
2. Structure and Properties of Spider Silk
3. Natural Synthesis of Spider Fibers
- The “tail” zone, which is responsible for the synthesis and secretion of spider web proteins;
- Lumen (bag) used for protein accumulation;
- Fiber alignment channel;
- Output for final fiber production.
| Major Ampullate Silk | Major ampullate silk is produced in the main ampullary glands. These fibers serve to allow escape from predators. Also, they are used for the web’s outer rim and spokes. In this way, the other threads can be attached to them. They have a strength five times greater than steel and three times greater than Kevlar [59]. |
| Minor Ampullate Silk | Minor ampullate silk is produced in the secondary ampullary glands. It has a role in the spiral formation of the network. Unlike MA fibers, it does not contain proline. Also, it has a reduced content of glutamate [59]. |
| Flagelliform Silk | Capture-spiral (flagelliform) silk is produced in the flagelliform glands. It is used for catching prey [59]. |
| Tubiliform Silk | Tubiliform (cylindriform) silk is produced in the tubiliform (cylindriform) glands. It is used for protective egg sacs [59]. |
| Aciniform Silk | Aciniform silk is produced in the aciniform glands. It is a wrapping silk usedfor the immobilization of prey [64]. |
| Pyriform Silk | Pyriform silk is produced in the pyriform glands. It functions like a glue, and connects the web to different materials [65]. |
| Aggregate Silk | Aggregate silk is made in the aggregate glands. It produces aqueous gluey substances, making the capture threads sticky [66]. |
| Glands | Type of Spider Silk | Composition |
|---|---|---|
| Aggregate | Aqueous cement | ASG1, ASG2 |
| Pyriform | Core fiber of capture spiral | PySp1, PySp2 |
| Tubuliform | Egg-case silk | TuSp1, ECP-1, ECP-2 |
| Flagelliform | Spiral silk | Flag |
| Aciniform | Capture silk | AcSp1 |
| Minor ampullate | Dragline silk, framework silk | MiSp1, MiSp2 |
| Major ampullate | Dragline silk, framework silk, radial silk | MaSp1, MaSp2 |
| Material | Tensile Strength (Mpa) | Elongation (%) | Toughness (kJ/kg) |
|---|---|---|---|
| Dragline (MA) silk | 4000 | 35 | 400 |
| Silkworm silk | 600 | 20 | 60 |
| Kevlar 49 | 3600 | 5 | 30 |
| Ligament | 150 | 5 | 5 |
| Bone | 160 | 3 | 3 |
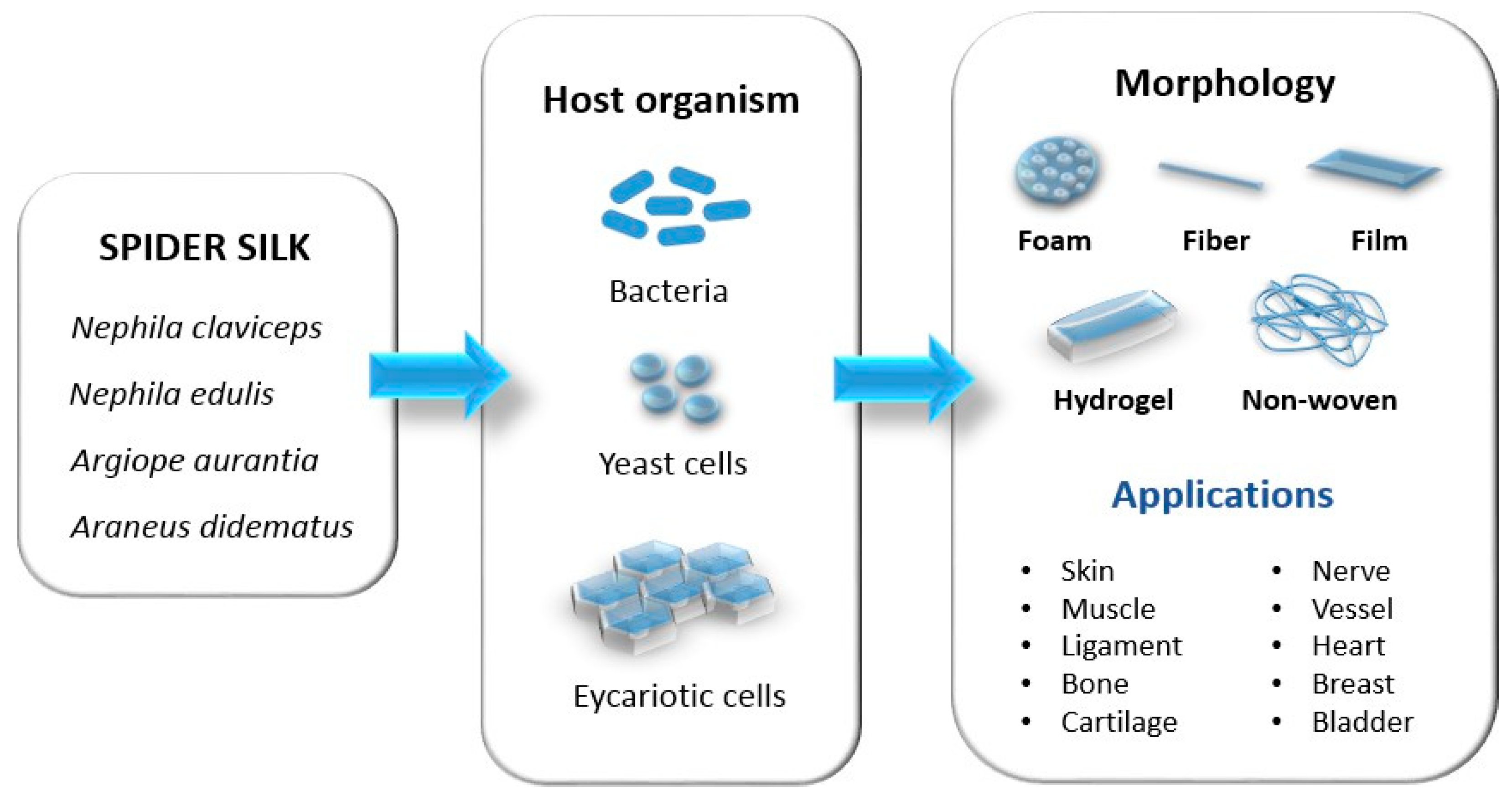
4. Recombinant Production of Spider Silk
- Determining the sequence of nucleotides in natural DNA (isolation of the desired sequence that encodes the target protein);
- Designing recombinant DNA;
- Selection of the vector that will enable the transmission of the desired sequence;
- Transmission of the vector into the host’s organism (bacteria, yeast, plants, insect cells, mammalian cells, and transgenic animals);
- Cultivation/production of proteins in the host organism;
- Isolation of the obtained proteins.
5. Spider Silk Structures
5.1. Spider Silk in Fiber form (1D)
5.2. Spider Silk Coatings (2D)
5.3. Three-Dimensional Constructs
6. Applications in Tissue Engineering
6.1. Skin Regeneration
6.2. Bone and Cartilage Tissue Repair
6.3. Vascularization
6.4. Ligament Repair
6.5. Muscle Tissue Repair
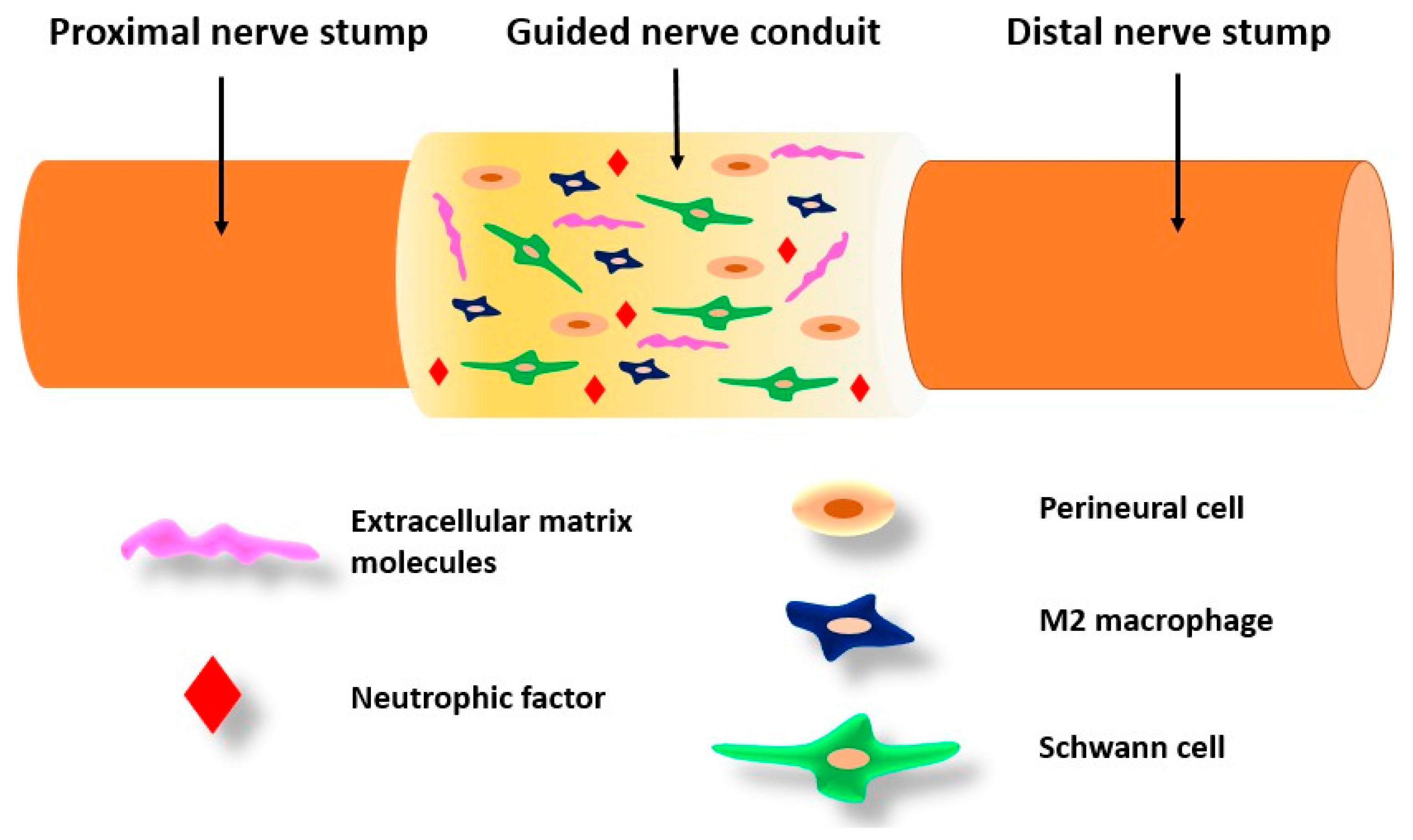
6.6. Repair of Peripheral Nerves
7. Spider Silk in Nanomedicine
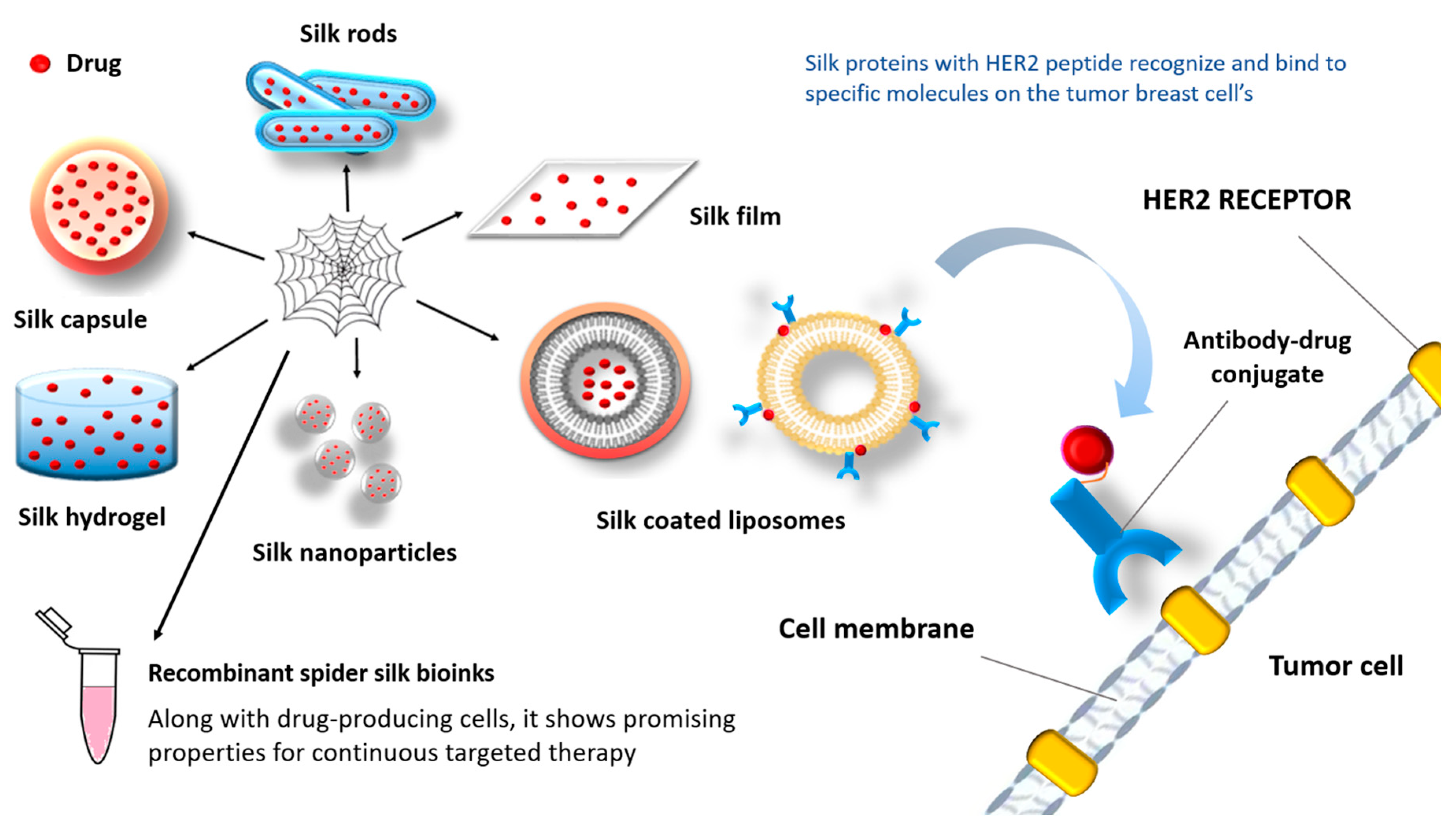
7.1. Drug Delivery Systems
7.2. Nanocomposites and Biomimetics
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Miserez, A.; Yu, J.; Mohammadi, P. Protein-Based Biological Materials: Molecular Design and Artificial Production. Chem. Rev. 2023, 123, 2049–2111. [Google Scholar] [CrossRef]
- Beppu, M.M.; Lopes, L.M.; Queiroz, C.D.S.; Moraes, M.A.D. Silk-Based Natural Biomaterials: Fundamentals and Biomedical Applications. In Handbook of Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 421–440. ISBN 978-0-323-99853-6. [Google Scholar]
- Babu, P.J.; Suamte, L. Applications of Silk-Based Biomaterials in Biomedicine and Biotechnology. Eng. Regen. 2024, 5, 56–69. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Santhosh, G. Biopolymer Matrices: Current Trends in Tissue Engineering. In Tailored Functional Materials for Clean and Sustainable Development; Apple Academic Press: New York, NY, USA, 2023; pp. 139–155. ISBN 978-1-00-339476-1. [Google Scholar]
- Dos Santos, F.V.; Siqueira, R.L.; De Morais Ramos, L.; Yoshioka, S.A.; Branciforti, M.C.; Correa, D.S. Silk Fibroin-Derived Electrospun Materials for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2024, 254, 127641. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Chowdhury, S.K.; Dey, S.; Moses, J.C.; Mandal, B.B. Silk: A Promising Biomaterial Opening New Vistas towards Affordable Healthcare Solutions. J. Indian Inst. Sci. 2019, 99, 445–487. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk Fibroin as Biomaterial for Bone Tissue Engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Cui, J.; Wu, S.; Geng, Z.; Su, J. Silk Fibroin-Based Biomaterials for Cartilage/Osteochondral Repair. Theranostics 2022, 12, 5103–5124. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wei, L.; Xu, Z.; Qin, L.; Yang, J.; Zou, Y.; Zhao, C.; Chen, L.; Hu, N. Evaluation and Application of Silk Fibroin Based Biomaterials to Promote Cartilage Regeneration in Osteoarthritis Therapy. Biomedicines 2023, 11, 2244. [Google Scholar] [CrossRef]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, H.; Yang, Y.; Zheng, Q.; Li, S.; Chen, Y. Bioactive Silk Fibroin-Based Hybrid Biomaterials for Musculoskeletal Engineering: Recent Progress and Perspectives. ACS Appl. Bio Mater. 2021, 4, 6630–6646. [Google Scholar] [CrossRef]
- Salehi, S.; Koeck, K.; Scheibel, T. Spider Silk for Tissue Engineering Applications. Molecules 2020, 25, 737. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem Cell-Based Tissue Engineering with Silk Biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef]
- Goel, A. Surgical Sutures—A Review. Off. Sci. J. Delhi Ophthalmol. Soc. 2015, 26, 159–162. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, L.; Mou, J.; Wu, D.; Zhou, P.; Xu, M. Mechanical Properties and Application Analysis of Spider Silk Bionic Material. e-Polymers 2020, 20, 443–457. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-Based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Hsia, Y.; Gnesa, E.; Tang, S.; Jeffery, F.; Geurts, P.; Zhao, L.; Franz, A.; Vierra, C. Identification and Synthesis of Novel Biomaterials Based on Spider Structural Silk Fibers. Appl. Phys. A 2011, 105, 301–309. [Google Scholar] [CrossRef]
- Xu, G.; Toh, G.W.; Du, N.; Liu, X.Y. Spider Silk: The Toughest Natural Polymer. In Natural Polymers. 1: Composites; RSC Green Chemistry; RSC Publishing: Cambridge, UK, 2012; ISBN 978-1-84973-402-8. [Google Scholar]
- DeFrancesco, L. Hanging on a Thread. Nat. Biotechnol. 2017, 35, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; DeSimone, E.; Scheibel, T. Biomedical applications of recombinant silk-based materials. Adv. Mater. 2018, 30, e1704636. [Google Scholar] [CrossRef]
- Foppiani, J.A.; Weidman, A.; Alvarez, A.H.; Valentine, L.; Devi, K.; Kaplan, D.L.; Lin, S.J. Clinical Use of Non-Suture Silk-Containing Products: A Systematic Review. Biomimetics 2023, 8, 45. [Google Scholar] [CrossRef]
- Nie, K.; Zhou, S.; Li, H.; Tian, J.; Shen, W.; Huang, W. Advanced Silk Materials for Musculoskeletal Tissue Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1199507. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Hu, Y.; Fei, Y.; Liu, H.; Huang, Z.; Wang, C.; Ruan, D.; Heng, B.C.; Chen, W.; et al. Systematic Review of Silk Scaffolds in Musculoskeletal Tissue Engineering Applications in the Recent Decade. ACS Biomater. Sci. Eng. 2021, 7, 817–840. [Google Scholar] [CrossRef]
- Hahn, J.; Gögele, C.; Schulze-Tanzil, G. Could an Anterior Cruciate Ligament Be Tissue-Engineered from Silk? Cells 2023, 12, 2350. [Google Scholar] [CrossRef]
- Croft, A.S.; Spessot, E.; Bhattacharjee, P.; Yang, Y.; Motta, A.; Wöltje, M.; Gantenbein, B. Biomedical Applications of Silk and Its Role for Intervertebral Disc Repair. JOR Spine 2022, 5, e1225. [Google Scholar] [CrossRef]
- Dey, S.; Jaiswal, C.; Shome, S.; Bhar, B.; Bandyopadhyay, A.; Manikumar, K.; Dadheech, R.; Mandal, B.B. Photocrosslinkable Silk-Based Biomaterials for Regenerative Medicine and Healthcare Applications. Regen. Eng. Transl. Med. 2023, 9, 181–201. [Google Scholar] [CrossRef]
- Intravaia, J.T.; Graham, T.; Kim, H.S.; Nanda, H.S.; Kumbar, S.G.; Nukavarapu, S.P. Smart Orthopedic Biomaterials and Implants. Curr. Opin. Biomed. Eng. 2023, 25, 100439. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive Polymeric Scaffolds for Tissue Engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Shafiq, M.; Li, J.; Yu, K.; Liu, Z.; Zhou, X.; Zhu, M. Recent Developments in Artificial Spider Silk and Functional Gel Fibers. SmartMat 2023, 4, e1189. [Google Scholar] [CrossRef]
- Schäfer, S.; Aavani, F.; Köpf, M.; Drinic, A.; Stürmer, E.K.; Fuest, S.; Grust, A.L.C.; Gosau, M.; Smeets, R. Silk Proteins in Reconstructive Surgery: Do They Possess an Inherent Antibacterial Activity? A Systematic Review. Wound Repair Regen. 2023, 31, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, Y.; Cheng, Q.; Yang, Z.; Wang, J.; Xu, Z.; Yang, M.; Shuai, Y. Silk Protein-Mediated Biomineralization: From Bioinspired Strategies and Advanced Functions to Biomedical Applications. ACS Appl. Mater. Interfaces 2023, 15, 33191–33206. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, R.; Yu, S.; Chen, J.; Kao, Y.; Wang, T.; Chang, P.; Sheu, H.; Chen, S.; Liu, W.; et al. Self-Healable Spider Dragline Silk Materials. Adv. Funct. Mater. 2023, 33, 2303571. [Google Scholar] [CrossRef]
- Jang, J.; Chae, S.; Yoon, J.; Kim, H.; Park, W. Organ Printing, 2nd ed.; IOP Publishing: Bristol, UK, 2023; ISBN 978-0-7503-5122-5. [Google Scholar]
- Pedde, R.D.; Mirani, B.; Navaei, A.; Styan, T.; Wong, S.; Mehrali, M.; Thakur, A.; Mohtaram, N.K.; Bayati, A.; Dolatshahi-Pirouz, A.; et al. Emerging Biofabrication Strategies for Engineering Complex Tissue Constructs. Adv. Mater. 2017, 29, 1606061. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Lee, O.J.; Lee, J.S.; Park, C.H. Three-Dimensional Digital Light-Processing Bioprinting Using Silk Fibroin-Based Bio-Ink: Recent Advancements in Biomedical Applications. Biomedicines 2022, 10, 3224. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Zhao, S.-X.; Li, J.-X.; Zhang, Y.-Q. Silk Fibroin Improves the Biological Properties of Egg White-Based Bioink for the Bioprinting of Tissue Engineering Materials. ACS Omega 2023, 8, 46685–46696. [Google Scholar] [CrossRef]
- Ye, L.; Liu, X.; Li, K.; Li, X.; Zhu, J.; Yang, S.; Xu, L.; Yang, M.; Yan, Y.; Yan, J. A Bioinspired Synthetic Fused Protein Adhesive from Barnacle Cement and Spider Dragline for Potential Biomedical Materials. Int. J. Biol. Macromol. 2023, 253, 127125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Reagan, M.R.; Kaplan, D.L. Electrospun Silk Biomaterial Scaffolds for Regenerative Medicine. Adv. Drug Deliv. Rev. 2009, 61, 988–1006. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Ostapchuk, G.; Catalano, P.N.; Hardy, J.G.; Evelson, P.A.; Orive, G.; Desimone, M.F. 4D Printing: The Development of Responsive Materials Using 3D-Printing Technology. Pharmaceutics 2023, 15, 2743. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Lewis, R. Structure of a protein superfiber: Spider dragline silk. Proc. Natl. Acad. Sci. USA 1990, 87, 7120–7124. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, F.; Knight, D. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef]
- Jorge, I.; Ruiz, V.; Lavado-García, J.; Vázquez, J.; Hayashi, C.; Rojo, F.J.; Atienza, J.M.; Elices, M.; Guinea, G.V.; Pérez-Rigueiro, J. Expression of Spidroin Proteins in the Silk Glands of Golden Orb-weaver Spiders. J. Exp. Zoolog. B Mol. Dev. Evol. 2022, 338, 241–253. [Google Scholar] [CrossRef]
- Babb, P.L.; Lahens, N.F.; Correa-Garhwal, S.M.; Nicholson, D.N.; Kim, E.J.; Hogenesch, J.B.; Kuntner, M.; Higgins, L.; Hayashi, C.Y.; Agnarsson, I.; et al. The Nephila Clavipes Genome Highlights the Diversity of Spider Silk Genes and Their Complex Expression. Nat. Genet. 2017, 49, 895–903. [Google Scholar] [CrossRef]
- Elices, M.; Guinea, G.V.; Plaza, G.R.; Real, J.I.; Pérez-Rigueiro, J. Example of Microprocessing in a Natural Polymeric Fiber: Role of Reeling Stress in Spider Silk. J. Mater. Res. 2006, 21, 1931–1938. [Google Scholar] [CrossRef]
- Du, N.; Liu, X.Y.; Narayanan, J.; Li, L.; Lim, M.L.; Li, D. Design of Superior Spider Silk: From Nanostructure to Mechanical Properties. Biophys. J. 2006, 91, 4528–4535. [Google Scholar] [CrossRef] [PubMed]
- Kojic, N.; Bico, J.; Clasen, C.; McKinley, G.H. Ex Vivo Rheology of Spider Silk. J. Exp. Biol. 2006, 209, 4355–4362. [Google Scholar] [CrossRef] [PubMed]
- Kojic, N.; Panzer, M.J.; Leisk, G.G.; Raja, W.K.; Kojic, M.; Kaplan, D.L. Ion Electrodiffusion Governs Silk Electrogelation. Soft Matter 2012, 8, 6897. [Google Scholar] [CrossRef]
- Pérez-Rigueiro, J.; Elices, M.; Plaza, G.R.; Guinea, G.V. Basic Principles in the Design of Spider Silk Fibers. Molecules 2021, 26, 1794. [Google Scholar] [CrossRef]
- Simmons, A.; Michal, C.; Jelinski, L. Molecular Orientation and Two-Component Nature of the Crystalline Fraction of Spider Dragline Silk. Science 1996, 271, 84–87. [Google Scholar] [CrossRef]
- Keten, S.; Buehler, M. Nanostructure and molecular mechanics of spider dragline silk protein assemblies. J. R. Soc. Interface 2010, 7, 1709–1721. [Google Scholar] [CrossRef]
- Work, R. Dimensions, Birefringences, and Force-Elongation Behavior of Major and Minor Ampullate Silk Fibers from Orb-Web-Spinning Spiders—The Effects of Wetting on These Properties. Text. Res. J. 1977, 47, 650–662. [Google Scholar] [CrossRef]
- Elices, M.; Pérez-Rigueiro, J.; Plaza, G.; Guinea, G. Recovery in Spider Silk Fibers. J. Appl. Polym. Sci. 2004, 92, 3537–3541. [Google Scholar] [CrossRef]
- Madurga, R.; Plaza, G.; Blackledge, T.; Guinea, G.; Elices, M.; Pérez-Rigueiro, J. Material Properties of Evolutionary Diverse Spider Silks Described by Variation in a Single Structural Parameter. Sci. Rep. 2016, 6, 18991. [Google Scholar] [CrossRef]
- Gu, L.; Jiang, Y.; Hu, J. Structure Design and Property of Spider Silk-Inspired Shape Memory Materials. Mater. Today Proc. 2019, 16, 1491–1496. [Google Scholar] [CrossRef]
- Blamires, S.; Lozano-Picazo, P.; Bruno, A.L.; Arnedo, M.; Ruiz-León, Y.; González-Nieto, D.; Rojo, F.J.; Elices, M.; Guinea, G.V.; Pérez-Rigueiro, J. The Spider Silk Standardization Initiative (S3I): A Powerful Tool to Harness Biological Variability and to Systematize the Characterization of Major Ampullate Silk Fibers Spun by Spiders from Suburban Sydney, Australia. J. Mech. Behav. Biomed. Mater. 2023, 140, 105729. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-J.; Kaplan, D.L. Mechanism of Silk Processing in Insects and Spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, B.; Greco, G.; Bäcklund, F.G.; Pugno, N.M.; Johansson, J.; Rising, A. Impact of Physio-Chemical Spinning Conditions on the Mechanical Properties of Biomimetic Spider Silk Fibers. Commun. Mater. 2022, 3, 83. [Google Scholar] [CrossRef]
- Gatwiri, J.; Kamweru, P.K. Spider Silk: A Natural Marvel of Mechanical and Structural Strength. Afr. J. Biol. Sci. 2021, 3, 1–16. [Google Scholar]
- Altman, G.H.; Chen, J.; Horan, R.L.; Horan, D.J. Method of Forming an Implantable Knitted Fabric Comprising Silk Fibroin Fibers. U.S. Patent No. 8,628,791, 24 March 2011. [Google Scholar]
- Gosline, J.; DeMont, M.; Denny, M. The Structure and Properties of Spider Silk. Endeavour 1986, 10, 37–43. [Google Scholar] [CrossRef]
- Tokareva, O.; Jacobsen, M.; Buehler, M.; Wong, J.; Kaplan, D. Structure–Function– Property–Design Interplay in Biopolymers: Spider Silk. Acta Biomater. 2014, 10, 1612–1626. [Google Scholar] [CrossRef]
- Roloff, F.; Strauß, S.; Vogt, P.M.; Bicker, G.; Radtke, C. Spider Silk as Guiding Biomaterial for Human Model Neurons. BioMed. Res. Int. 2014, 2014, 906819. [Google Scholar] [CrossRef]
- Tremblay, M.-L.; Xu, L.; Lefèvre, T.; Sarker, M.; Orrell, K.E.; Leclerc, J.; Rainey, J.K. Spider Wrapping Silk Fibre Architecture Arising from Its Modular Soluble Protein Precursor. Sci. Rep. 2015, 5, 11502. [Google Scholar] [CrossRef]
- Simmons, J.R.; Xu, L.; Rainey, J.K. Recombinant Pyriform Silk Fiber Mechanics Are Modulated by Wet-Spinning Conditions. ACS Biomater. Sci. Eng. 2019, 5, 4985–4993. [Google Scholar] [CrossRef]
- Moon, M.-J. Fine Structure of the Aggregate Silk Nodules in the Orb-Web Spider Nephila Clavata. Anim. Cells Syst. 2018, 22, 421–428. [Google Scholar] [CrossRef]
- Bittencourt, D.M.D.C.; Oliveira, P.; Michalczechen-Lacerda, V.A.; Rosinha, G.M.S.; Jones, J.A.; Rech, E.L. Bioengineering of Spider Silks for the Production of Biomedical Materials. Front. Bioeng. Biotechnol. 2022, 10, 958486. [Google Scholar] [CrossRef]
- Ramezaniaghdam, M.; Nahdi, N.D.; Reski, R. Recombinant Spider Silk: Promises and Bottlenecks. Front. Bioeng. Biotechnol. 2022, 10, 835637. [Google Scholar] [CrossRef]
- Heidebrecht, A.; Scheibel, T. Recombinant Production of Spider Silk Proteins. Adv. Appl. Microbiol. 2013, 82, 115–153. [Google Scholar]
- Humenik, M.; Smith, A.; Scheibel, T. Recombinant Spider Silks—Biopolymers with Potential for Future Applications. Polymers 2011, 3, 640–661. [Google Scholar] [CrossRef]
- Whittall, D.R.; Baker, K.V.; Breitling, R.; Takano, E. Host Systems for the Production of Recombinant Spider Silk. Trends Biotechnol. 2021, 39, 560–573. [Google Scholar] [CrossRef]
- Lin, S.; Chen, G.; Liu, X.; Meng, Q. Chimeric Spider Silk Proteins Mediated by Intein Result in Artificial Hybrid Silks. Biopolymers 2016, 105, 385–392. [Google Scholar] [CrossRef]
- Bowen, C.H.; Dai, B.; Sargent, C.J.; Bai, W.; Ladiwala, P.; Feng, H.; Huang, W.; Kaplan, D.L.; Galazka, J.M.; Zhang, F. Recombinant Spidroins Fully Replicate Primary Mechanical Properties of Natural Spider Silk. Biomacromolecules 2018, 19, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Pan, F.; Hu, C.-F.; Lee, S.Y.; Xia, X.-X.; Qian, Z.-G. Secretory Production of Spider Silk Proteins in Metabolically Engineered Corynebacterium Glutamicum for Spinning into Tough Fibers. Metab. Eng. 2022, 70, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Widmaier, D.M.; Tullman-Ercek, D.; Mirsky, E.A.; Hill, R.; Govindarajan, S.; Minshull, J.; Voigt, C.A. Engineering the Salmonella Type III Secretion System to Export Spider Silk Monomers. Mol. Syst. Biol. 2009, 5, 309. [Google Scholar] [CrossRef] [PubMed]
- Foong, C.P.; Higuchi-Takeuchi, M.; Malay, A.D.; Oktaviani, N.A.; Thagun, C.; Numata, K. A Marine Photosynthetic Microbial Cell Factory as a Platform for Spider Silk Production. Commun. Biol. 2020, 3, 357. [Google Scholar] [CrossRef]
- Jansson, R.; Lau, C.H.; Ishida, T.; Ramström, M.; Sandgren, M.; Hedhammar, M. Functionalized Silk Assembled from a Recombinant Spider Silk Fusion Protein (Z-4RepCT) Produced in the Methylotrophic Yeast Pichia pastoris. Biotechnol. J. 2016, 11, 687–699. [Google Scholar] [CrossRef]
- Sidoruk, K.V.; Davydova, L.I.; Kozlov, D.G.; Gubaidullin, D.G.; Glazunov, A.V.; Bogush, V.G.; Debabov, V.G. Fermentation Optimization of a Saccharomyces Cerevisiae Strain Producing 1F9 Recombinant Spidroin. Appl. Biochem. Microbiol. 2015, 51, 766–773. [Google Scholar] [CrossRef]
- Lazaris, A.; Arcidiacono, S.; Huang, Y.; Zhou, J.-F.; Duguay, F.; Chretien, N.; Welsh, E.A.; Soares, J.W.; Karatzas, C.N. Spider Silk Fibers Spun from Soluble Recombinant Silk Produced in Mammalian Cells. Science 2002, 295, 472–476. [Google Scholar] [CrossRef]
- Grip, S.; Rising, A.; Nimmervoll, H.; Storckenfeldt, E.; McQueen-Mason, S.J.; Pouchkina-Stantcheva, N.; Vollrath, F.; Engström, W.; Fernandez-Arias, A. Transient Expression of a Major Ampullate Spidroin 1 Gene Fragment from Euprosthenops sp. in Mammalian Cells. Cancer Genom. Proteom. 2006, 3, 83–87. [Google Scholar]
- Zhang, Y.; Hu, J.; Miao, Y.; Zhao, A.; Zhao, T.; Wu, D.; Liang, L.; Miikura, A.; Shiomi, K.; Kajiura, Z.; et al. Expression of EGFP-Spider Dragline Silk Fusion Protein in BmN Cells and Larvae of Silkworm Showed the Solubility Is Primary Limit for Dragline Proteins Yield. Mol. Biol. Rep. 2008, 35, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Schillberg, S.; Raven, N.; Spiegel, H.; Rasche, S.; Buntru, M. Critical Analysis of the Commercial Potential of Plants for the Production of Recombinant Proteins. Front. Plant Sci. 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Weichert, N.; Hauptmann, V.; Helmold, C.; Conrad, U. Seed-Specific Expression of Spider Silk Protein Multimers Causes Long-Term Stability. Front. Plant Sci. 2016, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.A.; Russo, J.; Gravgaard, C.; McCartney, H.; Gaines, W.; Marcotte, W.R. Spider Silk-like Proteins Derived from Transgenic Nicotiana Tabacum. Transgenic Res. 2016, 25, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Gührs, K.-H.; Grosse, F.; Conrad, U. Production of Spider Silk Proteins in Tobacco and Potato. Nat. Biotechnol. 2001, 19, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Hugie, M.R. Expression Systems for Synthetic Spider Silk Protein Production. Ph.D. Thesis, Utah State University, Logan, UT, USA, 2019. [Google Scholar] [CrossRef]
- Yang, J.; Barr, L.A.; Fahnestock, S.R.; Liu, Z.-B. High Yield Recombinant Silk-like Protein Production in Transgenic Plants through Protein Targeting. Transgenic Res. 2005, 14, 313–324. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, L.; Day, B.A.; Harris, T.I.; Oliveira, P.; Knittel, C.; Licon, A.L.; Gong, C.; Dion, G.; Lewis, R.V.; et al. CRISPR/Cas9 Initiated Transgenic Silkworms as a Natural Spinner of Spider Silk. Biomacromolecules 2019, 20, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.L.; Jones, J.A.; Bringhurst, H.N.; Copeland, C.G.; Addison, J.B.; Weber, W.S.; Mou, Q.; Yarger, J.L.; Lewis, R.V. Mechanical and Physical Properties of Recombinant Spider Silk Films Using Organic and Aqueous Solvents. Biomacromolecules 2014, 15, 3158–3170. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, S.; Piao, S.; An, T.; Wang, C. Production of Artificial Synthetic Spidroin Gene 4S-Transgenic Cloned Sheep Embryos Using Somatic Cell Nuclear Transfer. Anim. Biotechnol. 2021, 32, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Kuhbier, J.W.; Allmeling, C.; Reimers, K.; Hillmer, A.; Kasper, C.; Menger, B.; Brandes, G.; Guggenheim, M.; Vogt, P.M. Interactions between Spider Silk and Cells—NIH/3T3 Fibroblasts Seeded on Miniature Weaving Frames. PLoS ONE 2010, 5, e12032. [Google Scholar] [CrossRef] [PubMed]
- Kuhbier, J.W.; Reimers, K.; Kasper, C.; Allmeling, C.; Hillmer, A.; Menger, B.; Vogt, P.M.; Radtke, C. First Investigation of Spider Silk as a Braided Microsurgical Suture. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Hennecke, K.; Redeker, J.; Kuhbier, J.; Strauss, S.; Allmeling, C.; Kasper, C.; Vogt, P. Bundles of Spider Silk, Braided into Sutures, Resist Basic Cyclic Tests: Potential Use for Flexor Tendon Repair. PLoS ONE 2013, 8, e61100. [Google Scholar] [CrossRef]
- Allmeling, C.; Jokuszies, A.; Reimers, K.; Kall, S.; Vogt, P. Use of Spider Silk Fibres as an Innovative Material in a Biocompatible Artificial Nerve Conduit. J. Cell. Mol. Med. 2006, 10, 770–777. [Google Scholar] [CrossRef]
- Wendt, H.; Hillmer, A.; Reimers, K.; Kuhbier, J.; Schäfer-Nolte, F.; Allmeling, C.; Vogt, P. Artificial Skin—Culturing of Different Skin Cell Lines for Generating an Artificial Skin Substitute on Cross-Weaved Spider Silk Fibres. PLoS ONE 2011, 6, e21833. [Google Scholar] [CrossRef]
- Steins, A.; Dik, P.; Müller, W.; Vervoort, S.; Reimers, K.; Kuhbier, J.; Schepers, K. In Vitro Evaluation of Spider Silk Meshes as a Potential Biomaterial for Bladder Reconstruction. PLoS ONE 2015, 10, e0145240. [Google Scholar] [CrossRef]
- Trossmann, V.T.; Lentz, S.; Scheibel, T. Factors Influencing Properties of Spider Silk Coatings and Their Interactions within a Biological Environment. J. Funct. Biomater. 2023, 14, 434. [Google Scholar] [CrossRef]
- Borkner, C.B.; Elsner, M.B.; Scheibel, T. Coatings and Films Made of Silk Proteins. ACS Appl. Mater. Interfaces 2014, 6, 15611–15625. [Google Scholar] [CrossRef]
- Nilebäck, L.; Hedin, J.; Widhe, M.; Floderus, L.S.; Krona, A.; Bysell, H.; Hedhammar, M. Self-Assembly of Recombinant Silk as a Strategy for Chemical-Free Formation of Bioactive Coatings: A Real-Time Study. Biomacromolecules 2017, 18, 846–854. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Q.; Yang, W.; Wang, L.; Wang, J.; You, R.; Luo, Z.; Zhang, Q.; Yan, S. Development of a Bioactive Silk Fibroin Bilayer Scaffold for Wound Healing and Scar Inhibition. Int. J. Biol. Macromol. 2024, 255, 128350. [Google Scholar] [CrossRef] [PubMed]
- Krishnaji, S.T.; Huang, W.; Rabotyagova, O.; Kharlampieva, E.; Choi, I.; Tsukruk, V.V.; Naik, R.; Cebe, P.; Kaplan, D.L. Thin Film Assembly of Spider Silk-like Block Copolymers. Langmuir 2011, 27, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Lamprogiannis, L.; Karamitsos, A.; Karagkiozaki, V.; Tsinopoulos, I.; Gioti, M.; Fatouros, D.G.; Dimitrakos, S.; Logothetidis, S. Design and Fabrication of Drug-eluting Polymeric Thin Films for Applications in Ophthalmology. IET Nanobiotechnol. 2018, 12, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Zeplin, P.; Maksimovikj, N.; Jordan, M.; Nickel, J.; Lang, G.; Leimer, A.; Scheibel, T. Spider Silk Coatings as a Bioshield to Reduce Periprosthetic Fibrous Capsule Formation. Adv. Funct. Mater. 2014, 24, 2658–2666. [Google Scholar] [CrossRef]
- Borkner, C.B.; Wohlrab, S.; Möller, E.; Lang, G.; Scheibel, T. Surface Modification of Polymeric Biomaterials Using Recombinant Spider Silk Proteins. ACS Biomater. Sci. Eng. 2017, 3, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Zou, G.; Huang, J.; Ren, X.; Tian, Q.; Yu, Q.; Wang, P.; Yuan, Y.; Tang, W.; Wang, C.; et al. Water-Responsive Supercontractile Polymer Films for Bioelectronic Interfaces. Nature 2023, 624, 295–302. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Z.; Wei, M.; Chen, D.; Li, M. A Novel Scaffold from Recombinant Spider Silk Protein in Tissue Engineering. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2010; Volume 152. [Google Scholar]
- Johansson, U.; Widhe, M.; Shalaly, N.; Arregui, I.; Nilebäck, L.; Tasiopoulos, C.; Hedhammar, M. Assembly of Functionalized Silk Together with Cells to Obtain Proliferative 3D Cultures Integrated in a Network of ECM-like Microfibers. Sci. Rep. 2019, 9, 6291. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, S.; Chen, X.; Shahabi, S.; Nasiri, N. Resorbable Membranes for Guided Bone Regeneration: Critical Features, Potentials, and Limitations. ACS Mater. Au 2023, 3, 394–417. [Google Scholar] [CrossRef]
- Schacht, K.; Vogt, J.; Scheibel, T. Foams Made of Engineered Recombinant Spider Silk Proteins as 3D Scaffolds for Cell Growth. ACS Biomater. Sci. Eng. 2016, 2, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Rammensee, S.; Huemmerich, D.; Hermanson, K.; Scheibel, T.; Bausch, A. Rheological Characterization of Hydrogels Formed by Recombinantly Produced Spider Silk. Appl. Phys. 2006, 82, 261–264. [Google Scholar] [CrossRef]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef]
- Nie, L.; Sun, Y.; Okoro, O.V.; Deng, Y.; Jiang, G.; Shavandi, A. Click Chemistry for 3D Bioprinting. Mater. Horiz. 2023, 10, 2727–2763. [Google Scholar] [CrossRef]
- Withanage, S.; Savin, A.; Nikolaeva, V.; Kiseleva, A.; Dukhinova, M.; Krivoshapkin, P.; Krivoshapkina, E. Native Spider Silk-Based Antimicrobial Hydrogels for Biomedical Applications. Polymers 2021, 13, 1796. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Kapoor, S.; Kundu, S.C. Silk Protein-Based Hydrogels: Promising Advanced Materials for Biomedical Applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, L.; Cakić, S. A Review of Patents and Innovative Biopolymer-Based Hydrogels. Gels 2023, 9, 556. [Google Scholar] [CrossRef]
- Zahra, D.; Shokat, Z.; Ahmad, A.; Javaid, A.; Khurshid, M.; Ashfaq, U.A.; Nashwan, A.J. Exploring the Recent Developments of Alginate Silk Fibroin Material for Hydrogel Wound Dressing: A Review. Int. J. Biol. Macromol. 2023, 248, 125989. [Google Scholar] [CrossRef]
- Li, X.; Ullah, M.W.; Li, B.; Chen, H. Recent Progress in Advanced Hydrogel-Based Embolic Agents: From Rational Design Strategies to Improved Endovascular Embolization. Adv. Healthc. Mater. 2023, 12, 2202787. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, H.; Huang, Y.; Wei, Y.; Chen, J. Naturally Sourced Hydrogels: Emerging Fundamental Materials for next-Generation Healthcare Sensing. Chem. Soc. Rev. 2023, 52, 2992–3034. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Willner, I. Stimuli-Responsive Biomolecule-Based Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2020, 59, 15342–15377. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D Printing of Hydrogels: Rational Design Strategies and Emerging Biomedical Applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically Crosslinked Silk and Silk-Gelatin Hydrogels with Tunable Gelation Kinetics, Mechanical Properties and Bioactivity for Cell Culture and Encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional Silk Fibroin Hydrogels: Preparation, Properties and Applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef]
- Liu, L.; Tang, H.; Wang, Y. Polymeric Biomaterials: Advanced Drug Delivery Systems in Osteoarthritis Treatment. Heliyon 2023, 9, e21544. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Y.; Zhu, H.; Ma, T.; Sun, X.; Xiao, J. Microenvironment-Responsive Nanosystems for Osteoarthritis Therapy. Eng. Regen. 2024, 5, 92–110. [Google Scholar] [CrossRef]
- Shi, L.; Wang, F.; Zhu, W.; Xu, Z.; Fuchs, S.; Hilborn, J.; Zhu, L.; Ma, Q.; Wang, Y.; Weng, X.; et al. Self-Healing Silk Fibroin-Based Hydrogel for Bone Regeneration: Dynamic Metal-Ligand Self-Assembly Approach. Adv. Funct. Mater. 2017, 27, 1700591. [Google Scholar] [CrossRef]
- Zheng, H.; Lin, N.; He, Y.; Zuo, B. Self-Healing, Self-Adhesive Silk Fibroin Conductive Hydrogel as a Flexible Strain Sensor. ACS Appl. Mater. Interfaces 2021, 13, 40013–40031. [Google Scholar] [CrossRef]
- Ngadimin, K.D.; Stokes, A.; Gentile, P.; Ferreira, A.M. Biomimetic Hydrogels Designed for Cartilage Tissue Engineering. Biomater. Sci. 2021, 9, 4246–4259. [Google Scholar] [CrossRef]
- Hu, J.; Chen, B.; Guo, F.; Du, J.; Gu, P.; Lin, X.; Yang, W.; Zhang, H.; Lu, M.; Huang, Y.; et al. Injectable Silk Fibroin/Polyurethane Composite Hydrogel for Nucleus Pulposus Replacement. J. Mater. Sci. Mater. Med. 2012, 23, 711–722. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Guo, C.; Huang, Z.; Zhang, W.; Ma, F.; Wang, Z.; Kong, Q.; Wang, Y. Application and Development of Hydrogel Biomaterials for the Treatment of Intervertebral Disc Degeneration: A Literature Review. Front. Cell Dev. Biol. 2023, 11, 1286223. [Google Scholar] [CrossRef]
- Shanmugam, D.K.; Anitha, S.C.; Souresh, V.; Madhavan, Y.; Sampath, S.; Catakapatri Venugopal, D.; Saravanan, M. Current Advancements in the Development of Bionic Organs Using Regenerative Medicine and 3D Tissue Engineering. Mater. Technol. 2023, 38, 2242732. [Google Scholar] [CrossRef]
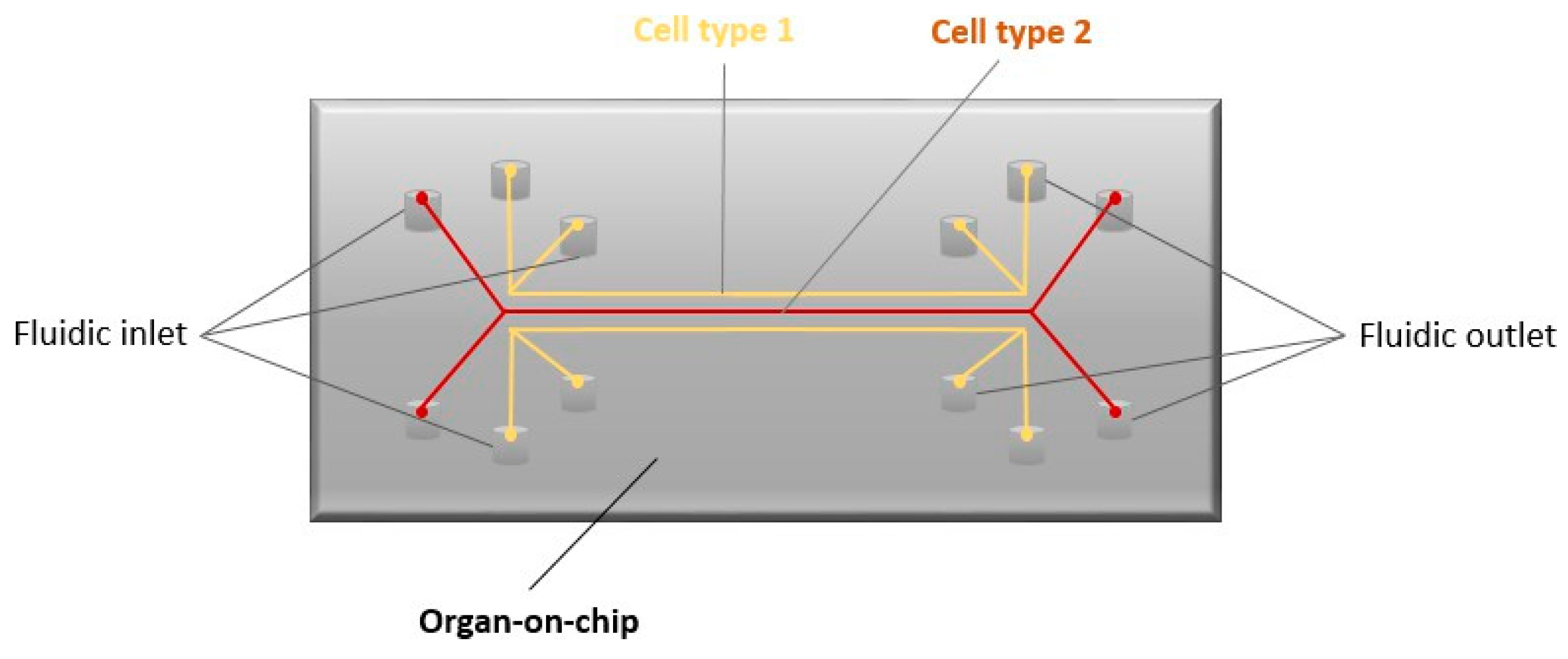
- Sontheimer-Phelps, A.; Hassell, B.; Ingber, D. Modelling Cancer in Microfluidic Human Organs-on Chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Bakirci, E.; Guenat, O.; Ahmad, S.; Gantenbein, B. Tissue Engineering Approaches for the Repair and Regeneration of the Anterior Cruciate Ligament: Towards 3D Bioprinted ACL-on-Chip. Eur. Cells Mater. 2022, 44, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In Vivo Bioresponses to Silk Proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Baoyong, L.; Jian, Z.; Denglong, C.; Min, L. Evaluation of a New Type of Wound Dressing Made from Recombinant Spider Silk Protein Using Rat Models. Burns 2010, 36, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Leonor, I.; Mano, J.; Reis, R.; Kaplan, D. Spider silk-bone sialoprotein fusion proteins for bone tissue engineering. Soft Matter 2011, 7, 4964–4973. [Google Scholar] [CrossRef]
- Dellaquila, A.; Greco, G.; Campodoni, E.; Mazzocchi, M.; Mazzolai, B.; Tampieri, A.; Sandri, M. Optimized Production of a High-Performance Hybrid Biomaterial: Biomineralized Spider Silk for Bone Tissue Engineering. J. Appl. Polym. Sci. 2019, 137, 48739. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Z.; Yin, Z.; Zhang, W.; Bai, L.; Su, J. Cell Unit-Inspired Natural Nano-Based Biomaterials as Versatile Building Blocks for Bone/Cartilage Regeneration. J. Nanobiotechnology 2023, 21, 293. [Google Scholar] [CrossRef]
- Dastagir, K.; Dastagir, N.; Limbourg, A.; Reimers, K.; Straub, S.; Vogt, P. In Vitro Construction of Artificial Blood Vessels Using Spider Silk as a Supporting Matrix. J. Mech. Behav. Biomed. Mater. 2020, 101, 103436. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, Y.; Chen, F.; Hu, X.; Cheng, W.; Lu, Q.; Kaplan, D.L. Biomimetic Vascular Grafts with Circumferentially and Axially Oriented Microporous Structures for Native Blood Vessel Regeneration. Adv. Funct. Mater. 2024, 34, 2308888. [Google Scholar] [CrossRef]
- Cattaneo, I.; Figliuzzi, M.; Azzollini, N.; Catto, V.; Farè, S.; Tanzi, M.C.; Alessandrino, A.; Freddi, G.; Remuzzi, A. In Vivo Regeneration of Elastic Lamina on Fibroin Biodegradable Vascular Scaffold. Int. J. Artif. Organs 2013, 36, 166–174. [Google Scholar] [CrossRef]
- Soffer, L.; Wang, X.; Zhang, X.; Kluge, J.; Dorfmann, L.; Kaplan, D.L.; Leisk, G. Silk-Based Electrospun Tubular Scaffolds for Tissue-Engineered Vascular Grafts. J. Biomater. Sci. Polym. Ed. 2008, 19, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Catto, V.; Farè, S.; Cattaneo, I.; Figliuzzi, M.; Alessandrino, A.; Freddi, G.; Remuzzi, A.; Tanzi, M.C. Small Diameter Electrospun Silk Fibroin Vascular Grafts: Mechanical Properties, in Vitro Biodegradability, and in Vivo Biocompatibility. Mater. Sci. Eng. C 2015, 54, 101–111. [Google Scholar] [CrossRef]
- Cardenas, L.; Sandoval, O.L.; Hurtado, D. Biomechanical Analysis of Spider Silk for Anterior Cruciate Ligament Reconstruction. ARPN J. Eng. Appl. Sci. 2016, 11, 52063642. [Google Scholar]
- Farè, S.; Torricelli, P.; Giavaresi, G.; Bertoldi, S.; Alessandrino, A.; Villa, T.; Fini, M.; Tanzi, M.C.; Freddi, G. In Vitro Study on Silk Fibroin Textile Structure for Anterior Cruciate Ligament Regeneration. Mater. Sci. Eng. C 2013, 33, 3601–3608. [Google Scholar] [CrossRef] [PubMed]
- Petzold, J.; Aigner, T.; Touska, F.; Zimmermann, K.; Scheibel, T.; Engel, F. Surface Features of Recombinant Spider Silk Protein eADF4(Κ16)-Made Materials Are Well-Suited for Cardiac Tissue Engineering. Adv. Funct. Mater. 2017, 27, 1701427. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Guo, B.; Ma, P.X. Nanofiber Yarn/Hydrogel Core–Shell Scaffolds Mimicking Native Skeletal Muscle Tissue for Guiding 3D Myoblast Alignment, Elongation, and Differentiation. ACS Nano 2015, 9, 9167–9179. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Guo, B.; Ma, P.X. Interwoven Aligned Conductive Nanofiber Yarn/Hydrogel Composite Scaffolds for Engineered 3D Cardiac Anisotropy. ACS Nano 2017, 11, 5646–5659. [Google Scholar] [CrossRef]
- Dong, R.; Ma, P.X.; Guo, B. Conductive Biomaterials for Muscle Tissue Engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef]
- Brooks, A.K.; Ramsey, R.G.; Zhang, N.; Yadavalli, V.K. Tunable Light-Actuated Interpenetrating Networks of Silk Fibroin and Gelatin for Tissue Engineering and Flexible Biodevices. ACS Biomater. Sci. Eng. 2023, 9, 5793–5803. [Google Scholar] [CrossRef]
- Allmeling, C.; Jokuszies, A.; Reimers, K.; Kall, S.; Choi, C.Y.; Brandes, G.; Kasper, C.; Scheper, T.; Guggenheim, M.; Vogt, P.M. Spider Silk Fibres in Artificial Nerve Constructs Promote Peripheral Nerve Regeneration. Cell Prolif. 2008, 41, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.; Welzel, G.; Haynl, C.; Schuster, S.; Scheibel, T. Recombinant Spider Silk and Collagen-Based Nerve Guidance Conduits Support Neuronal Cell Differentiation and Functionality In Vitro. ACS Appl. Bio Mater. 2019, 2, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Lewicka, M.; Rebellato, P.; Lewicki, J.; Uhlén, P.; Rising, A.; Hermanson, O. Recombinant Spider Silk Protein Matrices Facilitate Multi-Analysis of Calcium-Signaling in Neural Stem Cell-Derived AMPA-Responsive Neuron. BioRxiv 2019, 579292. [Google Scholar] [CrossRef]
- Hu, J. Spider Silks and Their Nanostructures. In Proceedings of the Global Summit on Nanomaterials: Applications and Properties Nanomaterials NANOMAT22, Dubai, United Arab Emirates, 23–25 March 2022. [Google Scholar]
- Zivic, F.; Grujovic, N.; Ul Ahad, I.; Brabazon, D. Introduction—The Current Status and Momentum. In Commercialization of Nanotechnologies—A Case Study Approach; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Sayin, D.; Gundogdu, G.; Kilic-Erkek, O.; Gundogdu, K.; Coban, H.S.; Abban-Mete, G. Silk Protein Sericin: A Promising Therapy for Achilles Tendinopathy—Evidence from an Experimental Rat Model. Clin. Rheumatol. 2023, 42, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- Kojic, N.; Pritchard, E.M.; Tao, H.; Brenckle, M.A.; Mondia, J.P.; Panilaitis, B.; Omenetto, F.; Kaplan, D.L. Focal Infection Treatment Using Laser-Mediated Heating of Injectable Silk Hydrogels with Gold Nanoparticles. Adv. Funct. Mater. 2012, 22, 3793–3798. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer Based Nanomaterials in Drug Delivery Systems: A Review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Rasekh, M.; Pisapia, F.; Nokhodchi, A. Innovative Applications of Electrospun Nanofibers in Cancer Research. J. Drug Deliv. Sci. Technol. 2024, 91, 105255. [Google Scholar] [CrossRef]
- Helfricht, N.; Klug, M.; Mark, A.; Kuznetsov, V.; Blum, C.; Scheibel, T.; Papastavrou, G. Surface Properties of Spider Silk Particles in Solution. Biomater. Sci. 2013, 1, 1166–1171. [Google Scholar] [CrossRef]
- Jastrzębska, K.; Kucharczyk, K.; Florczak, A.; Dondajewska, E.; Mackiewicz, A.; Dams-Kozlowska, H. Silk as an Innovative Biomaterial for Cancer Therapy. Rep. Pract. Oncol. Radiother. 2015, 20, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.; Hamad, M.; Hafez, M.; Wooley, K.; Elsabahy, M. Nanomedicine in Management of Hepatocellular Carcinoma: Challenges and Opportunities. Int. J. Cancer 2017, 140, 1475–1484. [Google Scholar] [CrossRef]
- Aquib, M.; Juthi, A.; Farooq, M.; Ali, M.; Janabi, A.; Bavi, S.; Wang, B. Advances in Local and Systemic Drug Delivery Systems for Post-Surgical Cancer Treatment. J. Mater. Chem. B 2020, 8, 8507–8518. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Jain, A.; Domb, A.; Khan, W. Biodegradable Polymers for Targeted Delivery of Anti-Cancer Drugs. Expert Opin. Drug Deliv. 2016, 13, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Park, J.; Karageorgiou, V.; Kim, U.; Valluzzi, R.; Cebe, P.; Kaplan, D. Water-Stable Silk Films with Reduced β-Sheet Content. Adv. Funct. Mater. 2015, 15, 1241–1247. [Google Scholar] [CrossRef]
- Laakkonen, P.; Vuorinen, K. Homing Peptides as Targeted Delivery Vehicles. Integr. Biol. 2010, 2, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Mieszawska-Czajkowska, A.; Kvenvold, L.; Kaplan, D. Silk-Based Nanocomplexes with Tumor-Homing Peptides for Tumor-Specific Gene Delivery. Macromol. Biosci. 2012, 12, 75–82. [Google Scholar] [CrossRef]
- Florczak, A.; Jastrzebska, K.; Mackiewicz, A.; Dams-Kozlowska, H. Blending Two Bioengineered Spider Silks to Develop Cancer Targeting Spheres. Mater. Chem. B 2017, 5, 3000–3011. [Google Scholar] [CrossRef]
- Hines, D.; Kaplan, D. Characterization of Small Molecule Controlled Release from Silk Films. Macromol. Chem. Phys. 2012, 214, 280–294. [Google Scholar] [CrossRef]
- Jastrzebska, K.; Florczak, A.; Kucharczyk, K.; Lin, Y.; Wang, Q.; Mackiewicz, A.; Dams-Kozlowska, H. Delivery of Chemotherapeutics Using Spheres Made of Bioengineered Spider Silks Derived from MaSp1 and MaSp2 Proteins. Nanomedicine 2018, 13, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Florczak, A.; Deptuch, T.; Kucharczyk, K.; Dams-Kozlowska, H. Systemic and Local Silk-Based Drug Delivery Systems for Cancer Therapy. Cancers 2021, 13, 5389. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Fan, D.; Chen, Y.; Zhao, Z.; He, X.; Li, G.; Lan, P. An Implantable and Controlled Drug-Release Silk Fibroin Nanofibrous Matrix to Advance the Treatment of Solid Tumour Cancers. Biomaterials 2016, 103, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hou, M.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Highly Porous Silk Fibroin Scaffold Packed in PEGDA/Sucrose Microneedles for Controllable Transdermal Drug Delivery. Biomacromolecules 2019, 20, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Tao, X.; Tan, G.; Tian, B.; Zhang, L.; Kundu, S.C.; Lu, S. Electro-Responsive Silk Fibroin Microneedles for Controlled Release of Insulin. Int. J. Biol. Macromol. 2023, 242, 124684. [Google Scholar] [CrossRef] [PubMed]
- Shchepelina, O.; Drachuk, I.; Gupta, M.; Lin, J.; Tsukruk, V. Silk-on-Silk Layer-by-Layer Microcapsules. Adv. Mater. 2011, 23, 4655–4660. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D. Silk Nanospheres and Microspheres from Silk/Pva Blend Films for Drug Delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef]
- Florczak, A.; Grzechowiak, I.; Deptuch, T.; Kucharczyk, K.; Kaminska, A.; Dams-Kozlowska, H. Silk Particles as Carriers of Therapeutic Molecules for Cancer Treatment. Materials 2020, 13, 4946. [Google Scholar] [CrossRef]
- Wongpinyochit, T.; Johnston, B.; Seib, F. Manufacture and Drug Delivery Applications of Silk Nanoparticles. J. Vis. Exp. 2016, 116, e54669. [Google Scholar]
- Cao, Y.; Liu, F.; Chen, Y.; Yu, T.; Lou, D.; Guo, Y.; Ran, H. Drug Release from Core-Shell PVA/Silk Fibroin Nanoparticles Fabricated by One-Step Electrospraying. Sci. Rep. 2017, 7, 11913. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Perez, A.; Montalban, M.; Aznar-Cervantes, S.; Cragnolini, F.; Cenis, J.; Villora, G. Production of Silk Fibroin Nanoparticles Using Ionic Liquids and High-Power Ultrasounds. J. Appl. Polym. Sci. 2014, 132, 41702–41709. [Google Scholar] [CrossRef]
- Wenk, E.; Wandrey, A.; Merkle, H.; Meinel, L. Silk Fibroin Spheres as a Platform for Controlled Drug Delivery. J. Controll. Release 2008, 132, 26–34. [Google Scholar] [CrossRef]
- Myung, S.; Kim, H.; Kim, Y.; Chen, P.; Jin, H. Fluorescent Silk Fibroin Nanoparticles Prepared Using a Reverse Microemulsion. Macromol. Res. 2008, 16, 604–608. [Google Scholar] [CrossRef]
- Solomun, J.; Totten, J.; Wongpinyochit, T.; Florence, A.; Seib, F. Manual Versus Microfluidic Assisted Nanoparticle Manufacture: Impact of Silk Fibroin Stock on Nanoparticle Characteristics. ACS Biomater. Sci. Eng. 2020, 6, 2796–2804. [Google Scholar] [CrossRef]
- Kazemimostaghim, M.; Rajkhowa, R.; Tsuzuki, T.; Wang, X. Ultrafine Silk Powder from Biocompatible Surfactant-Assisted Milling. Powder Technol. 2013, 185, 87–95. [Google Scholar] [CrossRef]
- Sun, N.; Lei, R.; Xu, J.; Kundu, S.; Cai, Y.; Yao, J.; Ni, Q. Fabricated Porous Silk Fibroin Particles for pHresponsive Drug Delivery and Targeting of Tumor Cells. J. Mater. Sci. 2019, 54, 3319–3330. [Google Scholar] [CrossRef]
- Subia, B.; Kundu, S. Drug Loading and Release on Tumor Cells Using Silk Fibroin-Albumin Nanoparticles as Carriers. Nanotechnology 2013, 24, 035103. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Z.; Ni, Y.; Xiong, Y.; Xu, W. Preparation and Characterization of a Novel Composite Based on Hyperbranched Polysilane and Fullerene. J. Appl. Polym. Sci. 2007, 105, 821–826. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, M.; Li, Y.; Chen, A.; Li, G.; Zhang, J.; Li, S. Formation of Curcumin Nanoparticles via Solution-Enhanced Dispersion by Supercritical CO2. Int. J. Nanomed. 2015, 10, 3171–3181. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Rybka, J.; Hilgendorff, M.; Krupinski, M.; Slachcinski, M.; Mackiewicz, A.; Dams-Kozlowska, H. Composite Spheres Made of Bioengineered Spider Silk and Iron Oxide Nanoparticles for Theranostics Applications. PLoS ONE 2019, 14, e0219790. [Google Scholar] [CrossRef]
- Herold, H.; Dobl, A.; Wohlrab, S.; Humenik, M.; Scheibel, T. Designed Spider Silk-Based Drug Carrier for Redox- or pH-Triggered Drug Release. Biomacromolecules 2020, 21, 4904–4912. [Google Scholar] [CrossRef]
- Colavite, P.; Sartori, A. Septic Arthritis: Immunopathogenesis, Experimental Models and Therapy. J. Venom. Anim. Toxins Trop. Dis. 2014, 20, 19. [Google Scholar] [CrossRef]
- Hasan, R.; Wohlers, A.; Shreffler, J.; Mulinti, P.; Ostlie, H.; Schaper, C.; Brooks, B.; Brooks, A. An Antibiotic-Releasing Bone Void Filling (ABVF) Putty for the Treatment of Osteomyelitis. Materials 2020, 13, 5080. [Google Scholar] [CrossRef]
- Mulinti, P.; Shreffler, J.; Hasan, R.; Dea, M.; Brooks, A.E. Infection Responsive Smart Delivery of Antibiotics Using Recombinant Spider Silk Nanospheres. Pharmaceutics 2021, 13, 1358. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga-Vélez, A.; Quintero-Martinez, A.; Orozco, L.M.; Sepúlveda-Arias, J.C. Silk Fibroin Nanocomposites as Tissue Engineering Scaffolds—A Systematic Review. Biomed. Pharmacother. 2021, 141, 111924. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Chouhan, D.; Konwarh, R.; Kumar, M.; Jadi, P.K.; Mandal, B.B. Comprehensive Review on Silk at Nanoscale for Regenerative Medicine and Allied Applications. ACS Biomater. Sci. Eng. 2019, 5, 2054–2078. [Google Scholar] [CrossRef]
- Mohammadi, P.; Aranko, S.; Landowski, C.; Ikkala, O.; Jaudzems, K.; Wagermaier, W.; Linder, M. Biomimetic Composites with Enhanced Toughening Using Silk-Inspired Triblock Proteins and Aligned Nanocellulose Reiforcements. Sci. Adv. 2019, 5, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Luca, V.; Nicola, P. Nanotube Superfiber Materials, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ornithopoulou, E.; Åstrand, C.; Gustafsson, L.; Crouzier, T.; Hedhammar, M. Self-Assembly of RGD-Functionalized Recombinant Spider Silk Protein into Microspheres in Physiological Buffer and in the Presence of Hyaluronic Acid. ACS Appl. Bio Mater. 2023, 6, 3696–3705. [Google Scholar] [CrossRef]
- Wang, X.; Ye, X.; Guo, J.; Dai, X.; Yu, S.; Zhong, B. Modeling the 3-Dimensional Structure of the Silkworm’s Spinning Apparatus in Silk Production. Acta Biomater. 2024, 174, 217–227. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, J.; Chen, S.; Jiao, X.; Li, X.; Chen, W. Synthesis, in Vitro Biocompatibility and Antibacterial Property of Novel Silk fibroin@Ag Spheres. Mater. Lett. 2024, 357, 135681. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S. ZIF-8 Nanoparticles Combined with Fibroin Protein Co-Modified TiO2 Nanotube Arrays to Construct a Drug Sustained-Release Platform. Mater. Lett. 2024, 356, 135541. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.; Lu, L.; Wang, H.; Zhao, Y.; Chai, R. Natural Multifunctional Silk Microcarriers for Noise-Induced Hearing Loss Therapy. Adv. Sci. 2024, 11, 2305215. [Google Scholar] [CrossRef]
- Li, X.; Hajinur Hirad, A.; Alarfaj, A.A.; Li, H.; Santhanam, R. A Convergent Fabrication of Graphene Oxide/Silk Fibroin/Hydroxyapatite Nanocomposites Delivery Improved Early Osteoblast Cell Adhesion and Bone Regeneration. Arab. J. Chem. 2024, 17, 105468. [Google Scholar] [CrossRef]
- Katti, K.S.; Jasuja, H.; Kar, S.; Katti, D.R. Nanostructured Biomaterials for in Vitro Models of Bone Metastasis Cancer. Curr. Opin. Biomed. Eng. 2021, 17, 100254. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branković, M.; Zivic, F.; Grujovic, N.; Stojadinovic, I.; Milenkovic, S.; Kotorcevic, N. Review of Spider Silk Applications in Biomedical and Tissue Engineering. Biomimetics 2024, 9, 169. https://doi.org/10.3390/biomimetics9030169
Branković M, Zivic F, Grujovic N, Stojadinovic I, Milenkovic S, Kotorcevic N. Review of Spider Silk Applications in Biomedical and Tissue Engineering. Biomimetics. 2024; 9(3):169. https://doi.org/10.3390/biomimetics9030169
Chicago/Turabian StyleBranković, Marija, Fatima Zivic, Nenad Grujovic, Ivan Stojadinovic, Strahinja Milenkovic, and Nikola Kotorcevic. 2024. "Review of Spider Silk Applications in Biomedical and Tissue Engineering" Biomimetics 9, no. 3: 169. https://doi.org/10.3390/biomimetics9030169
APA StyleBranković, M., Zivic, F., Grujovic, N., Stojadinovic, I., Milenkovic, S., & Kotorcevic, N. (2024). Review of Spider Silk Applications in Biomedical and Tissue Engineering. Biomimetics, 9(3), 169. https://doi.org/10.3390/biomimetics9030169






