Numerical Investigation of Odor-Guided Navigation in Flying Insects: Impact of Turbulence, Wingbeat-Induced Flow, and Schmidt Number on Odor Plume Structures
Abstract
:1. Introduction
2. Methodology
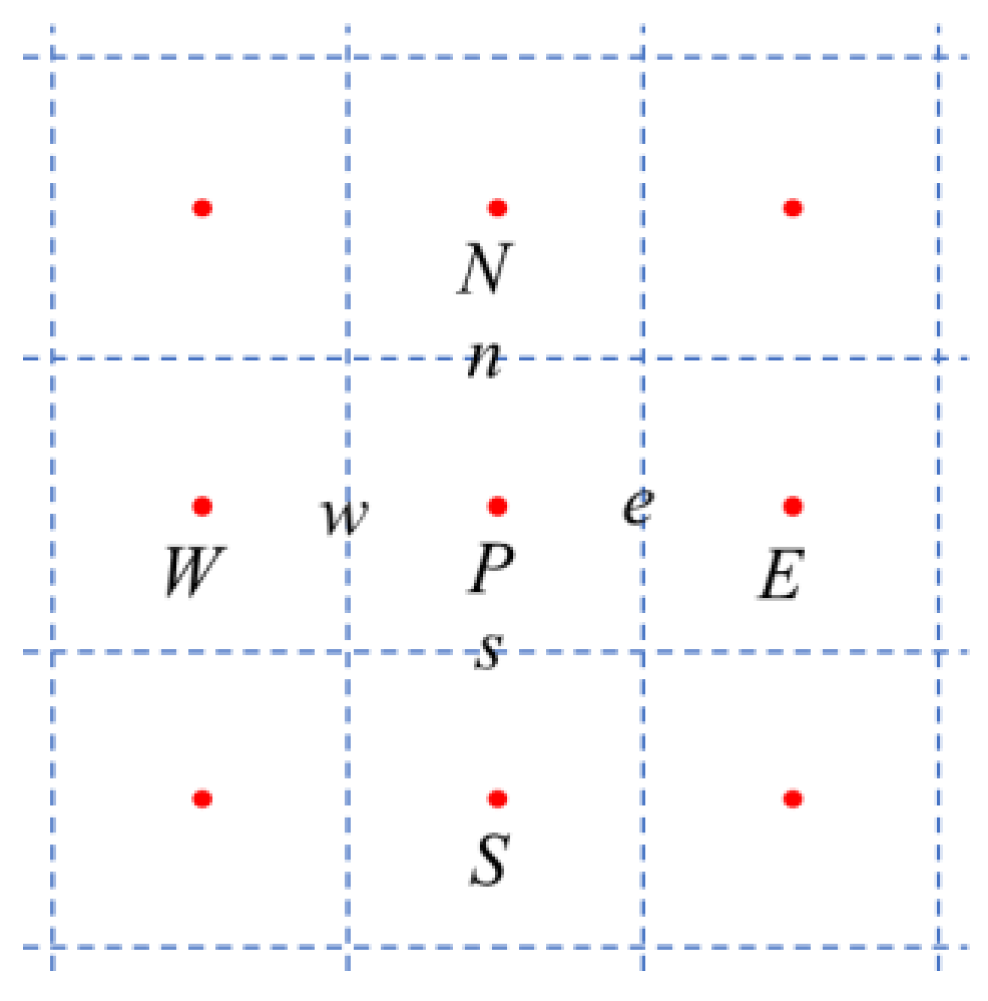
2.1. Governing Equations and Numerical Method
2.2. Odor Advection–Diffusion Equation
2.3. Wing Model with Torsional Spring
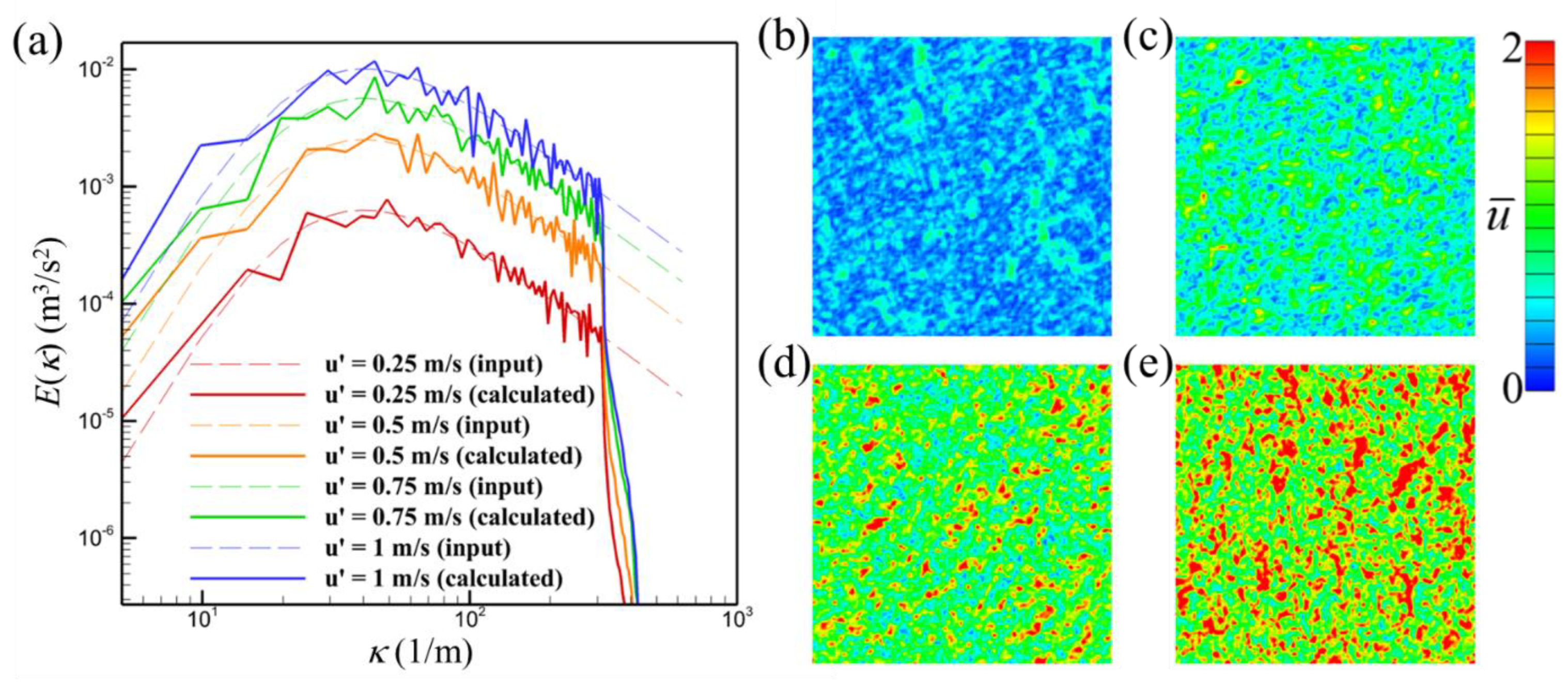
2.4. Turbulence Generator
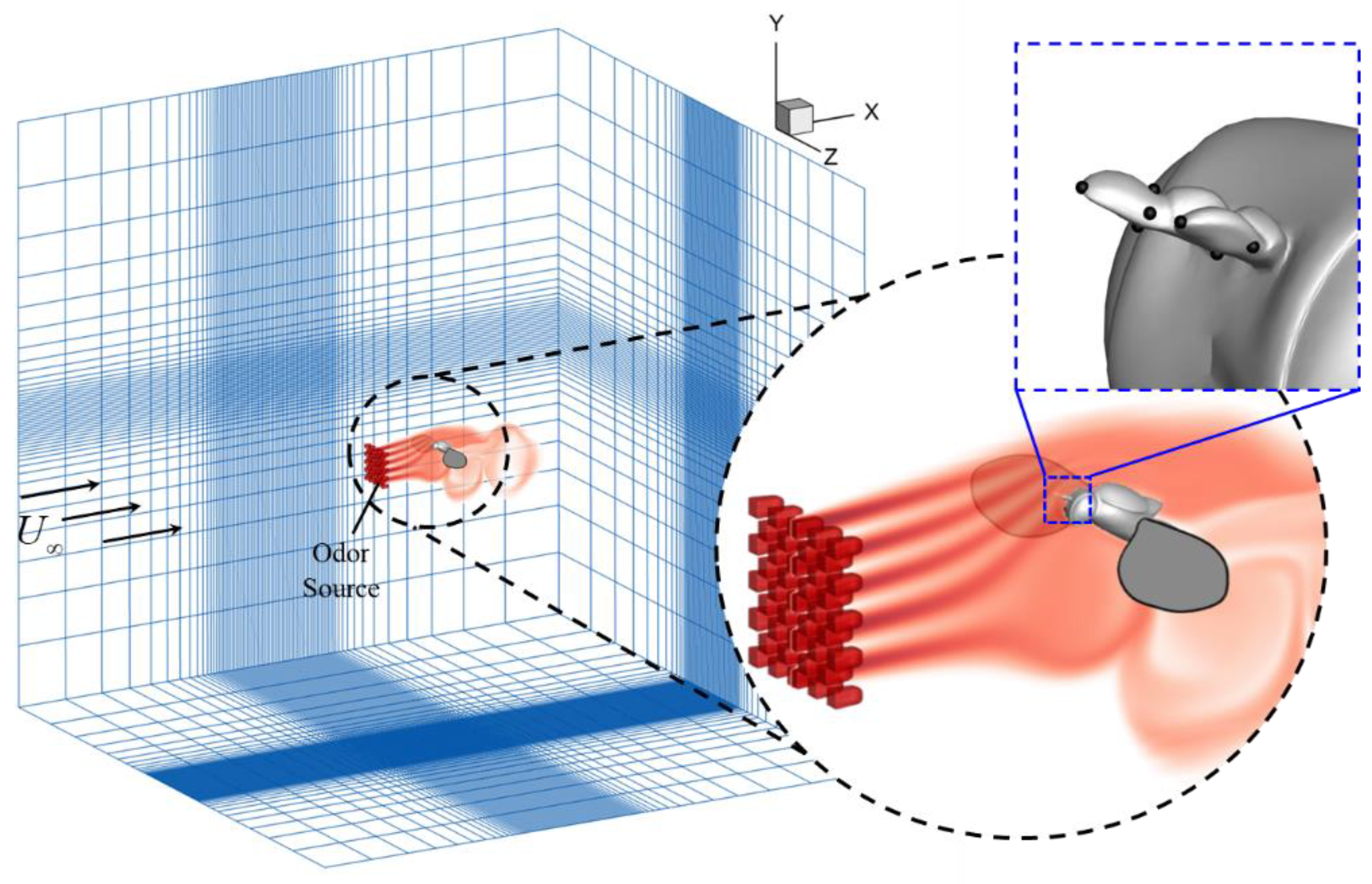
2.5. Simulation Setup
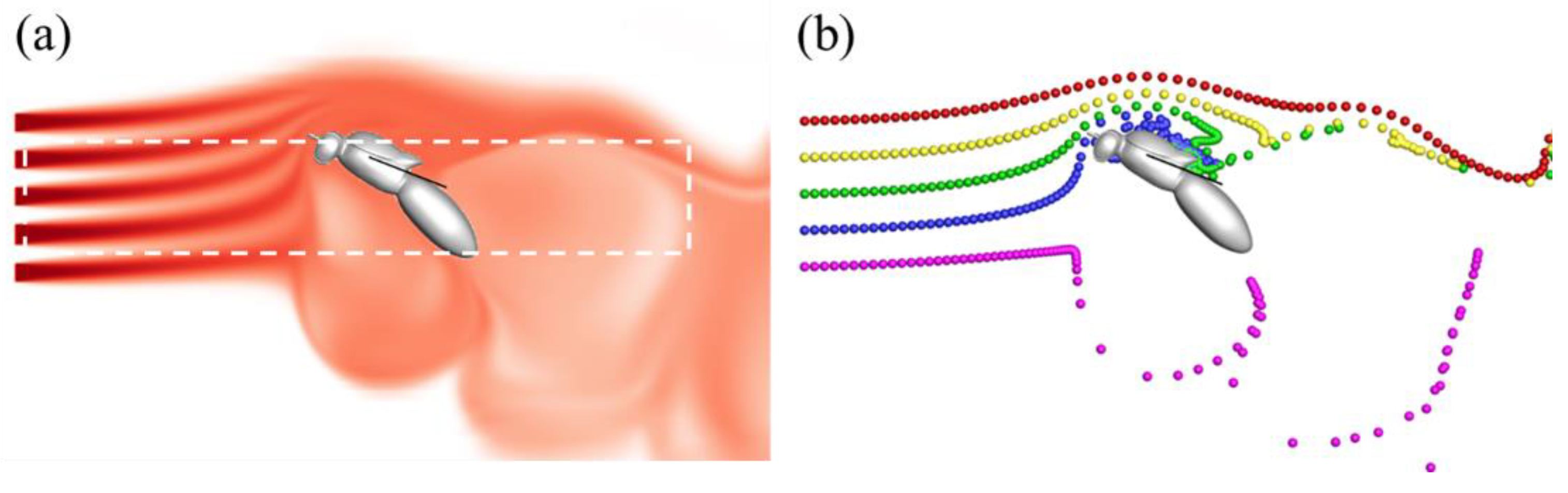
2.6. Evaluations of Olfactory Performance
3. Results and Discussion
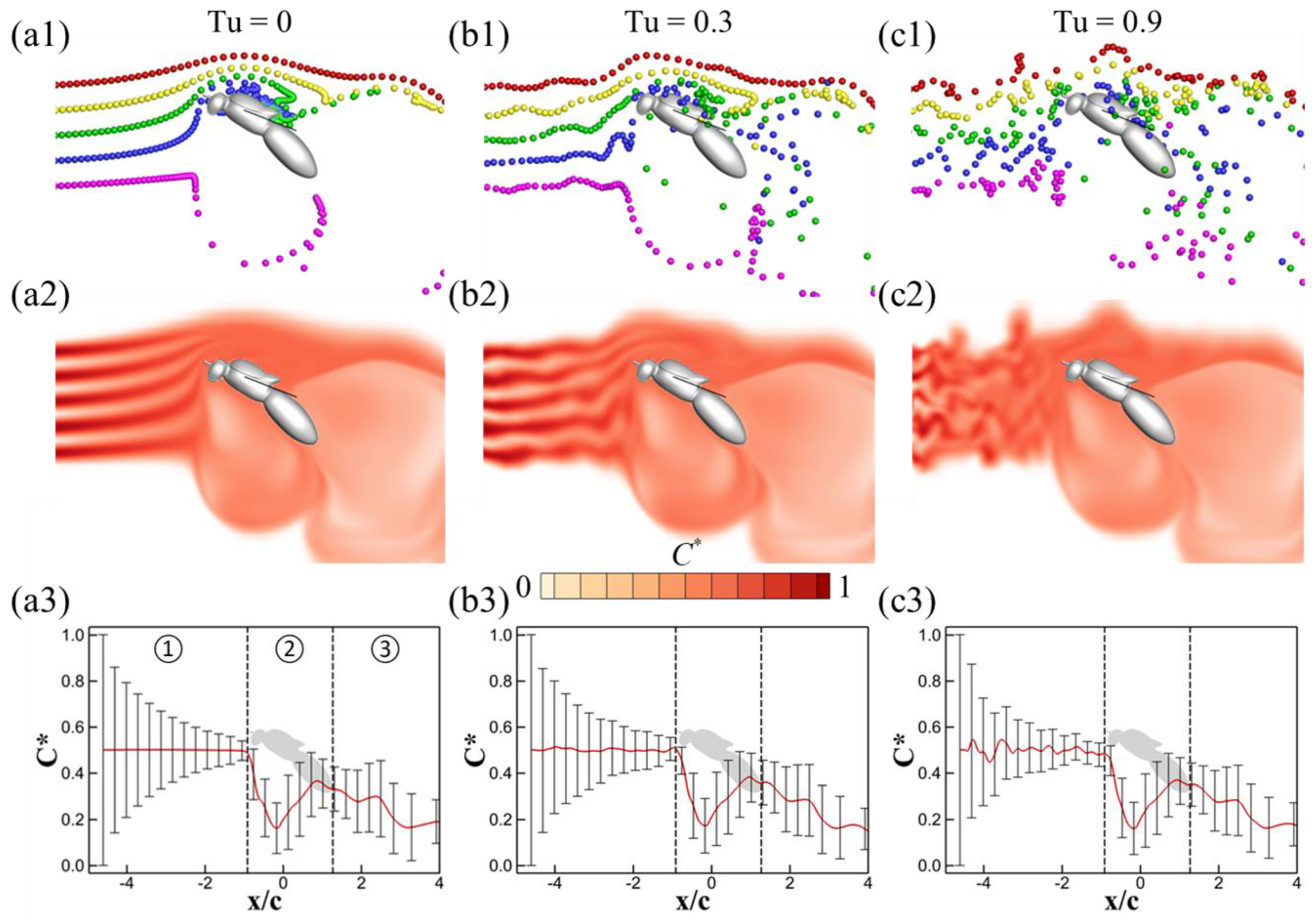
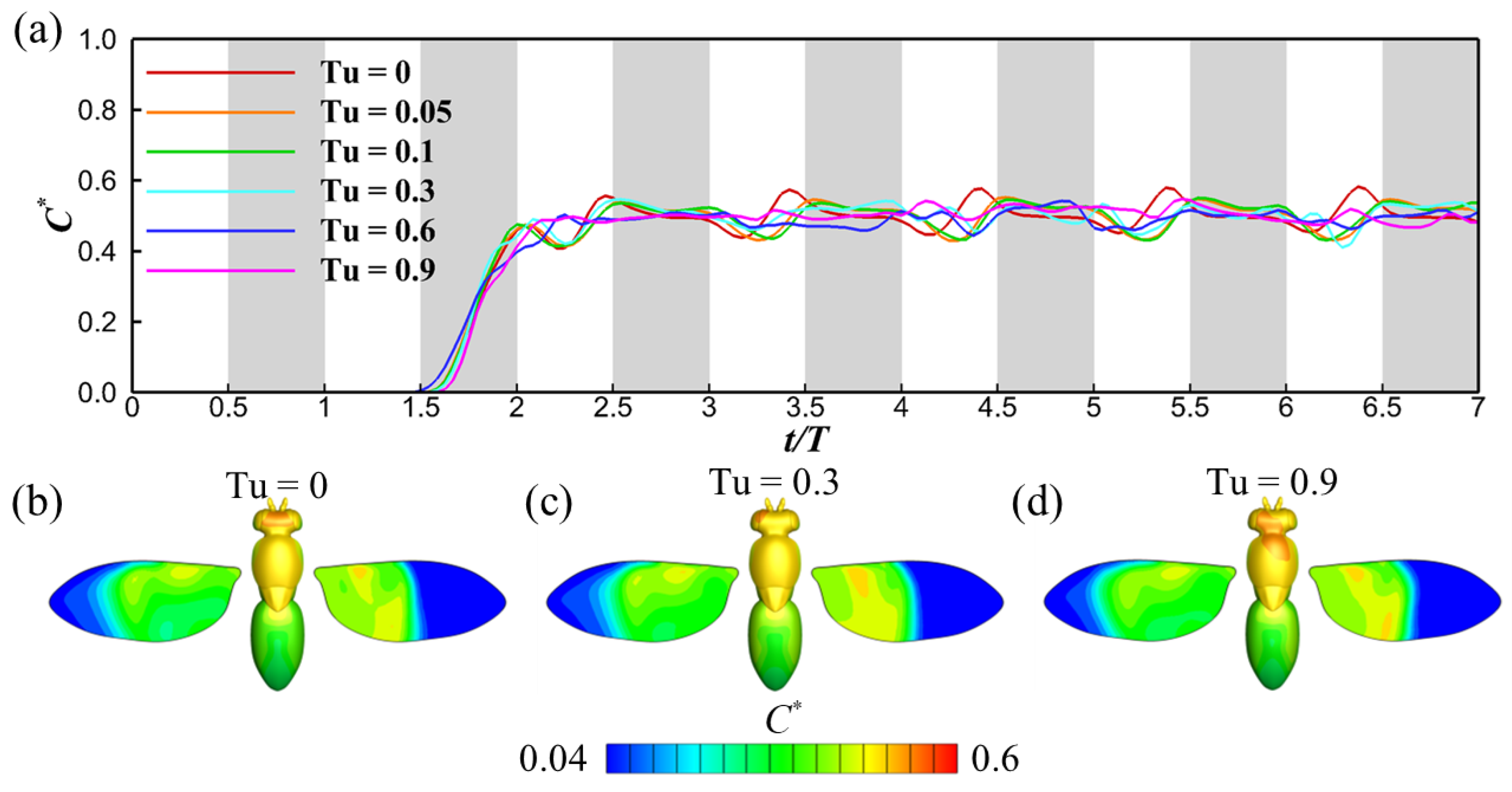
3.1. Effects of Turbulence Intensity
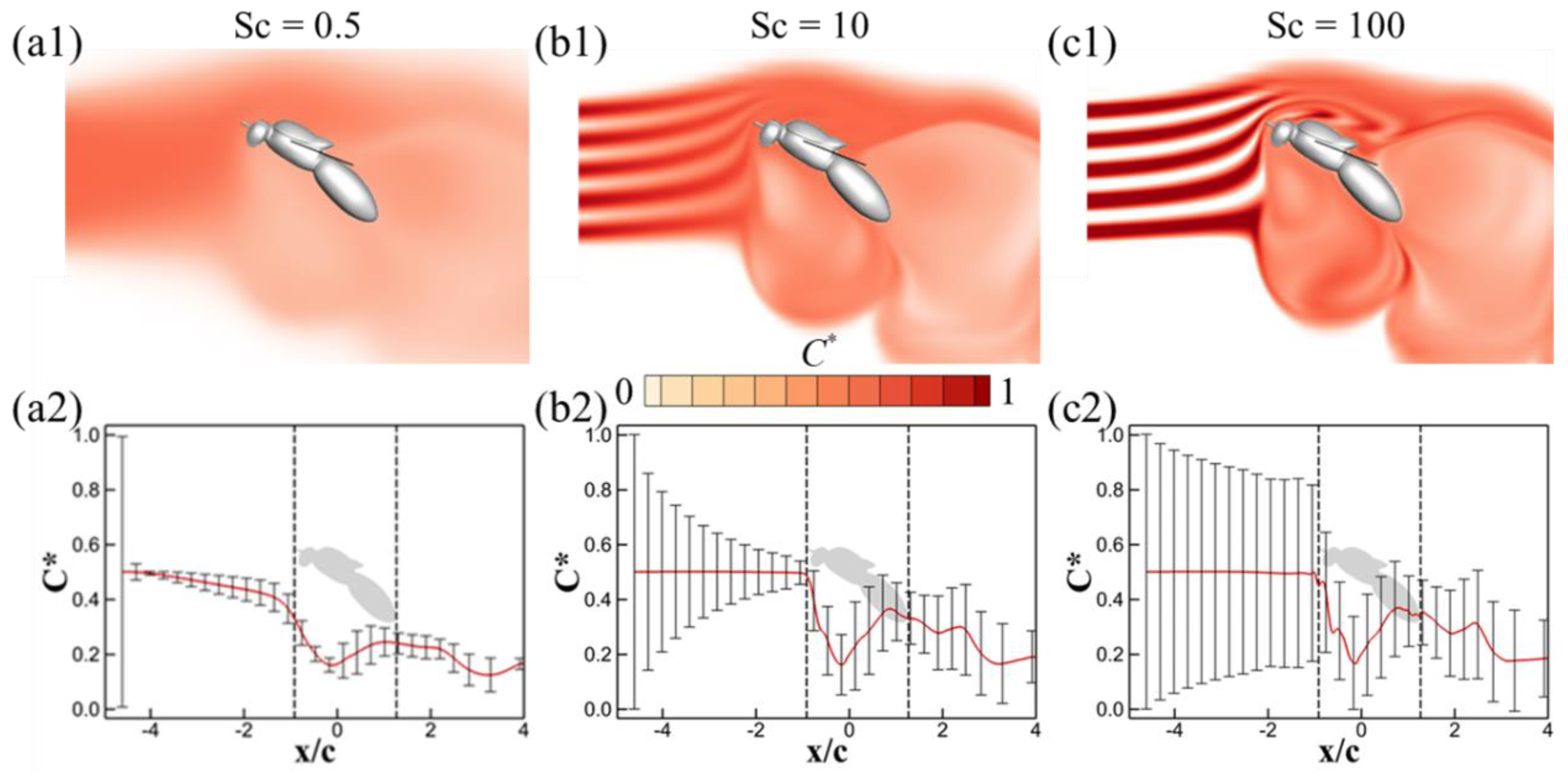
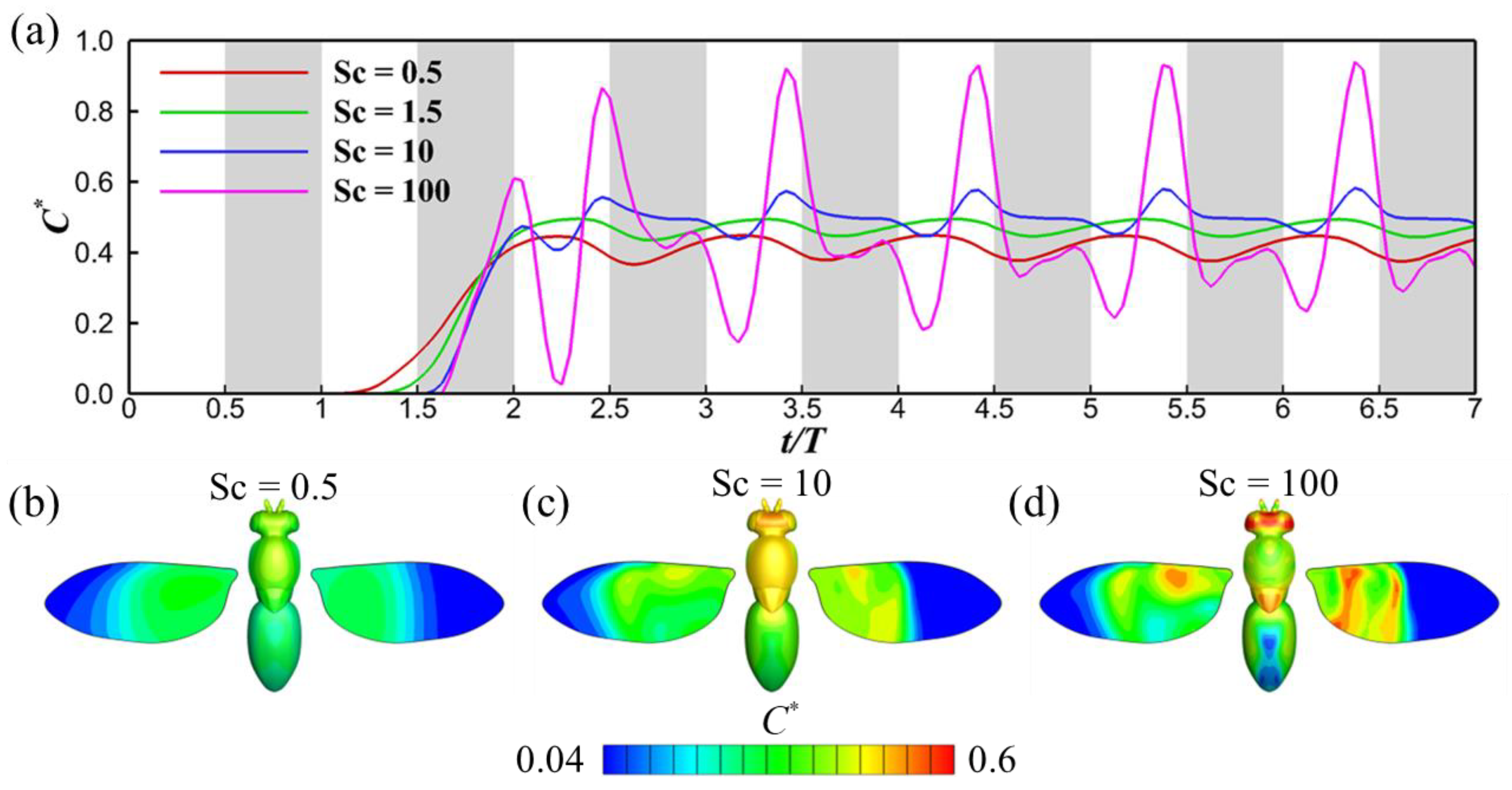
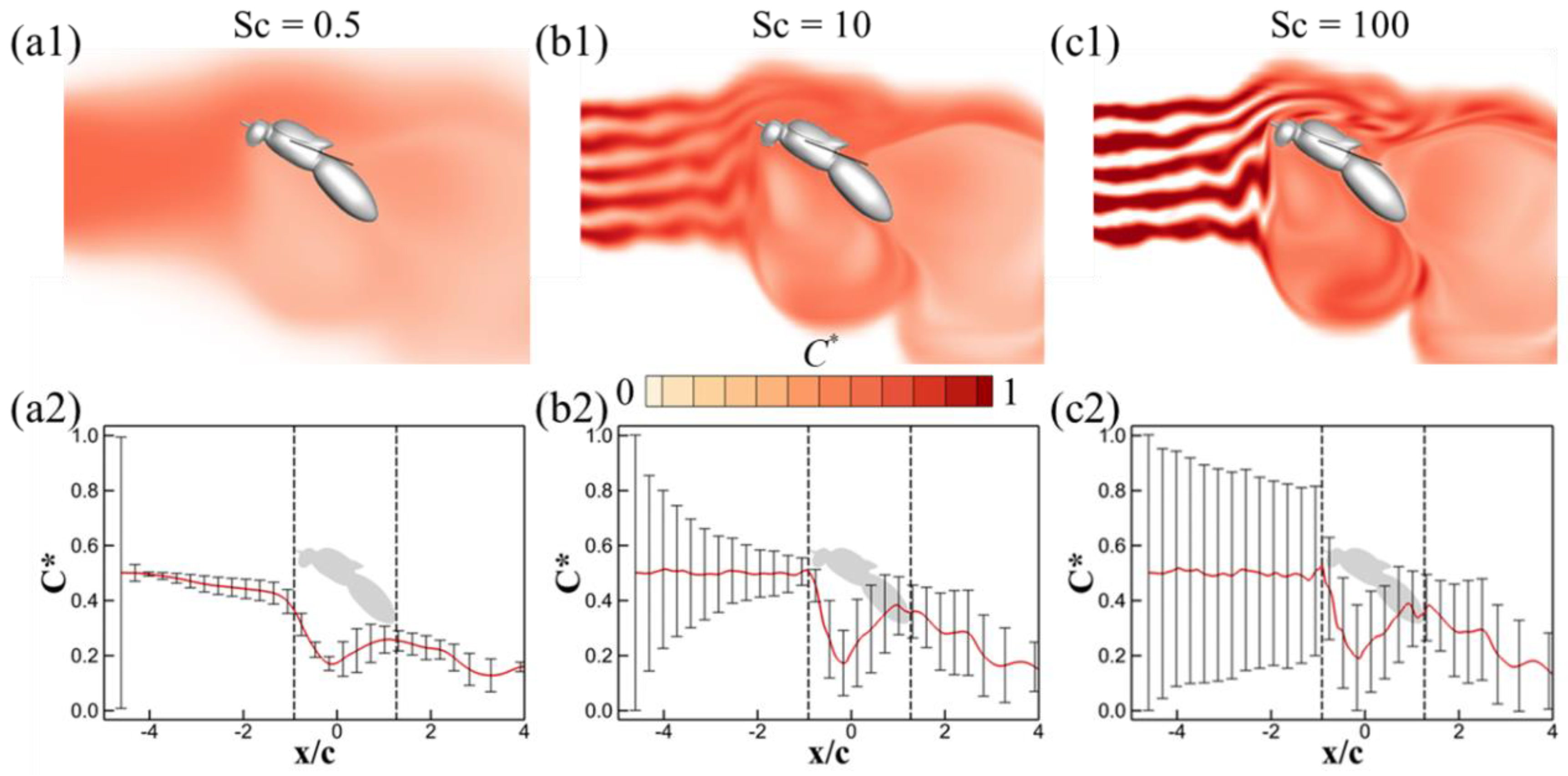
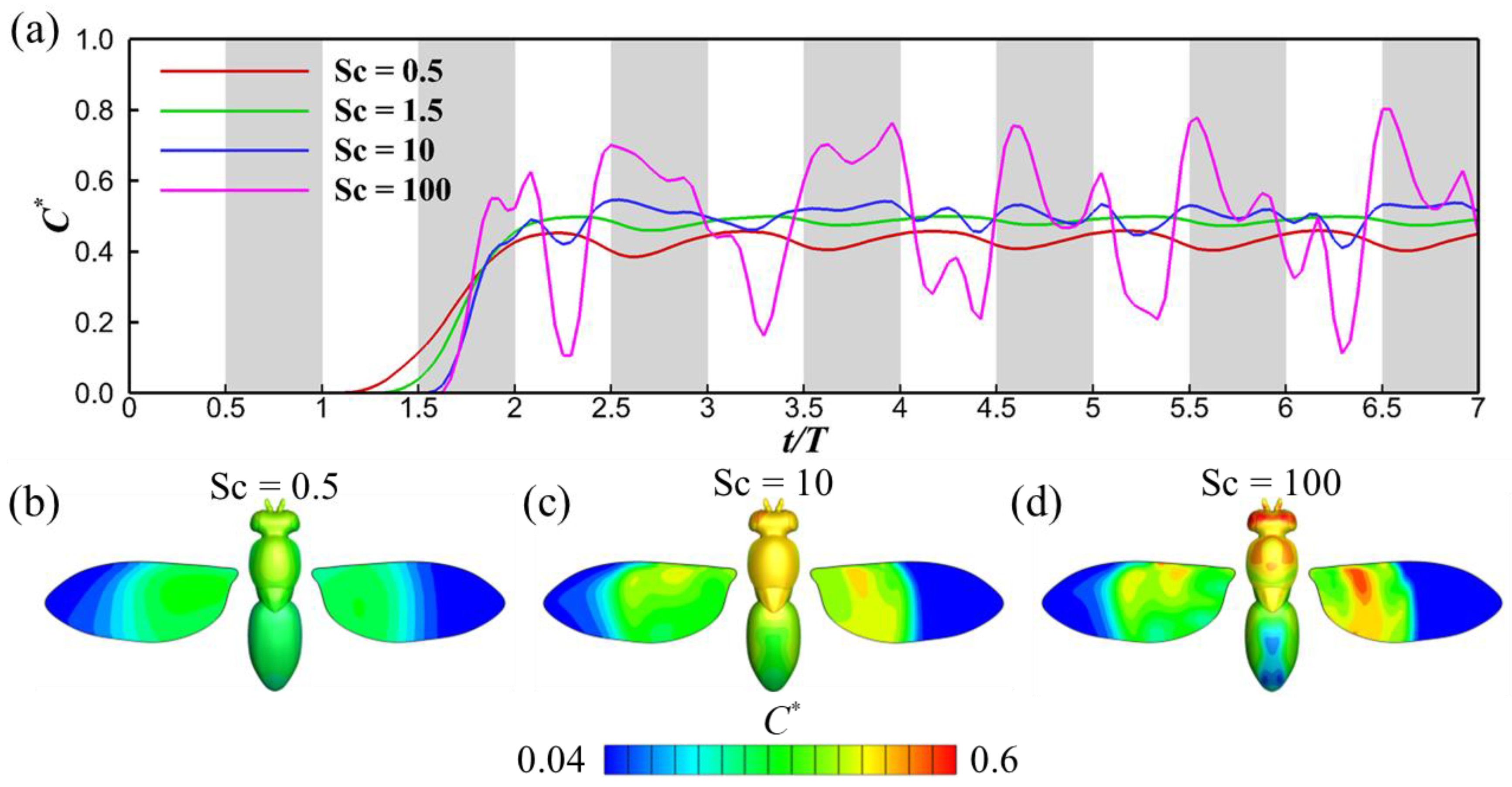
3.2. Effects of Schmidt Number
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pannunzi, M.; Nowotny, T. Odor stimuli: Not just chemical identity. Front. Physiol. 2019, 10, 1428. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.A. Chemical plume tracking behavior in animals and mobile robots. Navig. J. Inst. Navig. 2008, 55, 127–135. [Google Scholar] [CrossRef]
- Li, C.; Dong, H.; Zhao, K. A balance between aerodynamic and olfactory performance during flight in Drosophila. Nat. Commun. 2018, 9, 3215. [Google Scholar] [CrossRef] [PubMed]
- Talley, J.L.; White, E.B.; Willis, M.A. A comparison of odor plume-tracking behavior of walking and flying insects in different turbulent environments. J. Exp. Biol. 2023, 226, jeb244254. [Google Scholar] [CrossRef] [PubMed]
- Cardé, R.T.; Willis, M.A. Navigational strategies used by insects to find distant, wind-borne sources of odor. J. Chem. Ecol. 2008, 34, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Celani, A.; Villermaux, E.; Vergassola, M. Odor landscapes in turbulent environments. Phys. Rev. X 2014, 4, 041015. [Google Scholar] [CrossRef]
- Michaelis, B.T.; Leathers, K.W.; Bobkov, Y.V.; Ache, B.W.; Principe, J.C.; Baharloo, R.; Park, I.M.; Reidenbach, M.A. Odor tracking in aquatic organisms: The importance of temporal and spatial intermittency of the turbulent plume. Sci. Rep. 2020, 10, 7961. [Google Scholar] [CrossRef] [PubMed]
- Vickers, N.J. Mechanisms of animal navigation in odor plumes. Biol. Bull. 2000, 198, 203–212. [Google Scholar] [CrossRef]
- Justus, K.A.; Murlis, J.; Jones, C.; Cardé, R.T. Measurement of odor-plume structure in a wind tunnel using a photoionization detector and a tracer gas. Environ. Fluid Mech. 2002, 2, 115–142. [Google Scholar] [CrossRef]
- Atema, J. Eddy chemotaxis and odor landscapes: Exploration of nature with animal sensors. Biol. Bull. 1996, 191, 129–138. [Google Scholar] [CrossRef]
- Webster, D.; Weissburg, M. Chemosensory guidance cues in a turbulent chemical odor plume. Limnol. Oceanogr. 2001, 46, 1034–1047. [Google Scholar] [CrossRef]
- Connor, E.G.; McHugh, M.K.; Crimaldi, J.P. Quantification of airborne odor plumes using planar laser-induced fluorescence. Exp. Fluids 2018, 59, 137. [Google Scholar] [CrossRef]
- Wright, R. The olfactory guidance of flying insects. Can. Entomol. 1958, 90, 81–89. [Google Scholar] [CrossRef]
- Murlis, J.; Jones, C. Fine-scale structure of odour plumes in relation to insect orientation to distant pheromone and other attractant sources. Physiol. Entomol. 1981, 6, 71–86. [Google Scholar] [CrossRef]
- Elkinton, J.S.; Cardé, R.T. Odor dispersion. In Chemical Ecology of Insects; Springer: Berlin/Heidelberg, Germany, 1984; pp. 73–91. [Google Scholar]
- Mylne, K.R.; Mason, P. Concentration fluctuation measurements in a dispersing plume at a range of up to 1000 m. Q. J. R. Meteorol. Soc. 1991, 117, 177–206. [Google Scholar] [CrossRef]
- Mylne, K.R. Concentration fluctuation measurements in a plume dispersing in a stable surface layer. Bound.-Layer Meteorol. 1992, 60, 15–48. [Google Scholar] [CrossRef]
- Sane, S.P.; Jacobson, N.P. Induced airflow in flying insects II. Measurement of induced flow. J. Exp. Biol. 2006, 209, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Loudon, C.; Koehl, M. Sniffing by a silkworm moth: Wing fanning enhances air penetration through and pheromone interception by antennae. J. Exp. Biol. 2000, 203, 2977–2990. [Google Scholar] [CrossRef]
- Li, C. Effects of wing pitch kinematics on both aerodynamic and olfactory functions in an upwind surge. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2021, 235, 296–307. [Google Scholar] [CrossRef]
- Haverkamp, A.; Yon, F.; Keesey, I.W.; Mißbach, C.; Koenig, C.; Hansson, B.S.; Baldwin, I.T.; Knaden, M.; Kessler, D. Hawkmoths evaluate scenting flowers with the tip of their proboscis. elife 2016, 5, e15039. [Google Scholar] [CrossRef]
- Egea-Weiss, A.; Renner, A.; Kleineidam, C.J.; Szyszka, P. High precision of spike timing across olfactory receptor neurons allows rapid odor coding in Drosophila. iScience 2018, 4, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Szyszka, P.; Gerkin, R.C.; Galizia, C.G.; Smith, B.H. High-speed odor transduction and pulse tracking by insect olfactory receptor neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 16925–16930. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; van Breugel, F.; Dickinson, M.; Riffell, J.A.; Fairhall, A. History dependence in insect flight decisions during odor tracking. PLoS Comput. Biol. 2018, 14, e1005969. [Google Scholar] [CrossRef] [PubMed]
- Geier, M.; Bosch, O.J.; Boeckh, J. Influence of odour plume structure on upwind flight of mosquitoes towards hosts. J. Exp. Biol. 1999, 202, 1639–1648. [Google Scholar] [CrossRef]
- Mafra-Neto, A.; Cardé, R.T. Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature 1994, 369, 142–144. [Google Scholar] [CrossRef]
- Moore, P.A.; Atema, J. Spatial information in the three-dimensional fine structure of an aquatic odor plume. Biol. Bull. 1991, 181, 408–418. [Google Scholar] [CrossRef]
- Engels, T.; Kolomenskiy, D.; Schneider, K.; Farge, M.; Lehmann, F.-O.; Sesterhenn, J. Impact of turbulence on flying insects in tethered and free flight: High-resolution numerical experiments. Phys. Rev. Fluids 2019, 4, 013103. [Google Scholar] [CrossRef]
- Engels, T.; Kolomenskiy, D.; Schneider, K.; Lehmann, F.-O.; Sesterhenn, J. Bumblebee flight in heavy turbulence. Phys. Rev. Lett. 2016, 116, 028103. [Google Scholar] [CrossRef]
- Wan, H.; Dong, H.; Huang, G.P. Hovering hinge-connected flapping plate with passive deflection. AIAA J. 2012, 50, 2020–2027. [Google Scholar] [CrossRef]
- Mittal, R.; Dong, H.; Bozkurttas, M.; Najjar, F.; Vargas, A.; Von Loebbecke, A. A versatile sharp interface immersed boundary method for incompressible flows with complex boundaries. J. Comput. Phys. 2008, 227, 4825–4852. [Google Scholar] [CrossRef]
- Lee, C.; Su, Z.; Zhong, H.; Chen, S.; Zhou, M.; Wu, J. Experimental investigation of freely falling thin disks. Part 2. Transition of three-dimensional motion from zigzag to spiral. J. Fluid Mech. 2013, 732, 77–104. [Google Scholar] [CrossRef]
- Bergou, A.J.; Xu, S.; Wang, Z.J. Passive wing pitch reversal in insect flight. J. Fluid Mech. 2007, 591, 321–337. [Google Scholar] [CrossRef]
- Ishihara, D.; Yamashita, Y.; Horie, T.; Yoshida, S.; Niho, T. Passive maintenance of high angle of attack and its lift generation during flapping translation in crane fly wing. J. Exp. Biol. 2009, 212, 3882–3891. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.; Saad, T.; Sutherland, J.C. A fast turbulence generator using graphics Processing Units. In Proceedings of the 2018 Fluid Dynamics Conference, Zawiercie, Poland, 9–12 September 2018; p. 3559. [Google Scholar]
- Juves, C.B.D. A stochastic approach to compute subsonic-noise using linearized Euler’s equations. AIAA J. 1999, 105, 1872. [Google Scholar]
- Meng, X.; Liu, Y.; Sun, M. Aerodynamics of ascending flight in fruit flies. J. Bionic. Eng. 2017, 14, 75–87. [Google Scholar] [CrossRef]
- Lei, M.; Li, C. A Balance Between Odor Intensity and Odor Perception Range in Odor-Guided Flapping Flight. In Proceedings of the Fluids Engineering Division Summer Meeting, Toronto, Canada, 3–5 August 2022; Volume FEDSM2022-85407, p. V002T005A002. [Google Scholar]
- Zheng, L.; Hedrick, T.L.; Mittal, R. Time-varying wing-twist improves aerodynamic efficiency of forward flight in butterflies. PLoS ONE 2013, 8, e53060. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, S.; Hedrick, T.L.; Li, C. Aerodynamic explanation of flight speed limits in hawkmoth-like flapping-wing insects. Phys. Rev. Fluids 2022, 7, 093104. [Google Scholar] [CrossRef]

| Insects | Forward Flying Speed () | Wing Tip Velocity () | Advance Ratio () | Reynolds Number (Re) |
|---|---|---|---|---|
| Fruit fly (current) | 1.01 m/s | 3.2 m/s | 0.315 | 180 |
| Bumblebee [29] | 2.5 m/s | 8.75 m/s | 0.286 | 2042 |
| Butterfly [39] | 1.14 m/s | 2.79 m/s | 0.41 | 3455 |
| Hawkmoth [40] | 2.0 m/s | 4.88 m/s | 0.41 | 7335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, M.; Willis, M.A.; Schmidt, B.E.; Li, C. Numerical Investigation of Odor-Guided Navigation in Flying Insects: Impact of Turbulence, Wingbeat-Induced Flow, and Schmidt Number on Odor Plume Structures. Biomimetics 2023, 8, 593. https://doi.org/10.3390/biomimetics8080593
Lei M, Willis MA, Schmidt BE, Li C. Numerical Investigation of Odor-Guided Navigation in Flying Insects: Impact of Turbulence, Wingbeat-Induced Flow, and Schmidt Number on Odor Plume Structures. Biomimetics. 2023; 8(8):593. https://doi.org/10.3390/biomimetics8080593
Chicago/Turabian StyleLei, Menglong, Mark A. Willis, Bryan E. Schmidt, and Chengyu Li. 2023. "Numerical Investigation of Odor-Guided Navigation in Flying Insects: Impact of Turbulence, Wingbeat-Induced Flow, and Schmidt Number on Odor Plume Structures" Biomimetics 8, no. 8: 593. https://doi.org/10.3390/biomimetics8080593
APA StyleLei, M., Willis, M. A., Schmidt, B. E., & Li, C. (2023). Numerical Investigation of Odor-Guided Navigation in Flying Insects: Impact of Turbulence, Wingbeat-Induced Flow, and Schmidt Number on Odor Plume Structures. Biomimetics, 8(8), 593. https://doi.org/10.3390/biomimetics8080593






