1. Introduction
Mallards (Anas platyrhynchos) reside in various terrains, such as sandbars and reservoirs, throughout the year, with their survival in these soft-ground environments intricately linked to their anatomical structure. As bipedal avians, mallards’ unique toe configuration facilitates their mobility on yielding terrain. They possess four toes, of which the Ⅰ toe has undergone evolutionary diminution, rendering the Ⅱ, Ⅲ, and Ⅳ toes crucial for ambulation and bodily support. Additionally, their webbed feet serve pivotal roles in stabilizing sand, minimizing displacement, and augmenting the surface contact area, thereby optimizing movement on soft substrates like mudflats.
Benjamin et al. [
1] explored tendon functionality and its nutritional sustenance from tendon vasculature, emphasizing the critical interplay between tendons and fasciae. They highlighted how robust muscles contribute to tendons, ensuring the limb’s distal extremity remains unencumbered by mass and preserving its functional integrity. Biewener [
2] undertook an anatomical appraisal of equines, juxtaposing tendon elastic energy conservation with mechanical work. His findings revealed that elastic energy recovery accounted for approximately 40% of the mechanical effort during transitions from walking to a moderate trot in horses. This phenomenon underscores the specialized constitution of elongated tendons relative to diminutive pinnate muscle fibers, indicative of an evolutionary adaptation for efficient muscular force application and enhanced elastic energy recapture. Gangl et al. [
3] provided an exhaustive anatomical account of ostrich hind limbs, detailing the musculature and tendons affiliated with various skeletal components and discussing the structural principles underpinning the entire hind limb. Their insights particularly pertained to musculotendinous architectures instrumental in energy-efficient bipedal locomotion. Schaller et al. [
4] delved into the biomechanics of the ostrich’s intertarsal joint. Their research illuminated the presence of two critical ligaments at the junction, functioning akin to cords that facilitate the joint’s repositioning during extension or flexion. This study elucidated the correlation between intertarsal joint angular alterations and the requisite joint torque, laying the foundational groundwork for bionic flexible tensor-tendon joint design. Zhang et al. [
5] conducted an extensive anatomical analysis of the ostrich’s foot locomotor apparatus. Their investigation thoroughly examined the skeletal, tendinous, and ligamentous constituents, discovering that dual interphalangeal ligaments regulate the fourth toe’s movement, predominantly influenced by the lateral interphalangeal ligament, with negligible contribution from the internal counterpart. Clifton et al. [
6] evaluated the hindlimb musculature and webbing’s propulsive capabilities in aquatic avian species, noting that adept divers streamline their bodies by adjoining the femur and tibia to the thorax, concealed beneath the abdominal skin. They found that the lateral calf flexor undergoes near-isometric contraction during swimming, suggesting its role in averting hip and knee movements throughout the power stroke, thereby conserving a streamlined form. These studies collectively enhance our understanding of tendon and bone distribution via comprehensive anatomical exploration.
Weissengruber et al. [
7] investigated elephant footpads and found that the plantar configuration, which is mostly made up of connective tissue strips and adipocytes, plays a big role in how force is distributed and how mechanical energy is absorbed or stored during weight-bearing activities. EI-Gendy et al. [
8] dissected ostrich feet to discern structural-functional adaptations conducive to long-distance travel. Their analysis of the footpads, integral to ground contact, revealed four distinct pads: two on the third toe, one on the fourth, and one at the metatarsophalangeal joint. The study detailed papillae structures on the pads’ ventral surface and the longitudinal skeletal-cartilaginous formations beneath the phalanges, highlighting the soft tissues’ intricate arrangement. Different papillae orientations, lengths, and thicknesses were seen under electron microscopy. The collagen fibers’ parallel or slanted alignments in the pads may help ostriches absorb shock and run for long periods of time. Miao et al. [
9] explored the impact attenuation properties of German Shepherd Dogs’ footpads. They found the pad to have a complex, tripartite structure: the epithelium, dermis, and subcutis. The epithelium significantly mitigates ground impact forces, whereas the dermis and subcutis function as a hydrostatic system for energy storage, release, and dissipation, collaboratively catering to the biomechanical requisites of locomotion. Zhang et al. [
10] examined the ostrich footpad’s cushioning and vibration-damping capabilities, biomechanical testing, and finite element analysis. They determined that the footpad, comprising sequential skin, fascia, and toe cushion layers, diminishes stress from the exterior inward. With a minimum response frequency of 164.22 Hz, the footpad adeptly avoids resonance, significantly reducing peak acceleration through its composite material construction. Tian et al. [
11] analyzed the shock-absorbing mechanisms of goat hoof ball tissues and identified the primary components as the epidermis, dermis, dermal papillae, and subcutaneous tissue, with the dermal papillae dispersed within the dermis. Stress concentrations in the epidermis and uniform distribution in the dermis and dermal papillae suggest the epidermis’ role in diminishing ground impact forces, whereas the dermis facilitates energy storage, release, and dissipation, all collaboratively contributing to the hoof ball tissues’ cushioning effect. Collectively, these studies elucidate the footpads’ essential functions in shock absorption or energy conservation during animal locomotion.
Zhang et al. [
12] constructed a three-dimensional ostrich foot model. They acquired image data via CT scans and segmented these images with medical imaging and Mimics editing software (version 14.0). Geomagic Studio software (version 12.0) facilitated the geometric reconstruction of pertinent tissues, establishing a comprehensive three-dimensional model. In this process, bones, cartilage, and soft tissues that are important to the biomechanics of the ostrich foot were taken out, put back together, and extracted again. This made it possible to study the foot’s high-speed, heavy-duty, and shock-absorbing mechanisms in more detail. Rankin et al. [
13] explored the functional contributions of limb muscles and tendons to ostrich locomotion. Their findings indicated that muscle functions in support, propulsion, and braking were contingent on muscle type, location, and the bird’s gait. Additionally, the tendons’ connective and energy-storing roles could be replicated by spring-like structures, informing the design of bionic limbs and feet. Harrison et al. [
14] analyzed muscle forces, joint contact pressures, and the storage and use of elastic strain energy in horses’ distal forelimbs, proposing a simplified tendon-driven model. They found that the long flexor tendons in the forelimb’s distal region could store and utilize significant elastic strain energy, particularly while bearing weight. These studies collectively enhanced the understanding of the foot’s assembled structure and the reconstructed foot mechanism, with CT findings offering profound insights.
Lafortune et al. [
15] used an accelerometer-equipped force platform mounted on the wall to measure the shocks and external impact forces that the human calf could handle. This led to the idea of a human-ground impact mechanics model. In a distinct study, Taylor-Burt and Biewener [
16] investigated the kinematics of mallard ducks’ hind-limbs and the operational dynamics of crucial propulsive muscles during vertical takeoffs on land and in water. They discovered alterations in hindlimb kinematics and muscle functionality, with a notable surge in muscle power output during aquatic takeoffs. Expanding this research, Han et al. [
17] scrutinized the kinematic configurations of the mallard’s webbed foot. Their findings highlighted that mallards favor walking or grounded gaits at velocities under 5 km/h. The mallard’s toes moved in such a way that their distal ends made contact with the ground first, then their proximal ends swiftly followed, and their movements reversed when they lifted off. Intriguingly, the webbing exhibited a synchronized closing before lift-off and reopening before touch-down. This coordination suggests that mallard toes II, III, and IV operate as a cohesive system, with both the toes and webbing contributing collaboratively to terrestrial locomotion. Further, Han et al. [
18] observed mallards’ adaptive strategies during locomotion on soft terrain. They noted significant posture adjustments and a reduced touch-down angle, enabling the birds to navigate sinking challenges. Specifically, on sandy surfaces, the birds’ footfalls created depressions that facilitated sand stabilization and minimized displacement. Throughout this process, the mallards’ weight distribution across the extensive webbed area ensured minimal ground pressure, preventing significant sinking during sandy traverses. These behavioral locomotion studies underscore the importance of delving into the underlying mechanisms steering these complex, adaptive movements.
The prosthesis developed by Park et al. [
19] comprises two degrees of freedom at the knee and ankle joints. The knee joint’s movement is replicated using a DC motor, and a spring system is employed at the ankle to generate torque and control flexion angles. Additionally, they conducted topology and shape optimization of the foot and lower limb structures to attain an optimal shape and reduce weight. Sun et al. [
20] conducted an optimization of the leg and analyzed the impact of various design parameters on the performance of the optimized leg. They implemented this design on a quadruped robot and evaluated its motion performance. Furthermore, they developed a multi-objective topology optimization method that takes into account both the standing and walking positions of the flexible legs.
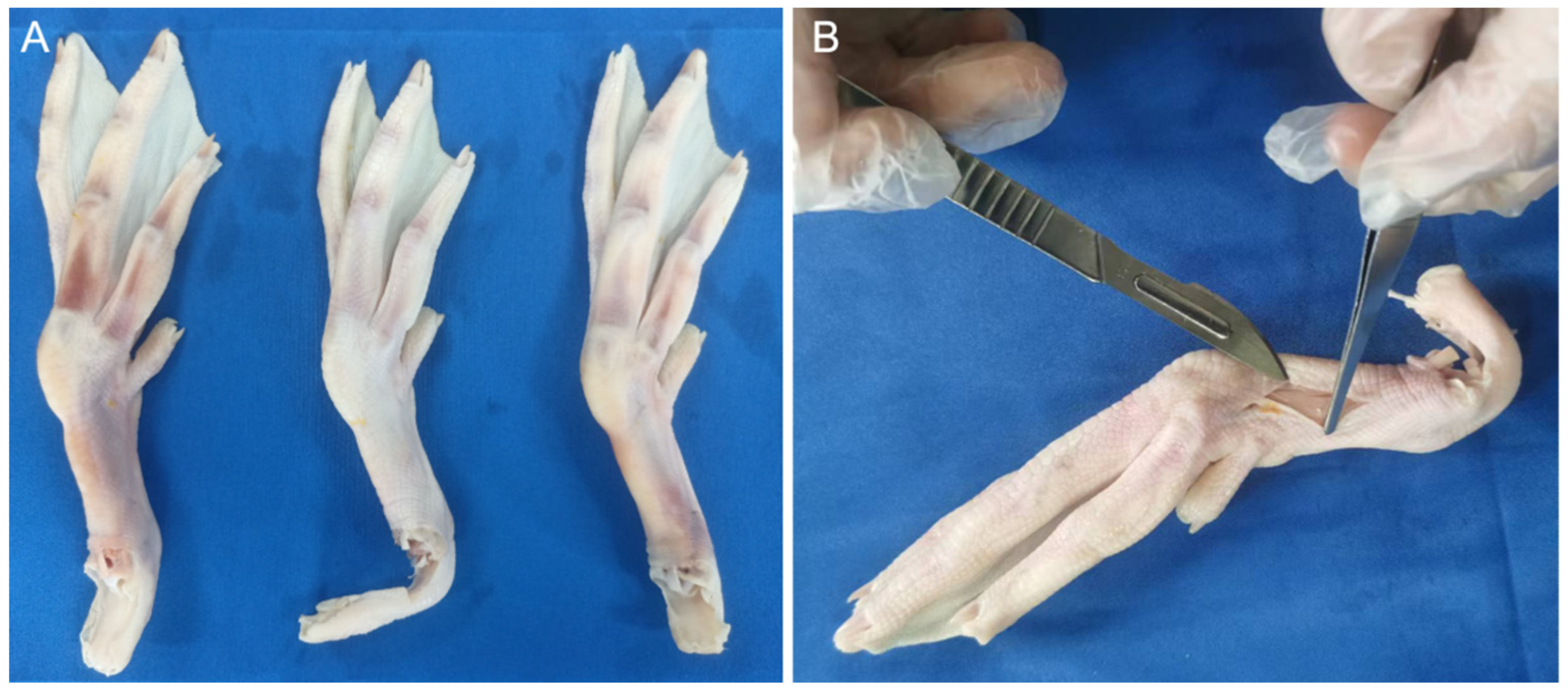
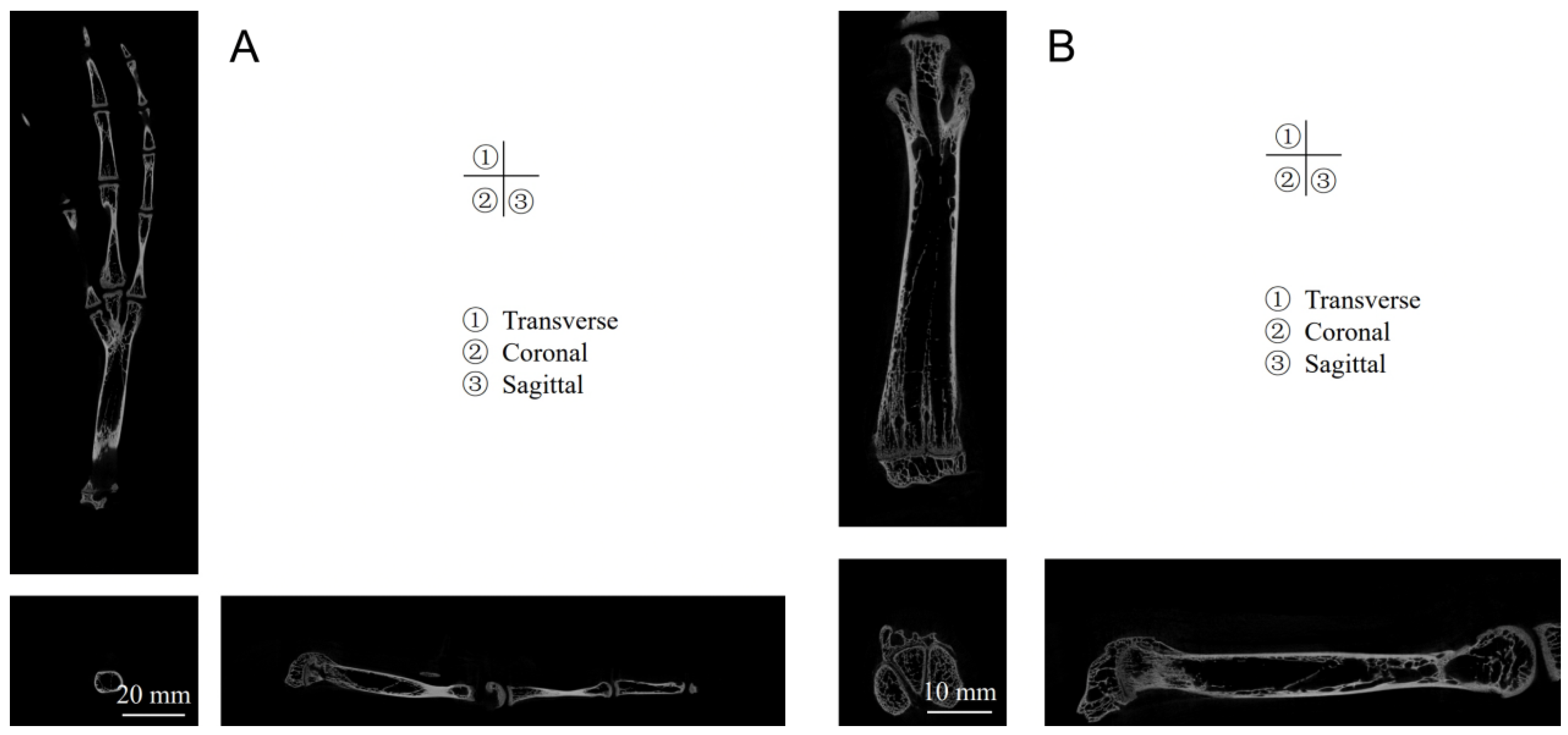
Current research on mallards predominantly focuses on the morphological and locomotive aspects of their webbed feet, with a noticeable paucity of investigations into the biological structure and material assembly mechanisms underlying the subsidence resistance of these webbed feet. The mallard’s adeptness at maneuvering through soft terrain is intricately tied to its unique foot structure and the synergistic assembly of rigid and pliable materials. This study aims to delve into the biological and material assembly characteristics of mallard webbed feet by acquiring pertinent biological structural parameters, such as those related to toes, bones, and joints, as well as the interconnected assembly of skin, fascia, tendons, and ligaments, employing CT scanning and gross anatomical methodologies. Utilizing a texture analyzer, we examined the correlation between the webbing opening angle and tarsometatarsal pressure, elucidated the biomechanical functions of the mallard’s webbed foot, and subsequently explored its subsidence resistance mechanisms. Drawing insights from the anatomical and kinematic analyses of the mallard, our objective extends to designing a bionic foot that replicates the functional properties of the mallard’s webbed foot.
5. Biomimetic Foot Design
5.1. Structure Composition
We have conceptualized a biomimetic foot in light of the mallard foot’s intricate anatomical structure and earlier kinematic studies [
17,
18]. This construct amalgamates a biomimetic webbing articulation mechanism, a segmental multi-toe elevation system, and synthetic webbing. The articulatory ensemble comprises a biomimetic tarsometatarsal component, torsion springs, a coiled column, a spiral slider, reciprocal blocks, and bearing supports. Concurrently, the multi-toe lift-off apparatus encompasses the second, third, and fourth digits. Mimicking the mallard’s skeletal configuration, the mechanical foot’s foundational structure replicates the precise bone arrangement, both in position and count. These synthetic bones are encased in
Supplementary Materials emulating skin and internal tissues. These elastic substances are strategically positioned on the digits, with interdigital materials simulating natural webbing. The foot’s heel region employs similarly pliant materials, designed to replicate the cushioning adipose pads found on the mallard’s sole.
The biomimetic foot adopts the proportional dimensions of a mallard’s foot. Based on the measured parameters of the toe bones, we mimic these proportions to design the sizes of the individual toe bones in the biomimetic foot. The material for the toe bones can be high-strength steel or other rigid materials, whereas the material for the biomimetic webbed foot is high-performance TPU material, renowned for its exceptional flexibility. The combination of rigid toe bones and the pliable webbed foot pad forms a rigid-flex coupling assembly. The biomimetic foot possesses variable surface area functionality, primarily suited for soft ground applications, offering excellent resistance to sinking. At present, the biomimetic foot has not fully applied the tendon-bone motion model. In the future, we will continue to research tendon-driven biomimetic feet.
5.2. Engineering Bionic Principle
As depicted in
Figure 12, the design emulates the mallard foot’s functionality, particularly the opening of the webbing via the second and fourth toes prior to ground contact and its closure post-lift-off. This mechanism involves the outward splaying of the second and fourth toes, initiated by the forward rotation of the bionic tarsometatarsal bone during ground contact. This action triggers the expansion of the bionic webbing, augmenting the contact area and enhancing the bionic foot’s sinkage resistance. At the same time that lift-off starts, the tarsometatarsal bones start to recoil, controlled by torsion springs. This causes the bionic second and fourth toes to close and the webbing to retract, which reduces aerodynamic drag while the bionic foot moves.
To emulate the sequential ground-release motion of a mallard’s three toes, the bionic foot incorporates multi-segmented replica toes. These artificial phalanges interconnect through a synergistic mechanism involving simulated joint heads and fossae. Joint restrictions are achieved via a carriage assembly, whereas tension spring preloading simulates the authentic joint’s tensile framework. This design ensures that the bionic foot achieves enhanced relaxation upon ground release and increased flexibility upon ground contact without necessitating an auxiliary control system.
In order to mimic the energy-saving effect observed in the mallard’s tendons, torsion springs are integrated into the metatarsophalangeal joints. This feature passively accumulates energy during forward motion and subsequently dispenses this stored energy upon ground release, facilitating effortless hindfoot lift-off. Additionally, biased tension springs are installed within the phalangeal joints, assisting the distal phalanges in ground detachment and reversion to their initial stance. This system also serves to dampen and diminish the vibrational impact experienced by the bionic foot upon touching the ground.
5.3. Working Process
Upon ground contact, the mallard foot initiates contact via the foot’s tip, subsequently followed by full palm grounding. The action of the second and fourth toes orchestrates the expansion of the interdigital webbing. The third toe, being the most extended, makes initial contact, guiding the rapid grounding of the foot’s palm through a pivot around the third toe’s tip. Concurrently, a forward rotation of the tarsometatarsal bone around the metatarsophalangeal joints ensues during this touchdown phase, resulting in a reduced joint angle. As lift-off commences, the elevation of the tarsal bones triggers the retraction of the hindfoot, progressively increasing the metatarsophalangeal joint angle. This sequence continues with the phalanges sequentially disengaging from the ground, commencing with the proximal and concluding with the distal toes. The lift-off phase culminates with this full retraction. During the swing phase, the second and fourth toes instigate a gradual closure, pulling the webbing inwards and extending the toes to facilitate webbing expansion in preparation for the subsequent stride cycle, thereby completing one full stride sequence.
In parallel, the bionic foot, upon ground contact, leads with the tip due to its forward center of gravity and the inherent angle of the bionic metatarsophalangeal joints. The full grounding of the palm follows this initial contact quickly. This transition is facilitated through a forward rotation of the bionic tarsometatarsal bone, inducing a clockwise motion of the helical column. This rotation translates to the helical slider instigating its motion, which, in turn, propels the push-pull block. Consequently, the simulated second and fourth toes undergo simultaneous extension, driving the expansion of the bionic webbing. This sequence augments the contact surface, mitigating sinking by distributing the load over a broader base.
During lift-off, the bionic foot initiates its ascent with the torsion spring at the tarsometatarsal bone reverting to its original position, thereby releasing stored energy. This action prompts the proximal phalanx to rotate around the first phalanx due to the metatarsophalangeal joint’s torsion spring, initiating ground detachment. Upon reaching the toe joints’ maximal rotational angle, the phalanx finalizes its lift-off sequence, commencing with the first phalangeal of the second, third, and fourth toes. After that, the torsion spring causes the remaining distal phalanges to separate one at a time until the last distal phalanx moves off the ground. The bionic foot’s lift-off phase is now complete. This progression is similar to the phalanges’ sequential detachment and reduces both adhesive ground forces and environmental perturbations.
When the bionic foot swings, the torsion spring at the tarsometatarsal bone re-engages, releasing any stored energy and starting the backward rotation of the tarsometatarsal bone. This motion induces a counterclockwise rotation in the helical column, propelling the helical slider and reinstating the push-pull block to its initial stance. Consequently, the bionic second and fourth toes commence closure, and the bionic webbing capitalizes on its elastic potential energy to facilitate its contraction. This process reduces aerial forward movement resistance, marking the completion of a singular stride cycle for the bionic foot.
Webbing dynamics in the bionic foot are controlled by the bionic tarsometatarsal bone’s forward rotation. The bionic metatarsophalangeal joint’s torsion spring controls the bone’s retraction. This passive modulation of the webbing confers dual advantages: the obviation of a control system and a reduction in energy expenditure. Through the rigid-flexible synergy between the toe and webbing, the bionic foot adeptly maximizes ground contact area, enhancing friction and sinkage resistance during touchdown. Conversely, it minimizes this area during lift-off to reduce aerial resistance and ground-touch vibration.
6. Conclusions
The material and structural assembly of the mallard foot showcase distinct characteristics: the skeleton forms the primary framework, establishing a supportive structure, whereas the tarsometatarsal bone exhibits attributes of a lightweight yet high-strength functional structure. Tendons, affixed above the bones, facilitate bone movement and are enveloped by tendon sheaths that safeguard the tendon tissues and minimize the friction encountered during tendon sliding. Encasing these components, the skin envelops both tendons and bones, supplemented by fat pads located on the plantar aspect of the foot to provide cushioning.
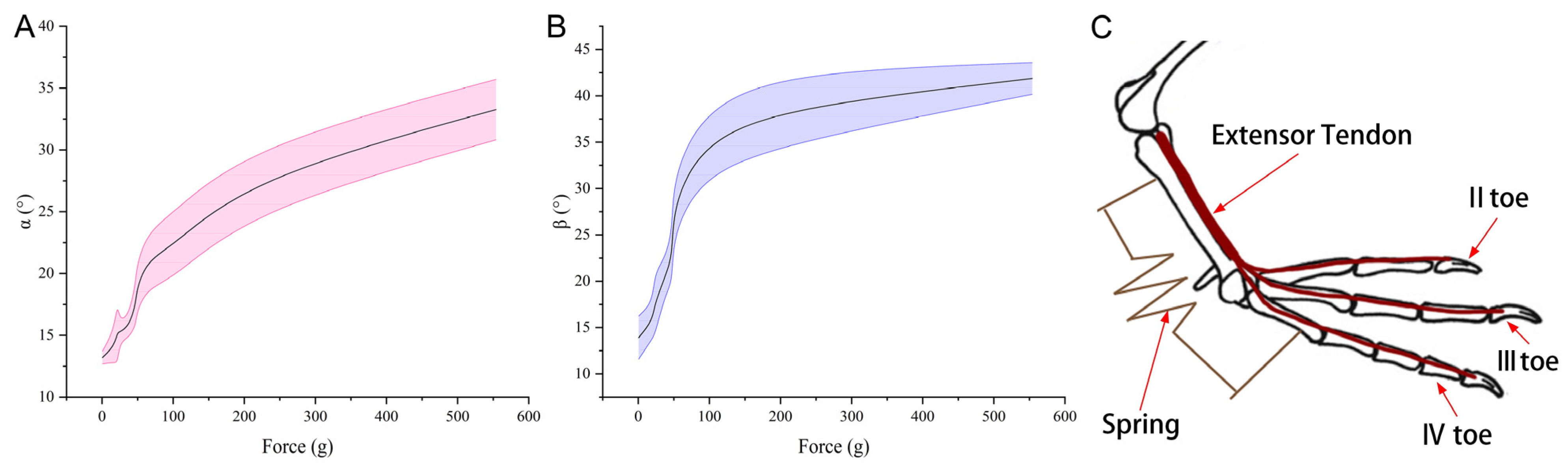
In terms of the osteomotor system, the mallard foot distinguishes itself primarily through its flexor tendons, which are more robust and distinctly segmented compared to the slimmer extensor tendons that converge into a single bundle at the proximal tarsometatarsal bones. During ambulatory activities, such as walking or grounded running, there is a harmonious interaction between the extensor tendon, located dorsally, and the flexor tendon, situated plantarly, orchestrating the toes’ flexion and extension movements.
Furthermore, the opening angles of the webbing are in direct proportion to the pressure exerted on the tarsometatarsal. This observation has led to the proposal of a biomechanical model. This study delved into the intricate bio-structural properties and material assembly of the toe and webbing, as well as the kinematic system encompassing the bones and muscles within the mallard’s foot. These insights contribute valuable data for future anatomical explorations into the feet of webbed birds but also inform the creation of a bionic foot prototype. On the basis of the mallard foot study, a bionic foot was designed, which provides a reference and a lesson for the future design of bionic feet.