Abstract
Dentin hypersensitivity (DH) is a very common dental problem that can have a negative impact on the quality of life and can lead to invasive dental procedures. Prevention of DH and control of symptoms are highly desirable. Hydroxyapatite (HAP) has been shown in vitro to block dentinal tubules and in vivo to be a safe and effective additive in oral care products that reduce DH clinically. This study’s aim was to conduct a systematic review and meta-analysis of the current evidence that HAP-containing oral care products reduce DH. Databases were searched, and only clinical trials in humans were included; studies conducted in vitro or on animals were not included. Publications in a foreign language were translated and included. We found 44 published clinical trials appropriate for systematic analysis. More than half of the trials had high-quality GRADE scores. HAP significantly reduced dentin hypersensitivity compared to placebo (39.5%; CI 95% [48.93; 30.06]), compared to fluoride (23%; CI 95% [34.18; 11.82]), and with a non-significant tendency compared to other desensitizing agents (10.2%; CI 95% [21.76; −19.26]). In conclusion, the meta-analysis showed that HAP added to oral care products is a more effective agent than fluoride in controlling dentin hypersensitivity and may be superior to other desensitizers.
1. Introduction
While enamel is acellular and comprised of nearly entirely inorganic mineral apatite, making it relatively inert, healthy teeth can respond to stimuli applied to the tooth such as thermal stimuli (ice and heat) and tactile because the underlying connective tissue, the dentin, is connected to the pulp complex, a highly innervated live tissue with nerve fibers capable of detecting stimuli and transmitting signals to the brain. Teeth become ‘extra’ sensitive for many reasons. For example, hairline cracks, poorly bonded restorations, or advanced carious lesions can cause pathological pain that brings patients into the dental office for treatment. Another very common problem of dental pain is called dentin hypersensitivity (DH), usually the result of exposure to the surface of dentin, generally at the cement-enamel junction. Gingival recession and loss of cementum from excessive tooth brushing or demineralization by highly acidic dietary (or external) acidic challenges can cause the entrances of dentin tubules to be exposed to the oral environment. Once this happens, even the mildest cold or airflow stimuli or focused tactile stimuli can result in sharp, severe pain. DH is, therefore, a “short, sharp pain arising from exposed dentin in response to stimuli, typically thermal, evaporative, tactile, osmotic, or chemical and which cannot be ascribed to any other form of dental defect or disease” [1,2,3]. It is this form of dentin pain that is the focus of this systematic review.
The current accepted theory of the induction of pain from DH is the “hydrodynamic theory” [4,5]. When the extracellular fluid in dentin tubules moves, the dentinal tubule processes of the odontoblasts can detect this movement. Through their close contact with the afferent pain nerve fibers in the tubules, the odontoblasts transmit the pain sensation. The movement of fluid can be created through desiccation, thermal changes (cold hot), physical force (fingernail or dental explorer), or osmotic pressures (dissolution of sugars).
DH is very common in the adult population. A recent systematic review showed that patients of all ages with permanent teeth have reported DH, with a prevalence of about one-third of the population at any given time across all studies [6].
The primary strategy for treating DH and maintaining reduced hypersensitivity is to physically cover the exposed dentin and prevent the movement of fluid in or out of the dentin tubules. Conservative procedures should be considered prior to using irreversible ones, such as dental fillings, or periodontal surgery [2]. Chemically occluding the entrances to the dental tubules, either with in-office procedures or with therapies administered at home, is a more conservative and effective approach. There are several agents that have been investigated in the past to conservatively treat DH [7,8]. There have been 8 systematic reviews of therapeutic treatment of dentin hypersensitivity published in the recent past that included studies on hydroxyapatite (HAP) as an active desensitization agent [8,9,10,11,12,13,14,15] (Supplementary Materials: Table S1). They included studies on HAP that ranged in number from just one to as many as 20 studies. All of them concluded that HAP was an effective dentin desensitizer. Two systematic reviews concluded that HAP was superior to other methods of controlling dentin hypersensitivity [11,13]. Those treatments that achieve dentin tubule occlusion with physical deposits for extended periods of time are considered better treatments than those that only achieve that on a short-term basis. Ingredients that encourage and speed up the natural remineralization process are also well suited for lowering DH symptoms. Biomimetic HAP seems to be one of those agents that fulfills both roles, since it is very similar to the HAP crystals between and intertwined within the collagen fiber bundles of dentin [16].
Since the most recent meta-analysis on the science of HAP reducing DH is 3 years old and we wanted to include foreign language studies, we conducted an updated systematic review and meta-analysis on all the RCTs where HAP was shown in clinical trials to reduce DH.
2. Materials and Methods
We used the PICO framework to guide the focus of this literature review. P: Patients—patients of all ages with healthy, non-carious dentitions with some level of dentin hypersensitivity. Patients undergoing periodontal or vital bleaching were not excluded. I: Intervention—the introduction of one of the following oral care products containing biomimetic HAP as an active ingredient; toothpaste, mouthwash, professional product or gel, either in-office professionally administered or self-administered at home. C: Comparison—no intervention (comparison to baseline), placebo controls (HAP-free oral care products), and positive controls (containing other desensitizing agents) were all considered. O: Outcome—a reduction in dentin hypersensitivity, which included reduction from tactile, cold air, ice water, heat, and electrical stimuli as measured by electric pulp testing, visual analogue scales, ordinal scale scores, or subject questionnaire self-assessments.
The following primary databases were searched: PubMed (Ovid Medline), EMBASE, Scopus, Cochrane Library, and Web of Science. Google Scholar was also searched. Two authors had a previous list of published papers. These provided 3 additional publications found outside of the search. The PRISMA guidelines for literature searches [17] were followed (see Supplementary Materials for the completed PRISMA-S checklist). We did not limit our search to English language publications. We found studies in the Korean, Italian, German, and Russian languages. These were translated using Google Translate. We searched the literature up to and including 1 May 2022. No studies on animals, in vitro or in situ human studies were included, even though mechanistic occlusion of exposed dentin tubules is the proposed mechanism of HAP desensitization. We also crosschecked the references that were reported in our previous comprehensive search [18]. For this updated meta-analysis on the efficacy of HAP in reducing DH, we were interested only in human clinical trials providing clinical evidence of efficacy in patients who could report changes in dentin hypersensitivity.
A qualitative analysis (synthesis) was completed for the studies that met the inclusion criteria. We rated the quality of the evidence using the guidelines in dentistry and GRADE graphics described by Richards et al. [19]. A Cochrane Risk of Bias (RoB) analysis using the methods of Sterne et al. [20] was conducted, and a table was generated.
For the meta-analysis, studies that met the inclusion criteria where at least two groups were compared were used. Where baseline data and data from the final examination were available, we calculated the mean reduction of DH (Schiff-Score, Wong–Baker, or VAS-Scale) for each group and then the difference between the groups. Those data were also calculated as the mean relative difference between the groups (in %), which were then used for the meta-analysis. Three different forest-plots were generated: HAP compared to placebo (1), HAP compared to fluoride (2), and HAP compared to other known actives for reducing DH (3). As (3) comprises many different active ingredients, weighting of the sample sizes was not performed for all analyses (1–3) to reduce the possible risk of bias. The calculation and meta-analysis were performed using the open-source software R, version 4.2.1 (R-project.org). We also used the packages dplyr and forestplot [21].
3. Results
Despite limiting our search to human dentin sensitivity and HAP, nearly 40,000 titles had to be screened in order to avoid missing any published studies. After duplicates and irrelevant papers, reviews, abstracts, book chapters, experiments conducted on animals, in vitro and in situ were all rejected (for not meeting our inclusion criteria), we found 44 relevant clinical trials where HAP was investigated clinically for the reduction of DH. Figure 1 summarizes the results of the search using the strategies outlined in the methods.
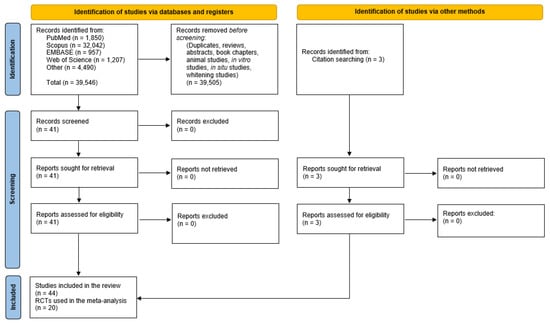
Figure 1.
Flow diagram summary of the systematic review search strategy and results.
A complete list of search words is provided in the Supplementary Materials (Table S2), along with the results of the number of citations found. The details of the 44 publications that met the inclusion criteria for the qualitative and quantitative synthesis are shown in Table 1. All retrieved studies were read in detail and assessed for quality. The GRADE assignments are shown in the table.

Table 1.
Summary of all clinical trials of hydroxyapatite (HAP) treatment of dentin sensitivity with GRADE assignments.
3.1. Testing Dentin Hypersensitivity (DH)
A variety of tests to illicit DH were used. The most common was an air blast using compressed air from a standard dental chair air–water syringe. Ice, or ice water, was also used as a cold stimulus, and tactile stimuli were used by applying a dental explorer. Most researchers have standardized their stimuli (same distance, isolation of neighboring teeth). Some studies evaluated DH at baseline, and many evaluated only one time point after the start of the clinical trials. Others tested DH at several time points at 2, 3, or even 4 weeks apart after measuring the baseline DH.
3.2. Dentin Hypersensitivity (DH) Scoring Results
Nearly all the studies involved using a patient response scoring system that involved a 4-point scale of increasing sensitivity severity (Schiff score [66]), a 10-point visual analogue scale (VAS), or one that required the subject to place a mark on a distance scale (e.g., 10 cm). One study [49] used an electric pulp tester, which eliminated the subjective aspects of dentin sensitivity reporting. Another study [37], which involved younger patients, used the Wong–Baker FACES pain rating scale [67]. The sensitivity tests were reproducible, accurate, and produced, in nearly all the clinical trials, changes in dentin hypersensitivity that showed statistically significant improvements in comfort in the subjects examined.
3.3. Qualitative Synthesis
3.3.1. GRADE Assignments
Of the 44 clinical trials found, half were double-blinded and randomized clinical trials (RCTs). The quality of those RCTs was rated as moderate to high. Some studies reported as RCTs were downgraded because they failed to provide the methods used for randomization. Some claimed they were blinded studies but did not provide the details of how the examiners or patients were blinded. These also received a lower GRADE score. Of the 44 trials, 11 were conducted to investigate the HAP application in an office setting with one or two applications. The others involved sending the subjects home with products to use. The length of the studies varied from a few days to 3 months. One study was conducted for 6 months. Three-month observation periods were used most often to evaluate the long-term efficacy of the test products.
From Table 1, it can be seen that all the studies except for one showed a statistically significant clinical benefit of HAP in reducing DH. In those studies where HAP was applied professionally, immediate relief of DH was achieved. HAP helped in the reduction of post-bleaching sensitivity in DH. At home application of HAP was in the form of gels in custom trays, but mostly it was in the form of toothpaste used twice a day. One study found that adding HAP to chewing gum worked to reduce DH. Compared to the placebo, HAP reduced DH from 6% to 80%. HAP was as good as or better than fluoride controls in reducing DH and as good or better than other desensitizing agents in reducing DH. This was confirmed in the meta-analyses of those studies that could be included in the meta-analyses (see Section 3.4 below).
3.3.2. Risk of Bias
The Risk of Bias (RoB) assignments of 44 clinical trials included in the qualitative synthesis are shown in Table 2. Of those, 23 had low risk of bias, 15 had high risk of bias, and the remainder fell in between those ratings.

Table 2.
Risk of Bias (RoB) assignments of the hydroxyapatite (HAP) and dentin hypersensitivity clinical trials.
3.4. Quantitative Synthesis—Meta-Analysis
Three separate forest plots were generated from the meta-analysis. These included a comparison between HAP and placebo (Figure 2), between HAP and fluoride (Figure 3), and between HAP and other desensitizers (Figure 4). The results showed that HAP worked as well or better than fluoride or other desensitizing agents in reducing DH. The degree of mean relative reduction ranged from 10.2% (CI 95% [21.76; −19.26]) reduction in the HAP-group compared to positive controls, mean relative reduction of 23% (CI 95% [34.18; 11.82]) in the HAP-group compared to fluoride, and mean relative reduction of 39.5% (CI 95% [48.93; 30.06]) in the HAP-group compared to placebo. Figure 2, Figure 3 and Figure 4 show the forest plots of the main outcome comparisons.
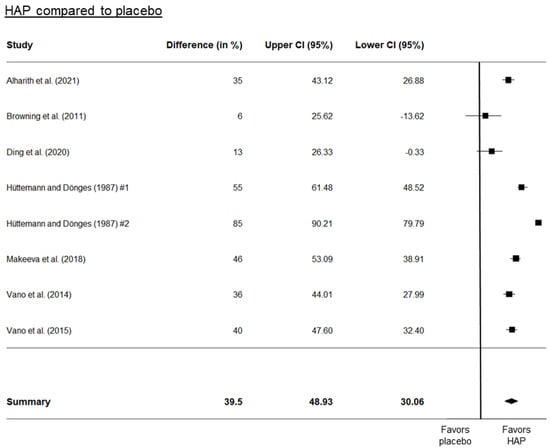
Figure 2.
Forest plot of HAP compared to placebo. Hüttemann and Dönges [40] tested the different particle sizes of HAP. #1 indicates a particle diameter of 6 µm, and #2 indicates a particle diameter of 2 µm. Details of the studies in this figure are in Table 1. Other references: Alharith [24], Browning [32], Ding [36], Makeeva [51], Vano [61,62].
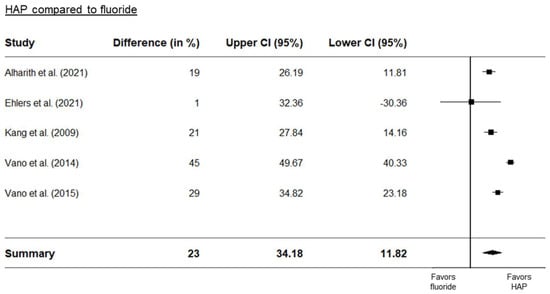
Figure 3.
Forest plot of HAP compared to fluoride. Details of the studies in this figure can be found in Table 1. References: Alharith [24], Ehlers [37], Kang [42]. Vano [61,62].
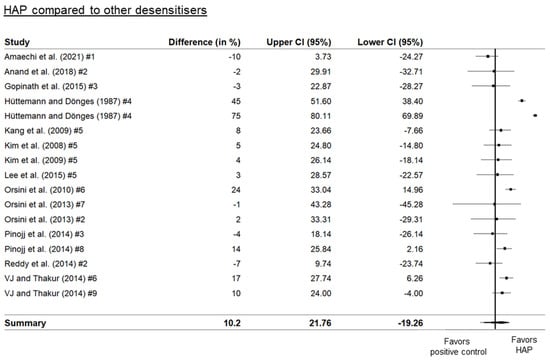
Figure 4.
Forest plot of HAP compared to other desensitizing agents. Numbers indicate different types of agents: #1: 20% silica, #2: arginine, #3: Bioglass (calcium sodium phosphosilicate), #4: 0.125% benzocaine, #5: SrCl2 combined with CaCO3, #6: KNO3, #7: strontium acetate, #8: casein-phosphoprotein amorphous calcium phosphate (CPP-ACP), #9: propolis. Details of the studies in this figure and the ingredient comparisons are provided in Table 1. References: Amaechi [27], Anand [29], Gopinath [38], Hüttemann [40], Kim [43,44], Lee [46], Orsini [52,53], Pinojj [55], Reddy [58], VJ and Thakur [64].
4. Discussion
Two of the most common clinical problems of dentition for which patients seek professional help are dental decay and hypersensitive teeth. While the former can lead to tooth loss and the latter is more of an annoyance, making consuming foods and beverages of different temperatures and sweetness very uncomfortable, both conditions can benefit from the attention of a preventive dentistry professional before the problem becomes too difficult to manage. In our last systematic review, we focused on the anti-caries efficacy of HAP in toothpaste to reduce dental caries in children [18]. Here, we have turned our attention to the ability of HAP toothpaste to manage dentin hypersensitivity in adults, a very common condition. The prevalence of DH varies greatly, but based on a meta-analysis [6], at least every third adult, on average, suffers, or has suffered from the condition.
HAP has been used for decades in toothpaste in Japan, where it was first developed, and in other countries (e.g., Germany), but it is a relatively new product in North America. Despite its widespread use in other dental applications, such as coating dental implants, bone repair, and periodontal surgery (see review by Chen et al. [68]), it has only recently become an accepted ingredient in oral care products in North America. Supplementary Material Table S3 lists those products for use in Canada approved by Health Canada for sensitive teeth. Most products for use in the USA can be purchased through online importers, such as Amazon.com.
4.1. How HAP Reduces Dentin Hypersensitivity (DH)
Many in vitro experiments have shown that HAP in toothpaste and other oral care products adhere to the exposed dentin surface, coat the surfaces with microscopic particles of HAP, and occlude open dentin tubules, thereby reducing fluid flow and blocking pain signals from the odontoblast processes to the brain [69]. In addition, studies have shown that a lower plaque pH (or lower pH from dietary exposure) encourages dissociation of the HAP particles into calcium and phosphate ions [66,70,71]. Existing HAP crystals grow in the presence of excess calcium and phosphate ions. These are provided by the dissociation of HAP particles supplied by the oral care products exposed to a lower pH. The calcium and phosphate ions diffuse further into the dentin tubules. The pH is higher in the tubules the further from the surface the ions diffuse, and eventually salivary buffers neutralize the weak acids. This encourages the mineral phase to remineralize, grow, and occlude the tubules further than the physical obstructions provided by the HAP particles, which are still intact. When added to oral care products that are used regularly at home simply by brushing teeth twice a day, HAP-particles can serve to block dentin tubules and contribute to their remineralization (Figure 5).
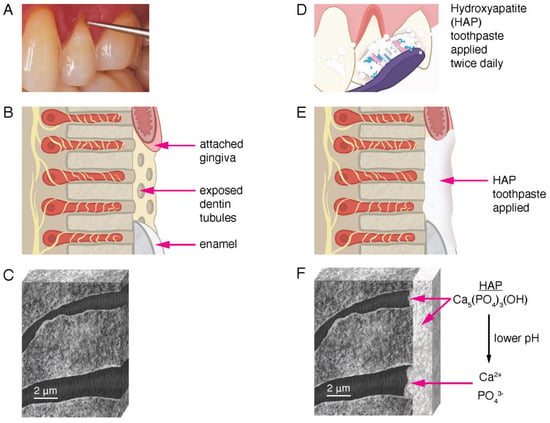
Figure 5.
Illustration of the mechanism of HAP reduction of dentin sensitivity. (A): Clinical photograph of gingival recession on tooth 24, showing exposed dentin at the cervical margin indicated by the tip of the explorer. Tactile sensitivity occurs when the explorer makes contact with the exposed dentin. (B): Drawing of the area in (A) showing exposed dentin tubules. The attached gingiva, exposed dentin tubules, and enamel are labeled (not to scale). The yellow structures are nerve endings extending into the dentin tubules in close proximity to the odontoblast processes. The movement of liquid through the tubule elicits dentin hypersensitivity. (C): Drawing of an actual SEM image at high magnification of a section of exposed and demineralized human dentin showing two open dentin tubules. (D): Drawing of toothbrush application of hydroxyapatite toothpaste to the area in (A). (E): Drawing of the same area in (B) showing a layer of HAP toothpaste after one application. (F): After multiple uses, the entire dentin surface (in the before illustration in (C)) is covered with a layer of HAP. The dentin tubules are also occluded with HAP minerals. Both intact HAP molecules and ions from the dissociation of HAP contribute to the remineralization of the dentin surface, reducing dentin sensitivity. The HAP layer is stable with continued use of HAP toothpaste, producing a long-term reduction in dentin hypersensitivity.
The oral care products used in the studies listed in Table 1 varied in their synthetic HAP particle sizes. HAP is synthesized in various processes, which leads to a variety of particle dimensions. Most particles are micrometer in size, and can sometimes be measured in nanometer widths, as shown in many SEM studies in vitro. With dentin tubule width in the µm range (Figure 5), the small dimensions of the nano- and micro-HAP particles explain how they can accumulate in the dentin tubules and eventually occlude them, reducing dentin hypersensitivity. Specific crystal morphology and size have not been studied in detail to determine what the optimum dimensions for biomimetic HAP should be for dentin tubule occlusion and dentin adherence.
4.2. Strength of Evidence and Results of the Meta-Analysis
Our meta-analysis clearly demonstrates that there are many well-conducted clinical studies that show a significant lowering of DH in patients who report (or test positive for) hypersensitive teeth due mainly to exposed root surfaces after gingival recession. Overall, a significant reduction of 39.5% (CI 95% [48.93; 30.06]) can be expected when HAP toothpaste is used exclusively for a few weeks, compared to the placebo. Evidence from well-conducted clinical trials that HAP is an effective dentin desensitizer comes from many studies already published and reviewed (see Supplementary Material Table S1, which contains recent systematic reviews). We have updated the literature in the present systematic review and meta-analysis, having found 44 clinical trials on HAP in oral care products to desensitize sensitive teeth. Our meta-analysis of 22 RCTs is the most up-to-date quantitative synthesis of the evidence, indicating that HAP is an effective dentin desensitizer.
Enax et al. [72] reviewed the safety of calcium phosphates, including biomimetic HAP, and it was concluded that HAP can be safely swallowed when used in oral care products. However, most subjects expectorate and rinse after using their toothpaste. Toothpastes are not the only method of applying HAP to sensitive teeth. At home custom tray application can be a method of application. Leaving the HAP in contact with exposed dentin for longer periods of time may increase its efficacy, but more RCTs are required to determine if this is truly the case. The use of HAP products in patients after vital bleaching or periodontal therapy, whitening teeth with carbamide peroxides either professionally, or with home use products, can increase dentin hypersensitivity [73]. Even though the hypersensitivity is transient and thought to be the result of inflammation of the pulp, designers of in-office vital bleaching gels are testing whether the addition of HAP to their products can reduce after-treatment DH [74]. Those patients who have already experienced exposed dentin before bleaching could benefit from using HAP gels and toothpastes.
There were 11 clinical trials in which the test HAP-containing product was applied professionally by the clinician. In some of those studies, the product applied (a toothpaste) was also used at home. Apart from one study [20], the trials involving professional application were not included in the meta-analysis because the data from these studies were not comparable. However, all studies where HAP was professionally applied showed a benefit of using HAP-products as professional (in-office) treatment with respect to reducing DH [23,24,25,31,35,39,47,49,60]. Patients with exposed dentin may have very uncomfortable hypersensitivity after vigorous periodontal therapy (root planing) or after periodontal surgery [75]. The studies we found showed that patients can be helped after their periodontal surgery to manage dentin hypersensitivity when their root surfaces have been exposed.
4.3. Enhancing HAP Efficiency
There is some evidence that adding other elements to HAP might improve its ability to occlude dentin tubules and provide more stability to deposited crystals. Examples include Zn, Mg, and both [76]. Fluoride is thought to promote the remineralization potential of tubules, and fortifying fluoride toothpastes with HAP to improve the desensitizing potential of fluoride is a strategy that has not been fully tested. Novamin (calcium sodium phosphosilicate) added to fluoride toothpaste seems to be an effective strategy [77], but the evidence suggests that HAP outperforms other methods of desensitization [13].
4.4. Additional Studies
After our search was completed, 4 additional studies appeared in the literature that were not included in this qualitative or quantitative synthesis [78,79,80,81]. These studies were consistent with HAP in reducing dentin hypersensitivity. In all, 48 trials have been published examining the effectiveness of HAP as a dentin-desensitizing additive in oral care products.
4.5. HAP Toothpaste Approved for Use in Canada
Government regulatory agencies have strict regulations for making claims on toothpaste packaging. Without clinical evidence, claims of dentin desensitizing cannot be made. In Canada, a number of toothpastes have received permission from Health Canada to be sold with claims to treat sensitive teeth. See Table S3 in the Supplementary Materials for this list and their characteristics.
5. Conclusions
Based on this systematic review and up-to-date meta-analysis, it can be concluded that hydroxyapatite is a safe biomimetic ingredient in oral care products for the reduction of dentin hypersensitivity, in addition to its already demonstrated anti-caries effects. Dental professionals can consider recommending hydroxyapatite-based oral care products as a primary strategy for the effective management of dentin hypersensitivity, which provides their patients with immediate and long-lasting relief from the dental pain caused by dentin hypersensitivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomimetics8010023/s1, Table S1: Previous systematic reviews on DH; Table S2: Search details; Table S3: Health Canada approved HAP toothpastes for DH; PRISMA checklist.
Author Contributions
Conceptualization: H.L., J.E. and F.M.; methodology, H.L., J.E. and F.M.; literature search, qualitative synthesis, H.L.; validation of qualitative synthesis, J.E. and F.M.; meta-analysis data extraction and forest plots, F.M.; illustrations, original draft preparation, H.L.; editing, H.L., J.E. and F.M.; supervision, corresponding author, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Kevin Limeback, BSc, BEd, for help with the design and artwork of Figure 5.
Conflicts of Interest
J.E. and F.M. are senior scientists and employees of Dr. Kurt Wolff GmbH & Co. KG in Germany.
References
- Holland, G.R.; Narhi, M.N.; Addy, M.; Gangarosa, L.; Orchardson, R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J. Clin. Periodontol. 1997, 24, 808–813. [Google Scholar] [CrossRef]
- Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J. Can. Dent. Assoc. 2003, 4, 221–226. [Google Scholar]
- Liu, X.X.; Tenenbaum, H.C.; Wilder, R.S.; Quock, R.; Hewlett, E.R.; Ren, Y.F. Pathogenesis, diagnosis and management of dentin hypersensitivity: An evidence-based overview for dental practitioners. BMC Oral. Health 2020, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Brannstrom, M.; Astrom, A. The hydrodynamics of dentin and its possible relationship to dentinal pain. Int. Dent. J. 1972, 22, 219–227. [Google Scholar] [PubMed]
- West, N.; Seong, J.; Davies, M. Dentine hypersensitivity. Monogr Oral Sci. 2014, 25, 108–122. [Google Scholar]
- Zeola, F.L.; Soares, P.V.; Cunha-Cruz, J. Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J. Dent. 2019, 81, 1–6. [Google Scholar] [CrossRef]
- Clark, D.; Levin, L. Non-surgical management of tooth hypersensitivity. Int. Dent. J. 2016, 66, 249–256. [Google Scholar] [CrossRef]
- Moraschini, V.; da Costa, L.S.; Dos Santos, G.O. Effectiveness for dentin hypersensitivity treatment of non-carious cervical lesions: A meta-analysis. Clin. Oral. Investig. 2018, 2, 617–631. [Google Scholar] [CrossRef]
- Hu, M.L.; Zheng, G.; Zhang, Y.D.; Yan, X.; Li, X.C.; Lin, H. Effect of desensitizing toothpastes on dentine hypersensitivity: A systematic review and meta-analysis. J. Dent. 2018, 75, 12–21. [Google Scholar] [CrossRef]
- Gul, H.; Ghaffar, M.A.; Kaleem, M.; Khan, A.S. Hydroxyapatite, a potent agent to reduce dentin hypersensitivity. J. Pak. Med. Assoc. 2021, 71, 2604–2610. [Google Scholar] [CrossRef]
- Alencar de Melo, C.; de Paula, B.L.F.; Guanipa Ortiz, M.I.; Baraúna Magno, M.; Martins Silva, C.; Cople Maia, L. Clinical efficacy of nano-hydroxyapatite in dentin hypersensitivity: A systematic review and meta-analysis. J. Dent. 2019, 82, 11–21. [Google Scholar] [CrossRef]
- Marto, C.M.; Baptista, P.A.; Nunes, T.; Pimenta, M.; Abrantes, A.M.; Pires, A.S.; Laranjo, M.; Carrilho, A.; Donato, H.; Botleho, M.F.; et al. Evaluation of the efficacy of dentin hypersensitivity treatments-A systematic review and follow-up analysis. J. Oral. Rehabil. 2019, 46, 952–990. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Zheng, G.; Lin, H.; Yang, M.; Zhang, Y.D.; Han, J.M. Network meta-analysis on the effect of desensitizing toothpastes on dentine hypersensitivity. J. Dent. 2019, 88, 103170. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.C.; Firmino, R.T.; Riva, J.J.; Ge, L.; Carrasco-Labra, A.; Brignardello-Petersen, R.; Colunga-Lozano, L.E.; Granville-Garcia, A.F.; Costa, F.O.; Yepes-Nuñez, J.J.; et al. Desensitizing Toothpastes for Dentin Hypersensitivity: A Network Meta-analysis. J. Dent. Res. 2020, 99, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Oubenyahya, H. Nano hydroxyapatite toothpaste as a treatment for dentine hypersensitivity: A systematic review. Saudi. J. Oral. Sci. 2021, 8, 122. [Google Scholar] [CrossRef]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Limeback, H.; Enax, J.; Meyer, F. Biomimetic hydroxyapatite and caries prevention: A systematic review and meta-analysis. Can. J. Dent. Hyg. 2021, 55, 148–159. [Google Scholar]
- Richards, D. Rating the quality of evidence in evidence-based dentistry. Evid. Based Dent. 2019, 20, 32–33. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovi, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Wickham, H.; François, F.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. R package version 0.7.6. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 15 June 2022).
- Al Asmari, D.; Khan, M.K. Evaluate efficacy of desensitizing toothpaste containing zinc-carbonate hydroxyapatite nanocrystals: Non-comparative eight-week clinical study. J. Int. Soc. Prev. Community Dent. 2019, 9, 566–570. [Google Scholar]
- Alencar, C.D.; Ortiz, M.I.; Silva, F.A.; Alves, E.B.; Araújo, J.L.; Silva, C.M. Effect of nanohydroxyapatite associated with photobiomodulation in the control of dentin hypersensitivity: A randomized, double-blind, placebo-controlled clinical trial. Am. J. Dent. 2020, 33, 138–144. [Google Scholar]
- Alharith, D.N.; Al-Omari, M.; Almnea, R.; Basri, R.; Alshehri, A.H.; Al-Nufiee, A.A. Clinical efficacy of single application of plain nano-hydroxyapatite paste in reducing dentine hypersensitivity–A randomized clinical trial. Saudi. Endod. J. 2021, 11, 24–30. [Google Scholar]
- Alsen, W.; Barngkgei, I.; Dayoub, S. Evaluation of desensitizing efficacy of nanohydroxyapatite on the treatment of dentine hypersensitivity following ultrasonic scaling: A randomized controlled trial. Braz. Dent. Sci. 2022, 25, e2737. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Lemke, K.C.; Saha, S.; Gelfond, J. Clinical efficacy in relieving dentin hypersensitivity of nanohydroxyapatite-containing cream: A randomized controlled trial. Open Dent. J. 2018, 12, 572–585. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Lemke, K.C.; Saha, S.; Luong, M.N.; Gelfond, J. Clinical efficacy of nanohydroxyapatite-containing toothpaste at relieving dentin hypersensitivity: An 8 weeks randomized control trial. BDJ Open 2021, 7, 23. [Google Scholar] [CrossRef]
- Amin, M.; Mehta, R.; Duseja, S.; Desai, K. Evaluation of the efficacy of comercially available nano hydroxypatite paste (Aclaim) as a desensitizing agent. Adv. Human Biol. 2015, 5, 34–38. [Google Scholar]
- Anand, S.; Rejula, F.; Sam, J.V.G.; Christaline, R.; Nair, M.G.; Dinakaran, S. Comparative evaluation of effect of nano-hydroxyapatite and 8% arginine containing toothpastes in managing dentin hypersensitivity: Double blind randomized clinical trial. Acta Medica 2017, 60, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Malpassi, M. Clinical trial of a 15% supermicronized hydroxyapatite gel for dentin hypersensitivity. G. Ital. Endod. 1991, 5, 43–47. [Google Scholar]
- Bevilacqua, F.M.; Catelan, A.; Araújo, G.S.A.; Saraceni, C.H.C.; Sampaio, J.E.C. Efficacy of a bioactive material and nanostructured desensitizing (Nano-hydroxyapatite associated with 9000 ppm SF and 5% PN) on dentin hypersensitivity treatment. Rev. Odontol. UNESP 2016, 45, 127–131. [Google Scholar] [CrossRef]
- Browning, W.D.; Cho, S.D.; Deschepper, E.J. Effect of a nano-hydroxyapatite paste on bleaching-related tooth sensitivity. J. Esthet. Restor. Dent. 2012, 24, 268–276. [Google Scholar] [CrossRef]
- Choi, Y.H.; Park, H.C.; Lee, S.M.; Son, H.J.; Choi, E.B.; Ha, J.Y.; Lee, J.Y.; Kim, K.K. Therapeutic effect of toothpaste containing hydroxyapatite and tribasic calcium phosphate on dentinal hypersensitivity. J. Life Sci. 2014, 24, 642–647. [Google Scholar] [CrossRef]
- da Silva, R.C.; Alencar, C.M.; Silva, B.H.R.; de Paula, B.L.F.; Barros, A.P.O.; da Silveira, A.D.S.; Silva, C.M. A clinical, randomised, double-blind study on the use of nano-hydroxyapatite and arginine during at-home tooth bleaching. J. Clin. Diag. Res. 2018, 12, ZC01–ZC05. [Google Scholar] [CrossRef]
- Douglas-de-Oliveira, D.W.; Oliveira, E.S.; Mota, A.F.; Pereira, V.H.; Bastos, V.O.; Glória, J.C.; Gonçalves, P.F.; Flecha, O.D. Effectiveness of three desensitizing dentifrices on cervical dentin hypersensitivity: A Pilot Clinical Trial. J. Int. Acad Periodontol. 2016, 18, 57–65. [Google Scholar] [PubMed]
- Ding, P.H.; Dai, A.; Hu, H.J.; Huang, J.P.; Liu, J.M.; Chen, L.L. Efficacy of nano-carbonate apatite dentifrice in relief from dentine hypersensitivity following non-surgical periodontal therapy: A randomized controlled trial. BMC Oral. Health 2020, 20, 170. [Google Scholar] [CrossRef]
- Ehlers, V.; Reuter, A.K.; Kehl, E.B.; Enax, J.; Meyer, F.; Schlecht, J.; Schmidtmann, I.; Deschner, J. Efficacy of a toothpaste based on microcrystalline hydroxyapatite on children with hypersensitivity caused by MIH: A randomised controlled trial. Oral. Health Prev. Dent. 2021, 19, 647–658. [Google Scholar]
- Gopinath, N.M.; John, J.; Nagappan, N.; Prabhu, S.; Kumar, E.S. Evaluation of dentifrice containing nano-hydroxyapatite for dentinal hypersensitivity: A randomized controlled trial. J. Int. Oral. Health 2015, 7, 118–122. [Google Scholar] [PubMed]
- Gümüştaş, B.; Dikmen, B. Effectiveness of remineralization agents on the prevention of dental bleaching induced sensitivity: A randomized clinical trial. Int. J. Dent. Hyg. 2021, 20, 1–8. [Google Scholar] [CrossRef]
- Hüttemann, R.W.; Dönges, H. Investigations for treating hypersensitive necks of teeth with hydroxyapatite. Dtsch Zahnärztl Z. 1987, 42, 486–488. [Google Scholar] [PubMed]
- Jena, A.; Shashirekha, G. Comparison of efficacy of three different desensitizing agents for in-office relief of dentin hypersensitivity: A 4 weeks clinical study. J. Conserv. Dent. 2015, 18, 389–393. [Google Scholar] [CrossRef]
- Kang, S.J.; Kwon, Y.H.; Park, J.B.; Herr, Y.; Chung, J.H. The effects of hydroxyapatite toothpaste on tooth hypersensitivity. J. Korean Acad. Periodontol. 2009, 39, 9–16. [Google Scholar] [CrossRef]
- Kim, M.S.; Chae, G.J.; Choi, S.H.; Chai, J.K.; Kim, C.K.; Cho, K.S. Effect of hydroxyapatite containing dentifrice on teeth hypersensitivity after periodontal therapy. J. Korean Acad. Periodontol. 2008, 38, 1–6. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, J.B.; Lee, C.W.; Koo, K.T.; Kim, T.I.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Chung, C.P.; Rhyu, I.C. The clinical effects of a hydroxyapatite containing toothpaste for dentine hypersensitivity. J. Korean Acad. Periodontol. 2009, 39, 87–94. [Google Scholar] [CrossRef]
- Kondyurova, E.V.; Lisevtsova, J.V.; Eliseykina, E.V.; Vilikotskiy, A.E.; Zakirova, S.A. Clinical evaluation of a dentifrice containing Nhap for the reduction of dentin hypersensitivity. Int. J. Oral. Dent. Health 2019, 5, 104. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Jung, H.-I.; Jung, B.-Y.; Cho, Y.-S.; Kwon, H.-K.; Kim, B.-I. Desensitizing efficacy of nano-carbonate apatite dentifrice and Er,Cr:YSGG laser:A randomized clinical trial. Photomed. Laser Surg. 2015, 33, 9–14. [Google Scholar] [CrossRef]
- Loguercio, A.D.; Tay, L.Y.; Herrera, D.R.; Bauer, J.; Reis, A. Effectiveness of nano-calcium phosphate paste on sensitivity during and after bleaching: A randomized clinical trial. Braz. Oral. Res. 2015, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Low, S.B.; Allen, E.P.; Kontogiorgos, E.D. Reduction in dental hypersensitivity with nano-hydroxyapatite, potassium nitrate, sodium monoflurophosphate and antioxidants. Open Dent. J. 2015, 9, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Maharani, D.A. Efficacy of a commercially available hydroxyapatite-containing toothpaste in reducing dentin hypersensitivity. Int. J. Clin. Prev. Dent. 2012, 8, 151–154. [Google Scholar]
- Makeeva, I.M.; Polyakova, M.A.; Avdeenko, O.E.; Paramonov, Y.O.; Kondrati’ev, S.A.; Pilyagina, A.A. Evaluation of the effectiveness of long-term use of Apadent Total Care toothpaste containing medical nano-hydroxyapatite. Stomatologii 2016, 95, 34–36. [Google Scholar] [CrossRef]
- Makeeva, I.M.; Polyakova, M.A.; Doroshina, V.Y.; Sokhova, I.A.; Arakelyan, M.G.; Makeeva, M.K. Efficiency of paste and suspension with nano-hydroxyapatite on the sensitivity of teeth with gingival recession. Stomatologiia 2018, 97, 23–27. [Google Scholar] [CrossRef]
- Orsini, G.; Procaccini, M.; Manzoli, L.; Giuliodori, F.; Lorenzini, A.; Putignano, A. A double-blind randomized-controlled trial comparing the desensitizing efficacy of a new dentifrice containing carbonate/hydroxyapatite nanocrystals and a sodium fluoride/potassium nitrate dentifrice. J. Clin. Periodontol. 2010, 37, 510–517. [Google Scholar] [CrossRef]
- Orsini, G.; Procaccini, M.; Manzoli, L.; Sparabombe, S.; Tiriduzzi, P.; Bambini, F.; Putignano, A. A 3-day randomized clinical trial to investigate the desensitizing properties of three dentifrices. J. Periodontol. 2013, 84, e65–e73. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Park, J.B.; Kwon, Y.H.; Herr, Y.; Chung, J.H. The effects of microcrystalline hydroxyapatite containing toothpaste in the control of tooth hypersensitivity. J. Korean Acad. Periodontol. 2005, 35, 577–590. [Google Scholar] [CrossRef]
- Pinojj, A.; Shetty, A.; Shetty, D.; Shetty, S. A comparison of clinical efficacy of dentifrices containing calcium sodium phosphosilicate, nanoparticle hydroxyapatite and a dentifrice containing casein phosphopeptide amorphous calcium phosphate on dentinal hypersensitivity: A comparative triple blind randomized study. Adv. Hum. Biol. 2014, 4, 57–64. [Google Scholar]
- Polyakova, M.; Sokhova, I.; Doroshina, V.; Arakelyan, M.; Novozhilova, N.; Babina, K. The effect of toothpastes containing hydroxyapatite, fluoroapatite, and Zn-Mg-hydroxyapatite nanocrystals on dentin hypersensitivity: A randomized clinical trial. J. Internat Soc. Prev. Commun. Dent. 2022, 12, 252. [Google Scholar]
- Porciani, P.F.; Chazine, M.; Grandini, S. A clinical study of the efficacy of a new chewing gum containing calcium hydroxyapatite in reducing dentin hypersensitivity. J. Clin. Dent. 2014, 25, 32–36. [Google Scholar] [PubMed]
- Reddy, S.; Prasad, M.G.S.; Prasad, S.; Bhowmik, N.; Ashwini, N.; Sravya, L.; Singh, S. The effect of pro-argin technology vs nano technology using commercially available dentifrice: A comparative study. Int. J. Appl. Dent. Sci. 2014, 1, 26–30. [Google Scholar]
- Seong, J.; Newcombe, R.G.; Foskett, H.L.; Davies, M.; West, N.X. A randomised controlled trial to compare the efficacy of an aluminium lactate/potassium nitrate/hydroxylapatite toothpaste with a control toothpaste for the prevention of dentine hypersensitivity. J. Dent. 2021, 108, 103619. [Google Scholar] [CrossRef]
- Shetty, S.; Kohad, R.; Yeltiwar, R. Hydroxyapatite as an in-office agent for tooth hypersensitivity: A clinical and scanning electron microscopic study. J. Periodontol. 2010, 81, 1781–1789. [Google Scholar] [CrossRef]
- Vano, M.; Derchi, G.; Barone, A.; Covani, U. Effectiveness of nano-hydroxyapatite toothpaste in reducing dentin hypersensitivity: A double-blind randomized controlled trial. Quintessence Internat. 2014, 45, 703–710. [Google Scholar]
- Vano, M.; Derchi, G.; Barone, A.; Genoves, A.; Covani, U. Tooth bleaching with hydrogen peroxide and nano-hydroxyapatite: A 9-month follow-up randomized clinical trial. Int. J. Dent. Hygiene. 2015, 13, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Vano, M.; Derchi, G.; Barone, A.; Pinna, R.; Usai, P.; Covani, U. Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: A double-blind randomized controlled trial. Clin. Oral. Investig. 2018, 22, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Narmatha, V.J.; Thakur, S. An in-vivo comparative study of the efficacy of Propolis, nano-hydroxyapatite and potassium nitrate-containingdesensitizing agents. RRJDS 2014, 2, 113–118. [Google Scholar]
- Wang, L.; Magalhães, A.C.; Francisconi-Dos-Rios, L.F.; Calabria, M.P.; Araújo, D.F.; Buzalaf, M.A.; Lauris, J.R.; Pereira, J.C. Treatment of dentin hypersensitivity using nano-hydroxyapatite pastes: A randomized three-month clinical trial. Oper. Dent. 2016, 41, E93–E101. [Google Scholar] [CrossRef]
- Schiff, T.; Dotson, M.; Cohen, S.; De Vizio, W.; McCool, J.; Volpe, A. Efficacy of a dentifrice containing potassium nitrate, soluble pyrophosphate, PVM/MA copolymer, and sodium fluoride on dentinal hypersensitivity: A twelve-week clinical study. J. Clin. Dent. 1994, 5, 87–92. [Google Scholar]
- Chambers, C.T.; Giesbrecht, K.; Craig, K.D.; Bennett, S.M.; Huntsman, E. A comparison of faces scales for the measurement of pediatric pain: Children’s and parents’ ratings. Pain 1999, 83, 25–35. [Google Scholar] [CrossRef]
- Chen, L.; Al-Bayatee, S.; Khurshid, Z.; Shavandi, A.; Brunton, P.; Ratnayake, J. Hydroxyapatite in oral care products-A review. Materials 2021, 14, 4865. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H. Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. J Endod. 1986, 12, 465–474. [Google Scholar] [CrossRef]
- Cieplik, F.; Rupp, C.M.; Hirsch, S.; Muehler, D.; Enax, J.; Meyer, F.; Hiller, K.-A.; Buchalla, W. Ca2+ release and buffering effects of synthetic hydroxyapatite following bacterial acid challenge. BMC Oral Health 2020, 20, 85. [Google Scholar]
- Enax, J.; Fabritius, H.-O.; Fabritius-Vilpoux, K.; Amaechi, B.T.; Meyer, F. Modes of action and clinical efficacy of particulate hydroxyapatite in preventive oral health care − state of the art. Open Dent. J. 2019, 13, 274–287. [Google Scholar] [CrossRef]
- Enax, J.; Meyer, F.; Schulze zur Wiesche, E.; Epple, M. On the application of calcium phosphate micro- and nanoparticles as food additive. Nanobiomaterials 2022, 12, 4075. [Google Scholar] [CrossRef] [PubMed]
- Maran, B.M.; Matos, T.P.; de Castro, A.D.S.; Vochikovski, L.; Amadori, A.L.; Loguercio, A.D.; Reis, A.; Berger, S.B. In-office bleaching with low/medium vs. high concentrate hydrogen peroxide: A systematic review and meta-analysis. J. Dent. 2020, 103, 103499. [Google Scholar] [CrossRef] [PubMed]
- Orilisi, G.; Tosco, V.; Monterubbianesi, R.; Notarstefano, V.; Özcan, M.; Putignano, A.; Orsini, G. ATR-FTIR, EDS and SEM evaluations of enamel structure after treatment with hydrogen peroxide bleaching agents loaded with nano-hydroxyapatite particles. PeerJ 2021, 29, e10606. [Google Scholar] [CrossRef] [PubMed]
- Canakçi, C.F.; Canakçi, V. Pain experienced by patients undergoing different periodontal therapies. J. Am. Dent. Assoc. 2007, 12, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Nikaido, T.; Abdou, A.; Matin, K.; Burrow, M.F.; Tagami, J. Inhibitory effect of zinc-containing desensitizer on bacterial biofilm formation and root dentin demineralization. Dent. Mater. J. 2019, 38, 940–946. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Mathews, S.M.; Ramalingam, K.; Mensinkai, P.K. Evaluation of nanohydroxyapatite-containing toothpaste for occluding dentin tubules. Am. J. Dent. 2015, 28, 33–39. [Google Scholar]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home oral care with biomimetic hydroxyapatite vs. conventional fluoridated toothpaste for the remineralization and desensitizing of white spot lesions: Randomized clinical trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef]
- Moharam, L.M.; Khadr, S.; Abdou, A.; Nagi, S.M. Effect of arginine and nano-hydroxyapatite application on the hypersensitivity and color change of bleached enamel: A randomized controlled clinical trial. J. Clin. Exp. Dent. 2022, 14, e499–e505. [Google Scholar] [CrossRef]
- Vlasova, N.; Samusenkov, V.; Novikova, I.; Nikolenko, D.; Nikolashvili, N.; Gor, I.; Danilina, A. Clinical efficacy of hydroxyapatite toothpaste containing Polyol Germanium Complex (PGC) with threonine in the treatment of dentine hypersensitivity. Saudi. Dent. J. 2022, 34, 310–314. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Trapani, B.; Gallo, S.; Radu, M.; Scribante, A. Biomimetic hydroxyapatite paste for molar-incisor hypomineralization: A randomized clinical trial. Oral. Dis. 2022, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).






