Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Type of Study. Case-control, cross-sectional, cohort studies, clinical trials, in vitro studies, reviews and meta-analyses from 2003 to 2023.
- Type of Participant. Participants who used zinc–carbonate hydroxyapatite toothpaste and/or mouthwash.
- Type of Intervention. Case-control, cross-sectional, cohort studies and clinical trials that have evaluated the possible benefits of zinc–carbonate hydroxyapatite toothpaste and/or mouthwash.
- Outcome Type. Each variable included in the studies was taken into account.
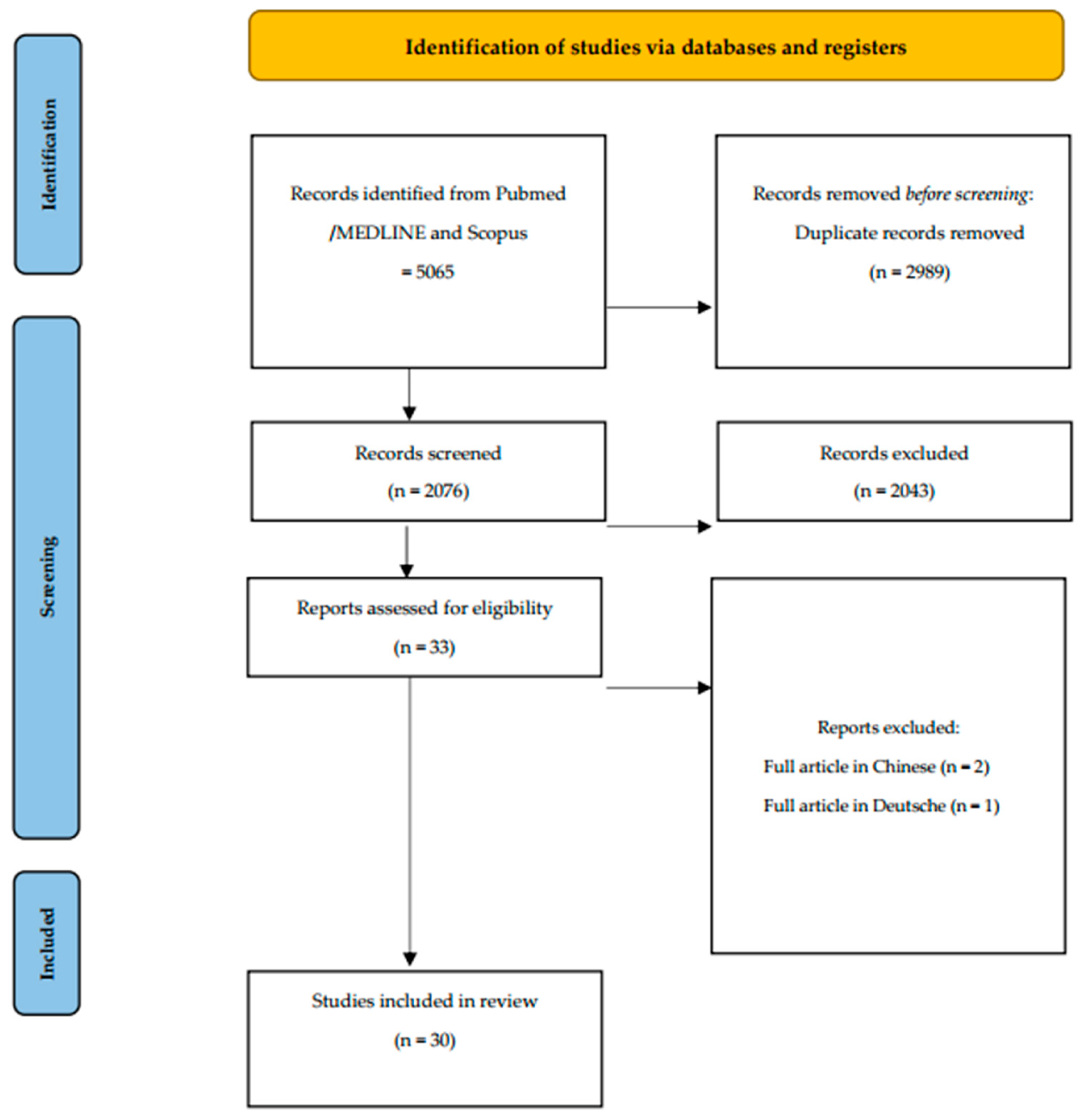
2.2. Search Strategy
2.3. Research
2.4. Screening and Selection of Articles
3. Results
3.1. Enamel Protection (Tooth Decay and/or Erosion) [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53], Table 1
| Articles | Study | Agents | Conclusion |
|---|---|---|---|
| Bossù et al., 2019 [33] | In vitro/clinical trial | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-HAp | The use of Biomimetic hydroxyapatite toothpastes has proven to be a valuable prevention measure against dental caries. |
| Poggio et al., 2010 [34] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (20%) ZnCO3/n-HAp | The toothpastes tested (Pronamel and BioRepair Plus) offer a degree of protection from erosive drinks. |
| Poggio et al., 2014 [35] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (20%) ZnCO3/n-Hap Zinc–carbonate hydroxyapatite toothpaste (24%) ZnCO3/n-HAp | Biorepair Plus-Total Protection® and Sensodyne Repair & Protect® provided higher protective effect against dentin demineralization. |
| Colombo et al., 2017 [36] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-HAp | In this study treatment of erosively challenged enamel with Zn-Hap toothpaste showed a clear protective effect. |
| Lombardini et al., 2014 [37] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (20%) ZnCO3/n-HAp Zinc–carbonate hydroxyapatite toothpaste (24%) ZnCO3/n-HAp | BioRepair Plus-Sensitive Teeth, Biorepair Plus-Total Protection and Sensodyne Repair & Protect provided lower effectiveness in protecting enamel against erosion. |
| Colombo et al., 2017 [38] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-HAp | The results of this study confirmed the potential benefit the Zn-HAP technology could provide in protecting enamel from erosive acid challenges. The treatment of erosively challenged enamel with Zn-Hap toothpaste showed a clear protective effect. |
| Bradna et al., 2016 [39] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (24%) ZnCO3/n-HAp | Results revealed that toothpastes with strong potential to form acid-resistant deposits on the enamel surface and having low abrasivity should be used for effective prevention of enamel erosion. |
| Aykut-Yetkiner et al., 2014 [40] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-Hap | Toothpastes with anti-erosive formulations reduced dentine erosion, especially under simulated extrinsic erosive conditions, but were not superior to a conventional fluoride toothpaste. |
| Ganss et al., 2011 [41] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-Hap | Conventional NaF toothpastes reduced erosive tissue loss, but had limited efficacy regarding the prevention of brushing abrasion. The special formulations were not superior or were even less effective. |
| Chandru et al., 2020 [42] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-HAp | As per statistical analysis, maximum remineralization of enamel blocks occurred after applying Colgate Sensitive Plus® toothpaste followed by BioRepair® tooth paste and Regenerate enamel Science™ toothpaste. The least remineralization potential was shown by control group. |
| Kensche et al., 2016 [43] | In vitro/clinical trial | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-HAp | The biomimetic materials reduced ion release, but their effect was less pronounced. |
| Kranz et al., 2022 [44] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-HAp | Treatment with Biorepair® did not affect enamel surfaces as proposed. Minor mineral precipitation and a reduction in surface roughness were detected on dentin surfaces only. |
| Alessandri Bonetti et al., 2014 [45] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (20%) ZnCO3/n-HAp | The lowest grade of damage was recorded in samples brushed with Zn-CHA. |
| Tschoppe et al., 2011 [46] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (24%) ZnCO3/n-HAp | With the in vitro conditions chosen, toothpastes containing n-HAp revealed higher remineralizing effects compared to amine fluoride toothpastes with bovine dentine, and comparable trends were obtained for enamel. |
| Hegazy et al., 2016 [47] | Clinical trial | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-Hap | Biorepair mouthwash can serve as a better alternative to different mouthwashes including both fluoride and chlorhexidine. This single mouthwash can serve as a multi-purpose mouthwash. |
| Lelli et al., 2014 [48] | In vitro/clinical trial | Zinc–carbonate hydroxyapatite toothpaste (20%) ZnCO3/n-HAp |
In conclusion, this study demonstrates that the toothpaste containing Zn-CHA structured microcrystals, unlike nitrate potassium/sodium fluoride and non-specified fluoride toothpastes, may promote enamel superficial repair by means of the formation of a protective biomimetic CHA coating. |
| Butera et al., 2021 [49] | Clinical trial | Zinc–carbonate hydroxyapatite toothpaste (20%) ZnCO3/n-HAp | The use of toothpaste containing Zn-carbonate hydroxyapatite could be proposed as a device for domiciliary oral hygiene because the deposition of hydroxyapatite on polymeric composite resin could prevent secondary caries on the margins of restorations. |
| Poggio et al., 2017 [50] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (20%) ZnCO3/n-HAp | Despite the limitations of this study, the protective pastes that showed the least weight loss due to acidic challenge are Biorepair and Regenerate. |
| Poggio et al., 2017 [51] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (20%) ZnCO3/n-HAp | Toothpaste with Zn-HAP resulted in significant enamel remineralization of erosively challenged enamel, indicating that these toothpastes could provide enamel health benefits relevant to enamel erosion. |
| Scribante et al., 2020 [52] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (30%) ZnCO3/n-HAp | The application of remineralizing solution induced a significant in vitro reduction of demineralized areas after the first week of application. |
| Butera et al., 2022 [53] | Clinical trial |
Zinc–carbonate hydroxyapatite toothpaste (20%) Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) | The use of the hydroxyapatite-based toothpaste, alone or in combination with the mouthwash containing hydroxyapatite, is an effective method for the domiciliary management of dental erosion in physically active individuals such as rugby players. |
3.2. Management of Dental Hypersensitivity [54,55,56,57,58,59,60,61,62], Table 2
| Articles | Study | Agents | Conclusion |
|---|---|---|---|
| Orsini et al., 2010 [54] | Clinical trial | Zinc–carbonate hydroxyapatite toothpaste (24%) ZnCO3/n-HAp | This study documented that a new dentifrice containing zinc-CHA crystals significantly reduced dentinal hypersensitivity after 4 and 8 weeks, supporting its utility in clinical practice. |
| Orsini et al., 2013 [55] | Clinical trial | Zinc–carbonate hydroxyapatite toothpaste (30%) ZnCO3/n-HAp | Rapid relief from DH with a zinc–carbonate hydroxyapatite dentifrice. |
| Peetsch et al., 2011 [56] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-Hap Zinc–carbonate hydroxyapatite mouthwash (undeclared percentage) ZnCO3/n-HAp | The original goal to occlude the l m-sized dentinal tubules may be achievable in all cases if agglomerates are broken up under mechanical stress, e. g. during tooth-brushing. In comparison to natural tooth mineral, the Biorepair products showed the highest chemical similarity. |
| Steinert et al., 2020 [57] | Clinical trial | Zinc–carbonate hydroxyapatite toothpaste (20%) ZnCO3/n-HAp | The tested toothpaste with biomimetic HAP is well-suited for individuals suffering from dentin hypersensitivity, because subjective symptoms of dentin hypersensitivity were reduced. |
| Al Asmari et al., 2019 [58] | Clinical trial | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-Hap | The results suggested that the use of Zn-CHA crystals dentifrice might be an effective therapy to reduce DH. |
| Abou Neel et al., 2021 [59] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-Hap | Both ESTP (eggshell toothpaste) and TNPsESTP (titanium dioxide particle eggshell toothpaste) showed significantly higher numbers of partially occluded dentinal tubules than Biorepair. |
| Pei et al., 2019 [60] | In vitro | Zinc–carbonate hydroxyapatite toothpaste (undeclared percentage) ZnCO3/n-Hap | Hydroxyapatite-containing desensitizing toothpastes could occlude dentinal tubules effectively with a certain degree of acid resistance. |
| Butera et al., 2022 [61] | Clinical trial | Zinc–carbonate hydroxyapatite toothpaste (30%) ZnCO3/n-HAp | The hydroxyapatite-based toothpaste tested caused a reduction of hypersensitivity/pain values higher than conventional fluoride toothpaste. |
| Butera et al., 2022 [62] | Clinical trial | Zinc–carbonate hydroxyapatite toothpaste (24%) ZnCO3/n-HAp | Biomimetic zinc-hydroxyapatite showed a desensitizing effect when used to treat MIH. |
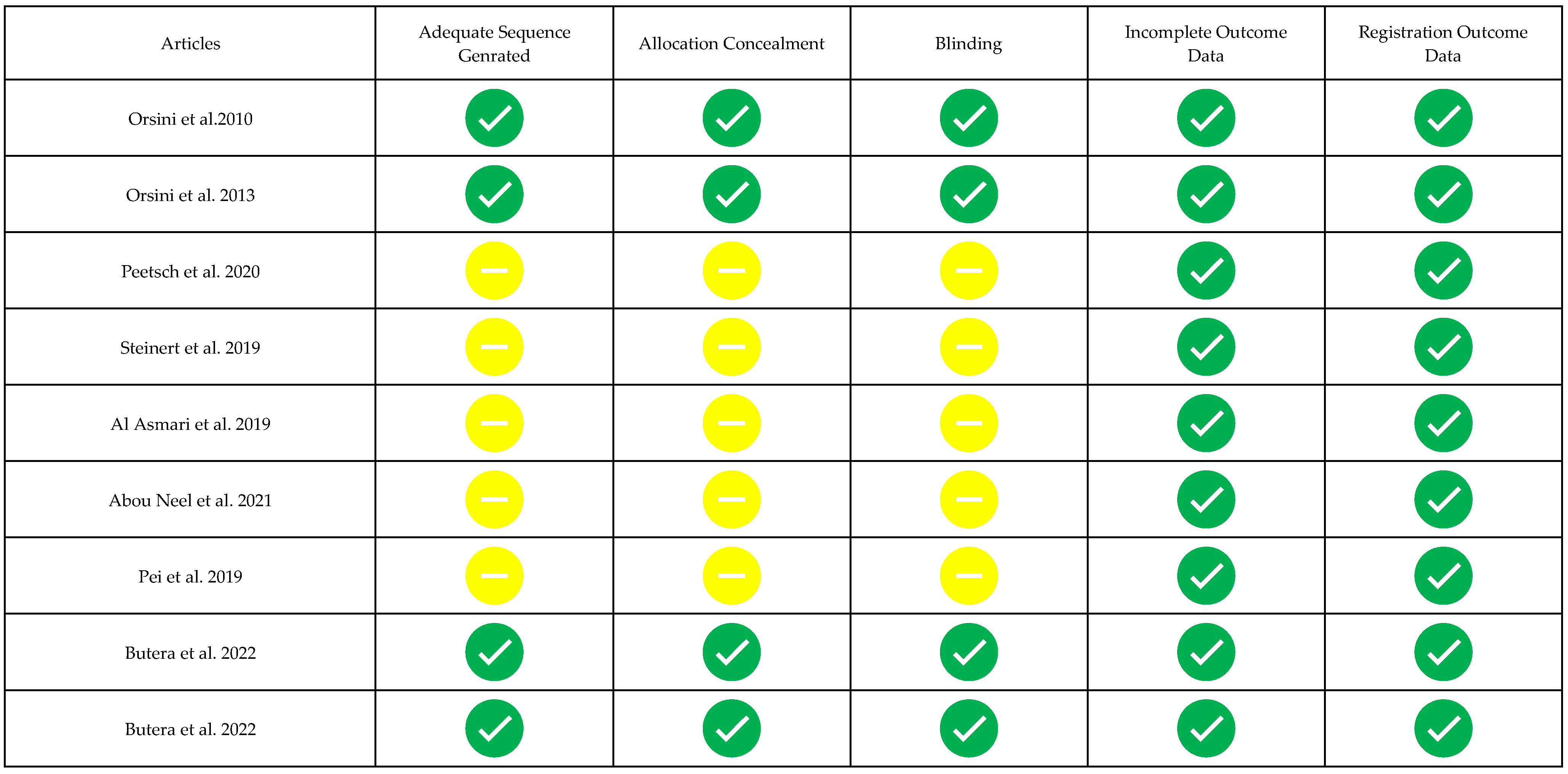
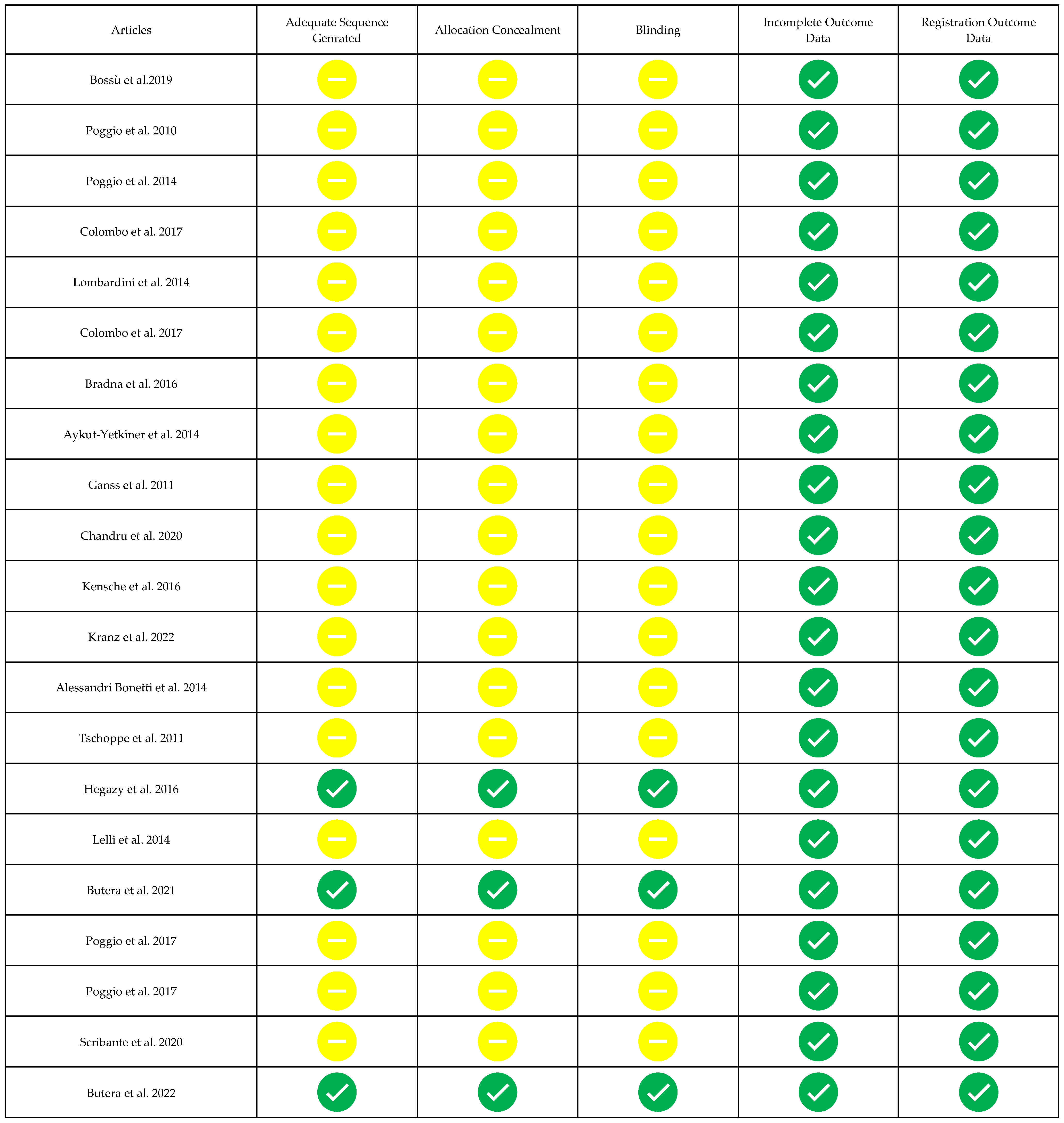
3.3. Risk of Bias of Single Studies
4. Discussion
4.1. Benefits for Dental Enamel
4.1.1. Protection of Enamel
4.1.2. Remineralizing Effect
4.2. Benefits for Dentin
4.3. Anti-Sensitivity Benefits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Figuero, E.; Nóbrega, D.F.; García-Gargallo, M.; Tenuta, L.M.A.; Herrera, D.; Carvalho, J.C. Mechanical and chemical plaque control in the simultaneous management of gingivitis and caries: A systematic review. J. Clin. Periodontol. 2017, 44, S116–S134. [Google Scholar] [CrossRef] [PubMed]
- Preda, C.; Butera, A.; Pelle, S.; Pautasso, E.; Chiesa, A.; Esposito, F.; Oldoini, G.; Scribante, A.; Genovesi, A.M.; Cosola, S. The efficacy of powered oscillating heads vs. Powered sonic action heads toothbrushes to maintain periodontal and peri-implant health: A narrative review. Int. J. Environ. Res. Public Health 2021, 18, 1468. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, M.; Worthington, H.V.; Deacon, S.A.; Deery, C.; Walmsley, A.D.; Robinson, P.G.; Glenny, A.M. Powered versus manual toothbrushing for oral health. Cochrane Database Syst. Rev. 2014, 2014, CD002281. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Enax, J. Hydroxyapatite in Oral Biofilm Management. Eur. J. Den. 2019, 13, 287–290. [Google Scholar] [CrossRef]
- Zampetti, P.; Scribante, A. Historical and bibliometric notes on the use of fluoride in caries prevention. Eur. J. Paediatr. Dent. 2020, 21, 148–152. [Google Scholar]
- Enax, J.; Amaechi, B.T.; Schulze ZurWiesche, E.; Meyer, F. Overview on Adjunct Ingredients Used in Hydroxyapatite-Based Oral Care Products. Biomimetics 2022, 7, 250. [Google Scholar] [CrossRef]
- Marinho, V.C.; Higgins, J.P.; Sheiham, A.; Logan, S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003, 2003, CD002278. [Google Scholar] [CrossRef]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef]
- Ten Cate, J.M.; Buzalaf, M.A.R. Fluoride Mode of Action: Once There Was an Observant Dentist. J. Dent. Res. 2019, 98, 725–730. [Google Scholar] [CrossRef]
- Kianoush, N.; Adler, C.J.; Nguyen, K.A.; Browne, G.V.; Simonian, M.; Hunter, N. Bacterial profile of dentine caries and the impact of pH on bacterial population diversity. PLoS ONE 2014, 9, e92940. [Google Scholar] [CrossRef]
- Warreth, A.; Abuhijleh, E.; Almaghribi, M.A.; Mahwal, G.; Ashawish, A. Tooth surface loss: A review of literature. Saudi Dent. J. 2020, 32, 53–60. [Google Scholar] [CrossRef]
- Ludovichetti, F.S.; Zambon, G.; Cimolai, M.; Gallo, M.; Signoriello, A.G.; Pezzato, L.; Bertolini, R.; Mazzoleni, S. Efficacy of Two Toothpaste in Preventing Tooth Erosive Lesions Associated with Gastroesophageal Reflux Disease. Appl. Sci. 2022, 12, 1023. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Weir, M.D.; Chow, L.C.; Xu, H.H. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J. Dent. Res. 2012, 91, 979–984. [Google Scholar] [CrossRef]
- Johansson, A.K.; Omar, R.; Carlsson, G.E.; Johansson, A. Dental erosion and its growing importance in clinical practice: From past to present. Int. J. Dent. 2012, 2012, 632907. [Google Scholar] [CrossRef]
- Cardoso, A.A.; de Sousa, E.T.; Steiner-Oliveira, C.; Parisotto, T.M.; Nobre-Dos-Santos, M. A high salivary calcium concentration is a protective factor for caries development during orthodontic treatment. J. Clin. Exp. Dent. 2020, 12, e209–e214. [Google Scholar] [CrossRef]
- Duckworth, R.M.; Huntington, E. On the relationship between calculus and caries. Monogr. Oral Sci. 2006, 19, 1–28. [Google Scholar]
- Saad, H.; Escoube, R.; Babajko, S.; Houari, S. Fluoride Intake Through Dental Care Products: A Systematic Review. Front. Oral Health 2022, 3, 916372. [Google Scholar] [CrossRef]
- Arifa, M.K.; Ephraim, R.; Rajamani, T. Recent Advances in Dental Hard Tissue Remineralization: A Review of Literature. Int. J. Clin. Pediatr. Dent. 2019, 12, 139–144. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. Natural enamel wear—a physiological source of hydroxylapatite nanoparticles for biofilm management and tooth repair? Med. Hypotheses 2010, 74, 670–672. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Roveri, N.; Battistella, E.; Bianchi, C.; Foltran, I.; Foresti, E.; Iafisco, M.; Lelli, M.; Naldoni, A.; Palazzo, B.; Rimondini, L. Surface Enamel Remineralization: Biomimetic Apatite Nanocrystals and Fluoride Ions Different Effects. J. Nanomater. 2009, 2009, 8. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Gawriolek, M.; Kaminska, I.; Otulakowska-Skrzynska, J.; Marczuk-Kolada, G.; Rzatowski, S.; Sokolowska, K.; Olszewska, A.; Schlagenhauf, U.; et al. Impact of a toothpaste with microcrystalline hydroxyapatite on the occurrence of early childhood caries: A 1-year randomized clinical trial. Sci. Rep. 2021, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Schlagenhauf, U.; Kunzelmann, K.H.; Hannig, C.; May, T.W.; Hösl, H.; Gratza, M.; Viergutz, G.; Nazet, M.; Schamberger, S.; Proff, P. Impact of a non-fluoridated microcrystalline hydroxyapatite dentifrice on enamel caries progression in highly caries-susceptible orthodontic patients: A randomized, controlled 6-month trial. J. Investig. Clin. Dent. 2019, 10, e12399. [Google Scholar] [CrossRef] [PubMed]
- Limeback, H.; Enax, J.; Meyer, F. Biomimetic hydroxyapatite and caries privation: A systematic review and meta-analysis. Can. J. Dent. Hyg. 2021, 55, 148–159. [Google Scholar]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef]
- Wierichs, R.J.; Westphal, S.; Lausch, J.; Meyer-Lueckel, H.; Esteves-Oliveira, M. Influence of highly concentrated fluoride dentifrices on remineralization characteristics of enamel in vitro. Clin. Oral Investig. 2018, 22, 2325–2334. [Google Scholar] [CrossRef]
- Fernandes, N.L.S.; Silva, J.G.V.C.; de Sousa, E.B.G.; D’Alpino, P.H.P.; de Oliveira, A.F.B.; de Jong, E.J.; Sampaio, F.C. Effectiveness of fluoride-containing toothpastes associated with different technologies to remineralize enamel after pH cycling: An in vitro study. BMC Oral Health 2022, 22, 489. [Google Scholar] [CrossRef]
- Da Silva, B.M.; Rios, D.; Foratori-Junior, G.A.; Magalhães, A.C.; Buzalaf, M.A.R.; Peres, S.C.S.; Honório, H.M. Effect of fluoride group on dental erosion associated or not with abrasion in human enamel: A systematic review with network metanalysis. Arch. Oral Biol. 2022, 144, 105568. [Google Scholar] [CrossRef]
- Indrapriyadharshini, K.; Madan Kumar, P.D.; Sharma, K.; Iyer, K. Remineralizing potential of CPP-ACP in white spot lesions—A systematic review. Indian J. Dent. Res. 2018, 29, 487–496. [Google Scholar] [CrossRef]
- Gupta, R.; Prakash, V. CPP-ACP complex as a new adjunctive agent for remineralisation: A review. Oral Health Prev. Dent. 2011, 9, 151–165. [Google Scholar]
- Reise, M.; Kranz, S.; Heyder, M.; Jandt, K.D.; Sigusch, B.W. Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Compared to Fluoride Products in an In-Vitro Demineralization Model. Materials 2021, 14, 5974. [Google Scholar] [CrossRef]
- Bossù, M.; Saccucci, M.; Salucci, A.; Di Giorgio, G.; Bruni, E.; Uccelletti, D.; Sarto, M.S.; Familiari, G.; Relucenti, M.; Polimeni, A. Enamel remineralization and repair results of Biomimetic Hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J. Nanobiotechnol. 2019, 17, 17. [Google Scholar] [CrossRef]
- Poggio, C.; Lombardini, M.; Colombo, M.; Bianchi, S. Impact of two toothpastes on repairing enamel erosion produced by a soft drink: An AFM in vitro study. J. Dent. 2010, 38, 868–874. [Google Scholar] [CrossRef]
- Poggio, C.; Lombardini, M.; Vigorelli, P.; Colombo, M.; Chiesa, M. The role of different toothpastes on preventing dentin erosion: An SEM and AFM study®. Scanning 2014, 36, 301–310. [Google Scholar] [CrossRef]
- Colombo, M.; Beltrami, R.; Rattalino, D.; Mirando, M.; Chiesa, M.; Poggio, C. Protective effects of a zinc-hydroxyapatite toothpaste on enamel erosion: SEM study. Ann. Stomatol. 2017, 7, 38–45. [Google Scholar] [CrossRef]
- Lombardini, M.; Ceci, M.; Colombo, M.; Bianchi, S.; Poggio, C. Preventive effect of different toothpastes on enamel erosion: AFM and SEM studies. Scanning 2014, 36, 401–410. [Google Scholar] [CrossRef]
- Colombo, M.; Mirando, M.; Rattalino, D.; Beltrami, R.; Chiesa, M.; Poggio, C. Remineralizing effect of a zinc-hydroxyapatite toothpaste on enamel erosion caused by soft drinks: Ultrastructural analysis. J. Clin. Exp. Dent. 2017, 9, e861–e868. [Google Scholar] [CrossRef]
- Bradna, P.; Vrbova, R.; Fialova, V.; Housova, D.; Gojisova, E. Formation of protective deposits by anti-erosive toothpastes-A microscopic study on enamel with artificial defects. Scanning 2016, 38, 380–388. [Google Scholar] [CrossRef]
- Aykut-Yetkiner, A.; Attin, T.; Wiegand, A. Prevention of dentine erosion by brushing with anti-erosive toothpastes. J. Dent. 2014, 42, 856–861. [Google Scholar] [CrossRef]
- Ganss, C.; Lussi, A.; Grunau, O.; Klimek, J.; Schlueter, N. Conventional and anti-erosion fluoride toothpastes: Effect on enamel erosion and erosion-abrasion. Caries Res. 2011, 45, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Chandru, T.P.; Yahiya, M.B.; Peedikayil, F.C.; Dhanesh, N.; Srikant, N.; Kottayi, S. Comparative evaluation of three different toothpastes on remineralization potential of initial enamel lesions: A scanning electron microscopic study. Indian J. Dent. Res. 2020, 31, 217–223. [Google Scholar] [PubMed]
- Kensche, A.; Pötschke, S.; Hannig, C.; Richter, G.; Hoth-Hannig, W.; Hannig, M. Influence of Calcium Phosphate and Apatite Containing Products on Enamel Erosion. Sci. World J. 2016, 2016, 7959273. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Heyder, M.; Mueller, S.; Guellmar, A.; Krafft, C.; Nietzsche, S.; Tschirpke, C.; Herold, V.; Sigusch, B.; Reise, M. Remineralization of Artificially Demineralized Human Enamel and Dentin Samples by Zinc-Carbonate Hydroxyapatite Nanocrystals. Materials 2022, 15, 7173. [Google Scholar] [CrossRef] [PubMed]
- Alessandri Bonetti, G.; Pazzi, E.; Zanarini, M.; Marchionni, S.; Checchi, L. The effect of zinc-carbonate hydroxyapatite versus fluoride on enamel surfaces after interproximal reduction. Scanning 2014, 36, 356–361. [Google Scholar] [CrossRef]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef]
- Hegazy, S.A.; Salama, R.I. Antiplaque and remineralizing effects of Biorepair mouthwash: A comparative clinical trial. Pediatr. Dent. J. 2016, 26, 89–94. [Google Scholar] [CrossRef]
- Lelli, M.; Putignano, A.; Marchetti, M.; Foltran, I.; Mangani, F.; Procaccini, M.; Roveri, N.; Orsini, G. Remineralization and repair of enamel surface by biomimetic Zn-carbonate hydroxyapatite containing toothpaste: A comparative in vivo study. Front. Physiol. 2014, 5, 333. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Gallo, S.; Lelli, M.; Tarterini, F.; Giglia, F.; Scribante, A. SEM/EDS Evaluation of the Mineral Deposition on a Polymeric Composite Resin of a Toothpaste Containing Biomimetic Zn-Carbonate Hydroxyapatite (microRepair®) in Oral Environment: A Randomized Clinical Trial. Polymers 2021, 13, 2740. [Google Scholar] [CrossRef]
- Poggio, C.; Gulino, C.; Mirando, M.; Colombo, M.; Pietrocola, G. Preventive effects of different protective agents on dentin erosion: An in vitro investigation. J. Clin. Exp. Dent. 2017, 9, e7–e12. [Google Scholar] [CrossRef]
- Poggio, C.; Gulino, C.; Mirando, M.; Colombo, M.; Pietrocola, G. Protective effect of zinc-hydroxyapatite toothpastes on enamel erosion: An in vitro study. J. Clin. Exp. Dent. 2017, 9, e118–e122. [Google Scholar] [CrossRef]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez YBaena, R.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength, and Hardness in In Vitro Tests. Biomed. Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Scardina, G.A.; Pezzullo, S.; Scribante, A. Home Oral Care Domiciliary Protocol for the Management of Dental Erosion in Rugby Players: A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 4893. [Google Scholar] [CrossRef]
- Orsini, G.; Procaccini, M.; Manzoli, L.; Giuliodori, F.; Lorenzini, A.; Putignano, A. A double-blind randomized-controlled trial comparing the desensitizing efficacy of a new dentifrice containing carbonate/hydroxyapatite nanocrystals and a sodium fluoride/potassium nitrate dentifrice. J. Clin. Periodontol. 2010, 37, 510–517. [Google Scholar] [CrossRef]
- Orsini, G.; Procaccini, M.; Manzoli, L.; Sparabombe, S.; Tiriduzzi, P.; Bambini, F.; Putignano, A. A 3-day randomized clinical trial to investigate the desensitizing properties of three dentifrices. J. Periodontol. 2013, 84, e65–e73. [Google Scholar] [CrossRef]
- Peetsch, A.; Epple, M. Characterization of the solid components of three desensitizing toothpastes and a mouth wash. Mat. Wiss. Werkst. 2011, 42, 131–135. [Google Scholar] [CrossRef]
- Steinert, S.; Zwanzig, K.; Doenges, H.; Kuchenbecker, J.; Meyer, F.; Enax, J. Daily Application of a Toothpaste with Biomimetic Hydroxyapatite and Its Subjective Impact on Dentin Hypersensitivity, Tooth Smoothness, Tooth Whitening, Gum Bleeding, and Feeling of Freshness. Biomimetics 2020, 5, 17. [Google Scholar] [CrossRef]
- Al Asmari, D.; Khan, M.K. Evaluate Efficacy of Desensitizing Toothpaste Containing Zinc-carbonate Hydroxyapatite Nanocrystals: Non-comparative Eight-week Clinical Study. J. Int. Soc. Prev. Community Dent. 2019, 9, 566–570. [Google Scholar]
- Abou Neel, E.A.; Bakhsh, T.A. An Eggshell-Based Toothpaste as a Cost-Effective Treatment of Dentin Hypersensitivity. Eur. J. Dent. 2021, 15, 733–740. [Google Scholar] [CrossRef]
- Pei, D.; Meng, Y.; Li, Y.; Liu, J.; Lu, Y. Influence of nano-hydroxyapatite containing desensitizing toothpastes on the sealing ability of dentinal tubules and bonding performance of self-etch adhesives. J. Mech. Behav. Biomed. Mater. 2019, 91, 38–44. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home Oral Care with Biomimetic Hydroxyapatite vs. Conventional Fluoridated Toothpaste for the Remineralization and Desensitizing of White Spot Lesions: Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Trapani, B.; Gallo, S.; Radu, M.; Scribante, A. Biomimetic hydroxyapatite paste for molar-incisor hypomineralization: A randomized clinical trial. Oral Dis. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Al-Bayatee, S.; Khurshid, Z.; Shavandi, A.; Brunton, P.; Ratnayake, J. Hydroxyapatite in Oral Care Products-A Review. Materials 2021, 14, 4865. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef]
- Fleet, M.E. The carbonate ion in hydroxyapatite: Recent X-ray and infrared results. Front. Biosci. 2013, 5, 643–652. [Google Scholar] [CrossRef]
- Mocanu, A.; Cadar, O.; Frangopol, P.T.; Petean, I.; Tomoaia, G.; Paltinean, G.A.; Racz, C.P.; Horovitz, O.; Tomoaia-Cotisel, M. Ion release from hydroxyapatite and substituted hydroxyapatites in different immersion liquids: In vitro experiments and theoretical modelling study. R. Soc. Open Sci. 2021, 8, 201785. [Google Scholar] [CrossRef]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef]
- Filip, D.G.; Surdu, V.-A.; Paduraru, A.V.; Andronescu, E. Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. J. Funct. Biomater. 2022, 13, 248. [Google Scholar] [CrossRef]
- Allaker, R.P. The Use of Antimicrobial Nanoparticles to Control Oral Infections. Nano-Antimicrobials 2011, 26, 395–425. [Google Scholar]
- Li, J.; Xie, X.; Wang, Y.; Yin, W.; Antoun, J.S.; Farella, M.; Mei, L. Long-term remineralizing effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) on early caries lesions in vivo: A systematic review. J. Dent. 2014, 42, 769–777. [Google Scholar] [CrossRef]
- Guanipa Ortiz, M.I.; Alencar, C.M.; Freitas De Paula, B.L.; Alves, E.B.; Nogueira Araújo, J.L.; Silva, C.M. Effect of the casein phosphopeptide-amorphous calcium phosphate fluoride (CPP-ACPF) and photobiomodulation (PBM) on dental hypersensitivity: A randomized controlled clinical trial. PLoS ONE 2019, 14, e0225501. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Quintini, M.; Lelli, M.; Tarterini, F.; Foltran, I.; Scribante, A. Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review. Biomimetics 2023, 8, 71. https://doi.org/10.3390/biomimetics8010071
Butera A, Maiorani C, Gallo S, Pascadopoli M, Quintini M, Lelli M, Tarterini F, Foltran I, Scribante A. Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review. Biomimetics. 2023; 8(1):71. https://doi.org/10.3390/biomimetics8010071
Chicago/Turabian StyleButera, Andrea, Carolina Maiorani, Simone Gallo, Maurizio Pascadopoli, Martina Quintini, Marco Lelli, Fabrizio Tarterini, Ismaela Foltran, and Andrea Scribante. 2023. "Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review" Biomimetics 8, no. 1: 71. https://doi.org/10.3390/biomimetics8010071
APA StyleButera, A., Maiorani, C., Gallo, S., Pascadopoli, M., Quintini, M., Lelli, M., Tarterini, F., Foltran, I., & Scribante, A. (2023). Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review. Biomimetics, 8(1), 71. https://doi.org/10.3390/biomimetics8010071