Convergent Evolution of Adhesive Properties in Leaf Insect Eggs and Plant Seeds: Cross-Kingdom Bioinspiration
Abstract
1. Introduction
- How does the morphology differ between P. philippinicum eggs and C. grandis seeds?
- What influence does the substrate surface roughness have on the adhesion of C. grandis seeds?
- What influence does the surface chemistry have on the adhesion of these seeds?
- What are the similarities and differences between both examples in terms of their adhesive performance and the repeatability of adhesion?
2. Materials and Methods
2.1. Specimens
2.2. Microscopic Visualization
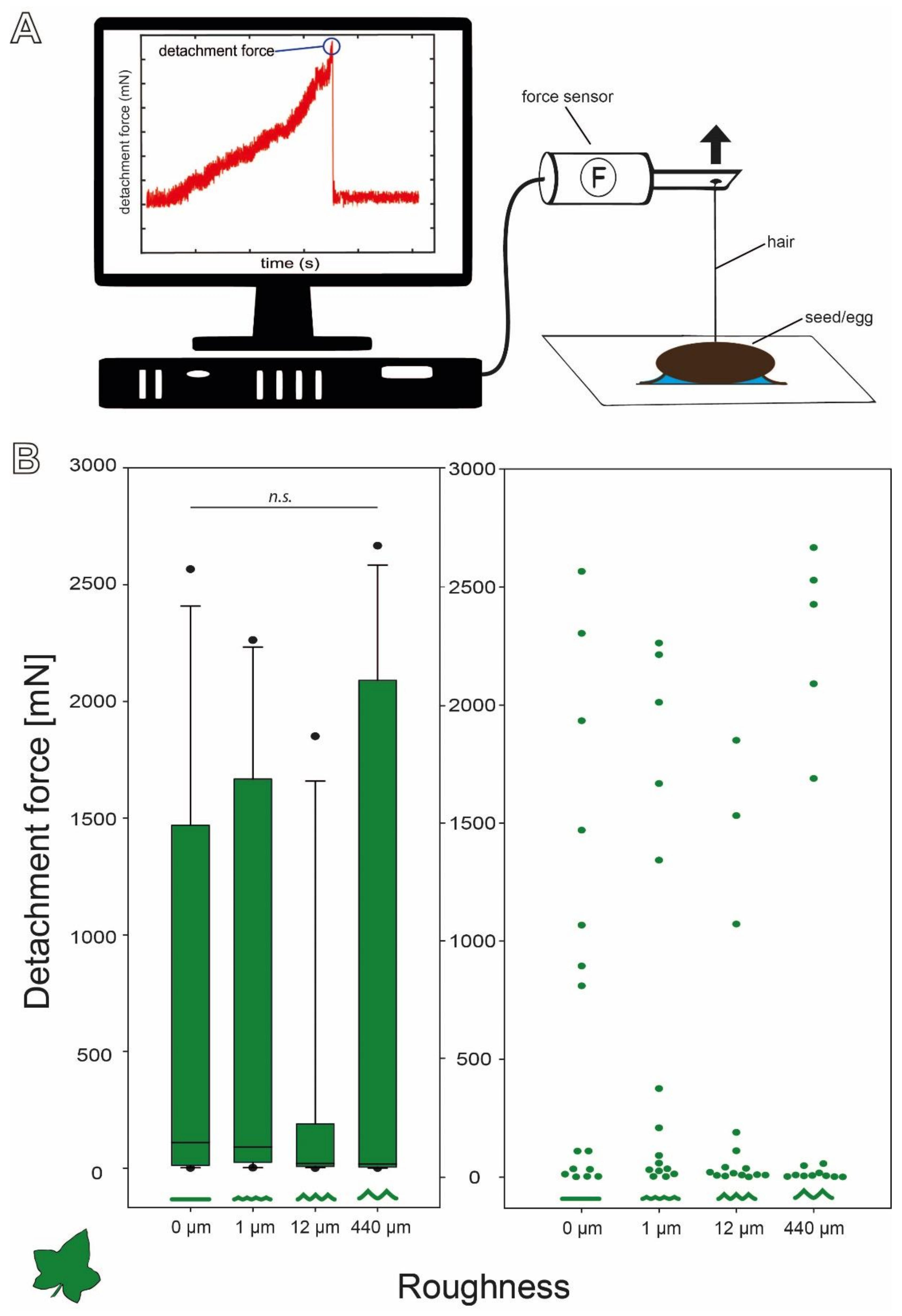
2.3. Detachment Force Measurements
- Surface roughness
- 2.
- Surface chemistry
- 3.
- Cyclic repetitions of attachment
2.4. Substrate Preparation
2.4.1. Glass
2.4.2. Epoxy Resin
2.5. Statistical Analysis
3. Results
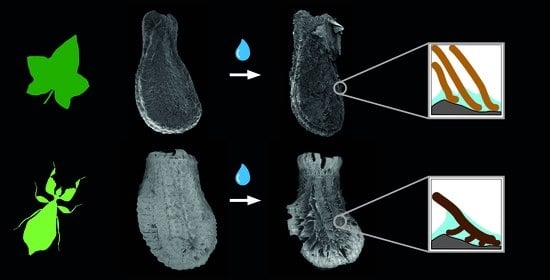
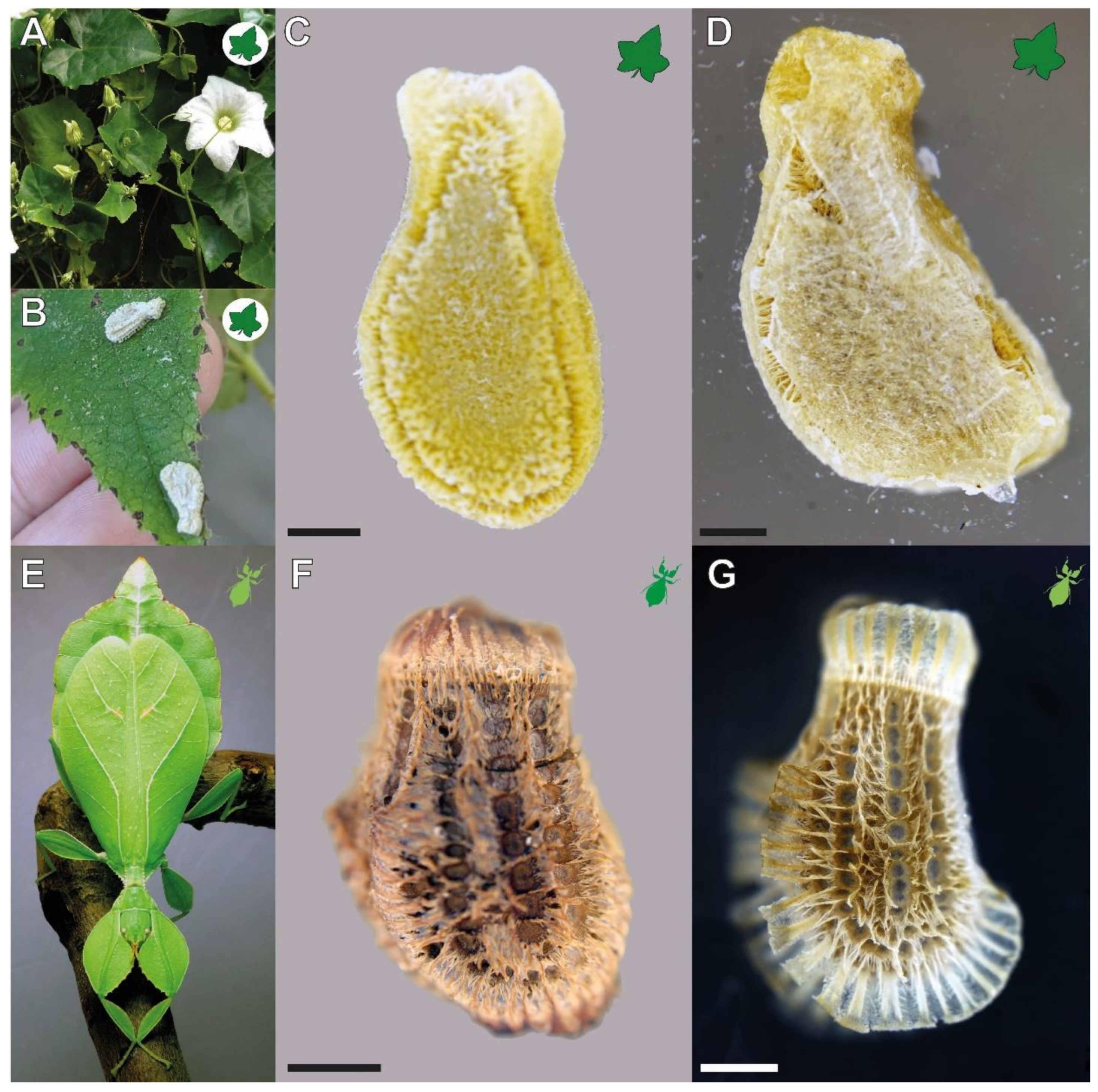
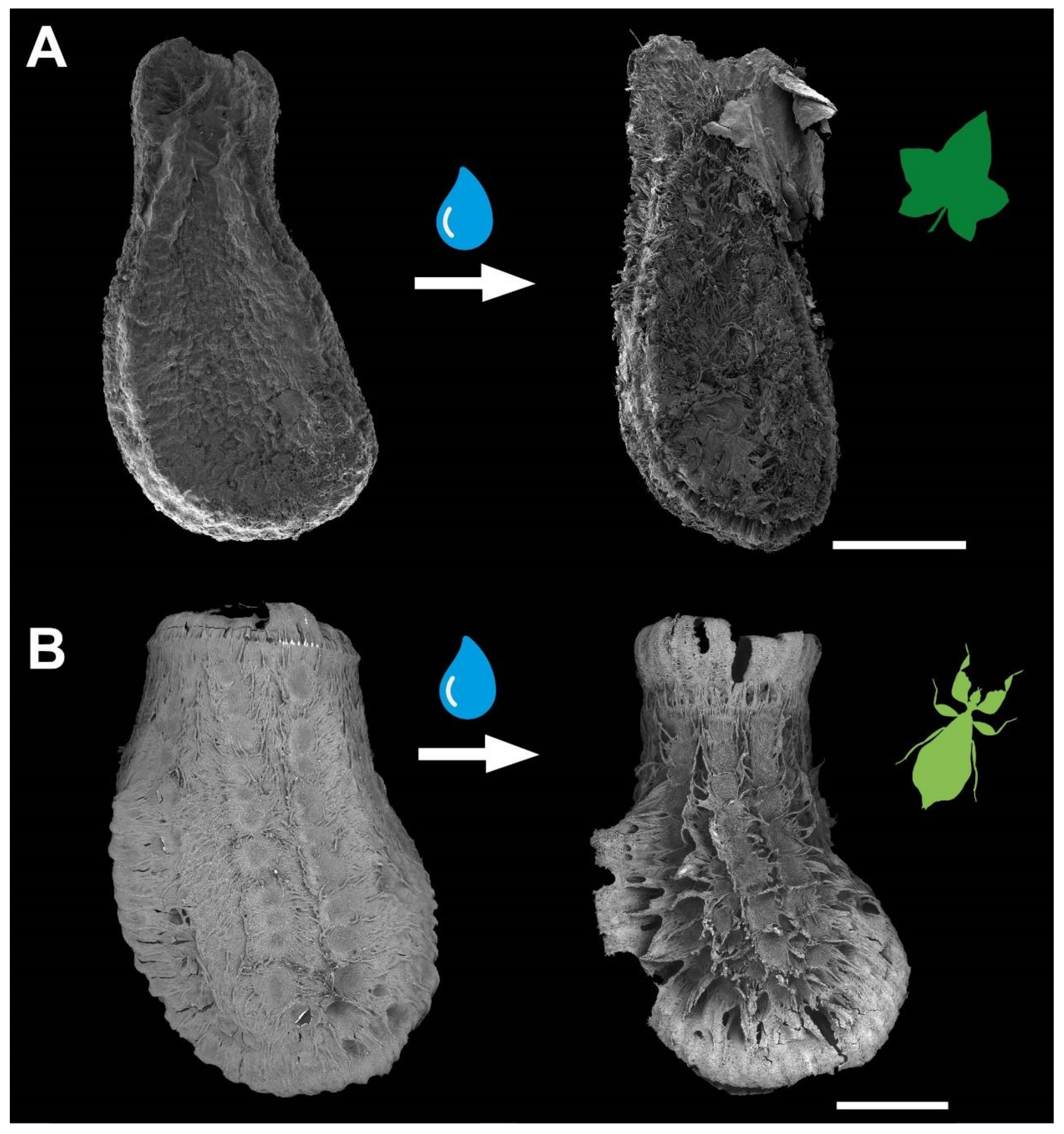
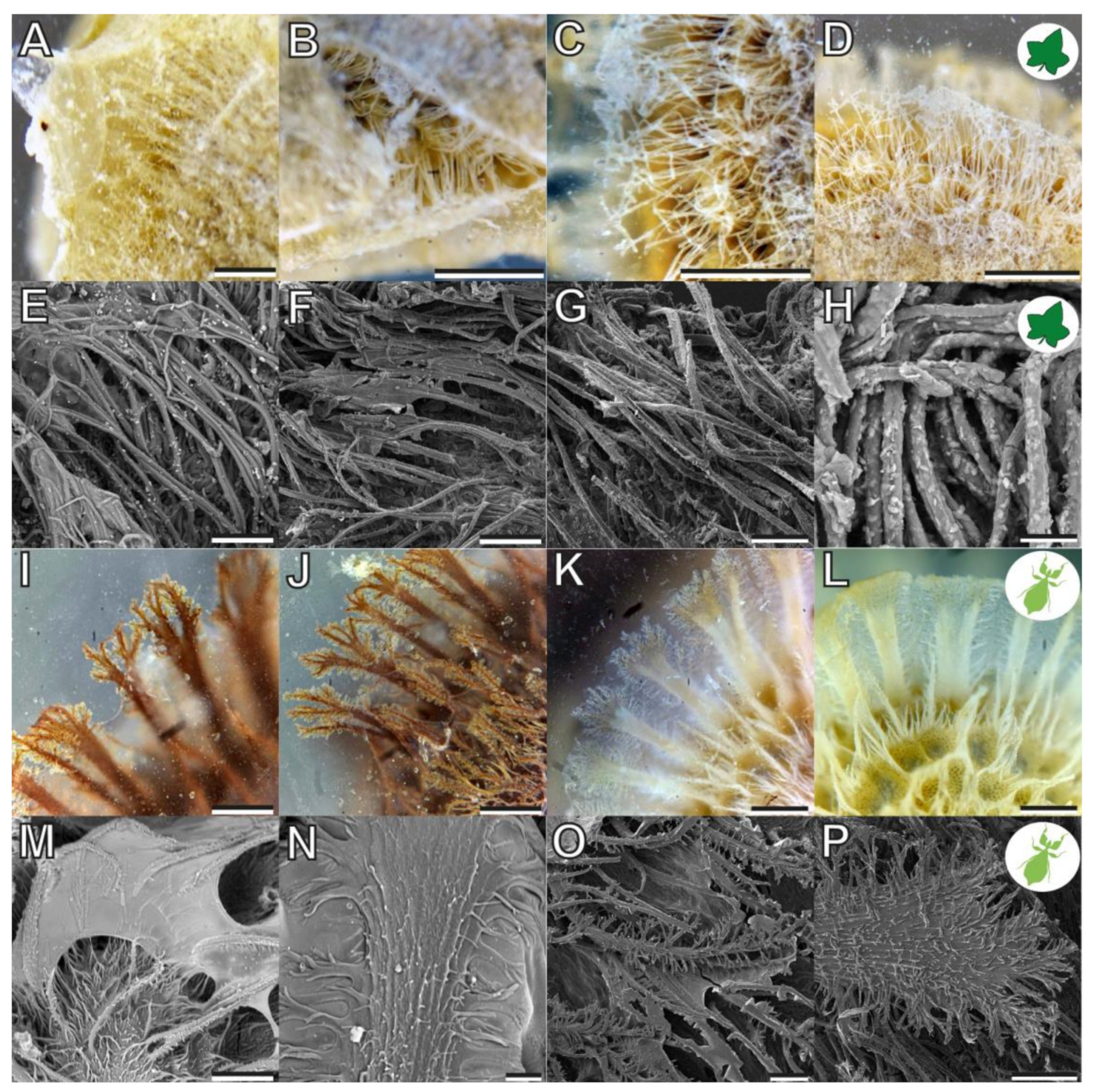
3.1. Morphology
3.2. Adhesion of C. grandis Seeds
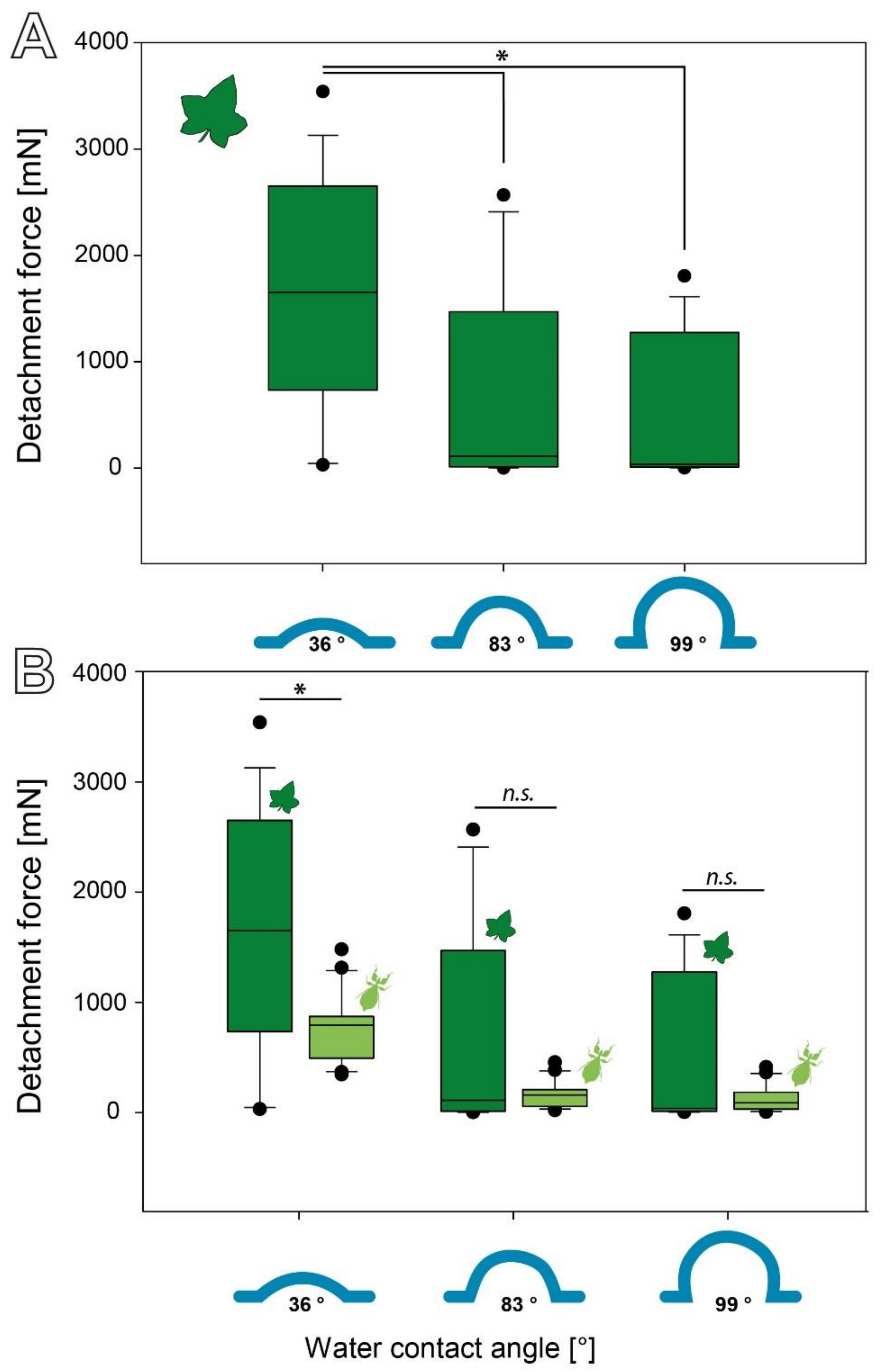
3.2.1. Influence of Substrate Roughness
3.2.2. Influence of Surface Chemistry
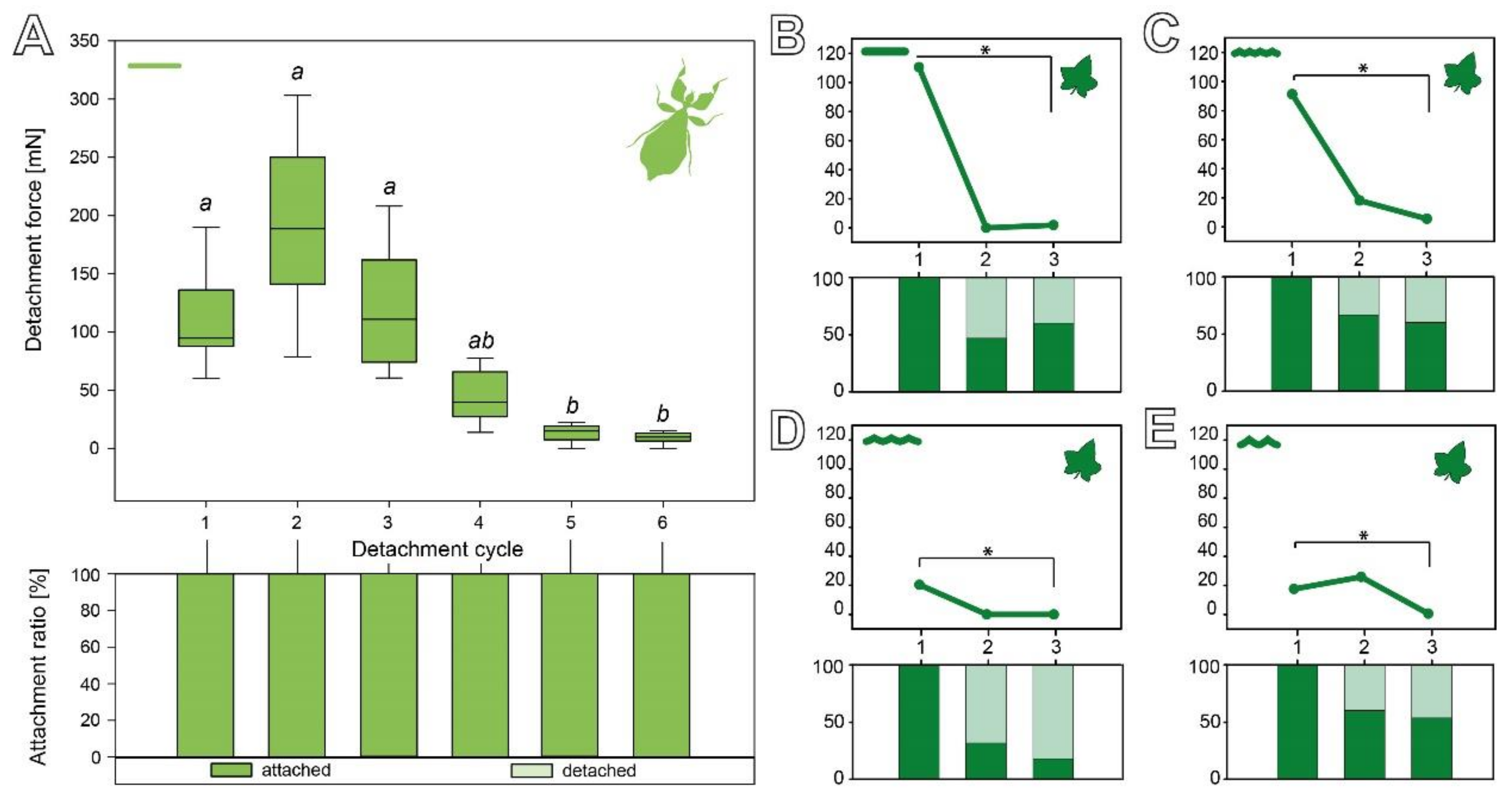
3.2.3. Cyclic Repetition
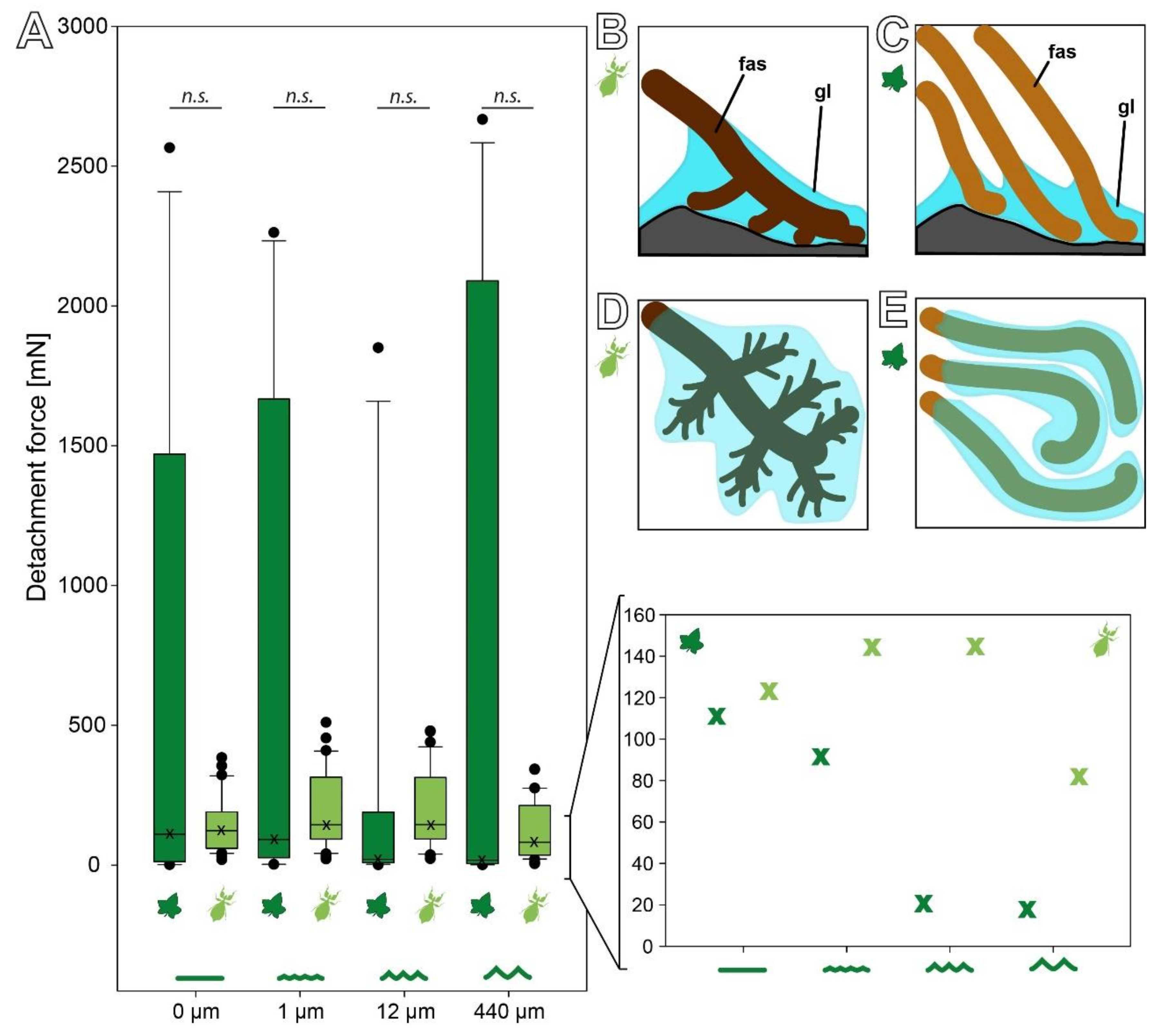
3.3. Comparison of the Attachment Capability between Eggs and Seeds
4. Discussion
4.1. Comparison between Seed and Egg Adhesive Systems
4.1.1. Morphology
4.1.2. Adhesive Performance
4.1.3. Ecological Differences
4.2. Biomimetic Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baur, F.; Gorb, S.N. How the Bee Releases Its Leg Attachment Devices. In Biona Report; Wisser, A., Nachtigall, W., Eds.; Gustav Fischer: Stuttgart, Germany, 2000; Volume 15, pp. 295–297. [Google Scholar]
- Ng, L.; Elgar, M.A.; Stuart-Fox, D. From bioinspired to bioinformed: Benefits of greater engagement from biologists. Front. Ecol. Evol. 2021, 9, e11234. [Google Scholar] [CrossRef]
- Bell, G. Selection; Oxford University Press: Oxford, UK, 2007; 699p. [Google Scholar]
- Langowski, J.K.A.; Sharma, P.; Shoushtari, A.L. In the soft grip of nature. Sci. Robot. 2020, 5, abd9120. [Google Scholar] [CrossRef] [PubMed]
- Winand, J.; Büscher, T.H.; Gorb, S.N. Learning From Nature: A Review on Biological Gripping Principles and Their Application to Robotics. In Soft Robotics; Monkman, G., Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; pp. 21–59. [Google Scholar]
- Hughes, J.; Culha, U.; Giardina, F.; Guenther, F.; Rosendo, A.; Iida, F. Soft manipulators and grippers: A review. Front. Robot. AI 2016, 3, 20. [Google Scholar] [CrossRef]
- Büscher, T.H.; Gorb, S.N. Physical constraints lead to parallel evolution of micro- and nanostructures of animal adhesive pads: A review. Beilstein J. Nanotechnol. 2021, 12, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Beutel, R.G.; Gorb, S.N. Evolution of attachment structures and phylogeny of Hexapoda (Arthropoda). Zoology 2001, 104, 68. [Google Scholar]
- Gorb, S.N. Attachment Devices of Insect Cuticle; Springer: Dordrecht, The Netherlands, 2001; 305p. [Google Scholar]
- Büscher, T.H.; Buckley, T.R.; Grohmann, C.; Gorb, S.N.; Bradler, S. The evolution of tarsal adhesive microstructures in stick and leaf insects (Phasmatodea). Front. Ecol. Evol. 2018, 6, 69. [Google Scholar] [CrossRef]
- Büscher, T.H.; Gorb, S.N. Subdivision of the neotropical Prisopodinae Brunner von Wattenwyl, 1893 based on features of tarsal attachment pads (Insecta, Phasmatodea). ZooKeys 2017, 645, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Büscher, T.H.; Grohmann, C.; Bradler, S.; Gorb, S.N. Tarsal attachment pads in Phasmatodea (Hexapoda: Insecta). Zoologica 2019, 164, 1–94. [Google Scholar]
- Büscher, T.H.; Kryuchkov, M.; Katanaev, V.L.; Gorb, S.N. Versatility of Turing patterns potentiates rapid evolution in tarsal attachment microstructures of stick and leaf insects (Phasmatodea). J. R. Soc. Interface 2018, 15, 20180281. [Google Scholar] [CrossRef] [PubMed]
- Büscher, T.; Lohar, R.; Kaul, M.-C.; Gorb, S.N. Multifunctional adhesives on the eggs of the leaf insect Phyllium philippinicum (Phasmatodea: Phylliidae): Solvent influence and biomimetic implications. Biomimetics 2020, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Büscher, T.H.; Quigley, E.; Gorb, S.N. Adhesion performance in the eggs of the Philippine leaf insect Phyllium philippinicum (Phasmatodea: Phylliidae). Insects 2020, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Tau’olunga. Coccinia Grandis Fruit. Available online: https://commons.wikimedia.org/wiki/Coccinia_grandis#/media/File:Coccinia_grandis_fruit.jpg (accessed on 15 May 2022).
- Voigt, D.; Gorb, S.N. Egg attachment of the asparagus beetle Crioceris asparagi to the crystalline waxy surface of Asparagus officinalis. Proc. R. Soc. B 2010, 277, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Kang, V.; Johnston, R.; van de Kamp, T.; Faragó, T.; Federle, W. Morphology of powerful suction organs from blepharicerid larvae living in raging torrents. BMC Zool. 2019, 4, 187. [Google Scholar] [CrossRef]
- Kang, V.; White, R.T.; Chen, S.; Federle, W. Extreme suction attachment performance from specialised insects living in mountain streams (Diptera: Blephariceridae). eLife 2021, 10, 63250. [Google Scholar] [CrossRef]
- Büscher, T.H.; Petersen, D.S.; Bijma, N.N.; Bäumler, F.; Pirk, C.W.W.; Büsse, S.; Heepe, L.; Gorb, S.N. The exceptional attachment ability of the ectoparasitic bee louse Braula coeca (Diptera, Braulidae) on the honeybee. Physiol. Entomol. 2021, 19, 170. [Google Scholar] [CrossRef]
- Beutel, R.G.; Gorb, S.N. A revised interpretation of the evolution of attachment structures in Hexapoda with special emphasis on Mantophasmatodea. Arthropod Syst. Phylogeny 2006, 64, 3–25. [Google Scholar]
- Petersen, D.S.; Kreuter, N.; Heepe, L.; Büsse, S.; Wellbrock, A.H.; Witte, K.; Gorb, S.N. Holding tight to feathers–structural specializations and attachment properties of the avian ectoparasite Crataerina pallida (Diptera, Hippoboscidae). J. Exp. Biol. 2019, 221, jeb179242. [Google Scholar]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Gorb, E.V.; Gorb, S.N. Attachment ability of the polyphagous bug Nezara viridula (Heteroptera: Pentatomidae) to different host plant surfaces. Sci. Rep. 2018, 8, 10975. [Google Scholar] [CrossRef]
- Holstein, N. Monograph of Coccinia (Cucurbitaceae). PhytoKeys 2015, 54, 1–166. [Google Scholar] [CrossRef]
- O’Hanlon, J.C.; Jones, B.R.; Bulbert, M.W. The dynamic eggs of the Phasmatodea and their apparent convergence with plants. Naturwissenschaften 2020, 107, 34. [Google Scholar] [CrossRef] [PubMed]
- Brock, P.D.; Büscher, T.H. Stick and Leaf Insects of the World; NAP Editions: Verrieres le Buisson, France, 2022. [Google Scholar]
- Hinton, H.E. Biology of Insect Eggs; Pergamon Press: Oxford, UK, 1981; Volume 1. [Google Scholar]
- Moscona, A. Studies of the egg of Bacillus libanicus (Orthoptera, Phasmidae). I. The egg envelopes. Q. J. Microsc. Sci. 1950, 91, 183–193. [Google Scholar] [PubMed]
- Van de Kamp, T.; Greven, H. Struktur des spezialisierten und unspezialisierten Chorion des Eis der Stabschr ecke Malacomorpha cyllarum (Phasmatodea). Entomol. Gen. 2008, 31, 64–74. [Google Scholar] [CrossRef]
- Mazzini, M.; Carcupino, M.; Fausto, A.M. Egg chorion architecture in stick insects (Phasmatodea). Int. J. Insect Morphol. Embryol. 1993, 22, 391–415. [Google Scholar] [CrossRef]
- Moscona, A. Studies of the egg of Bacillus libanicus (Orthoptera, Phasmidae). Q. J. Microsc. Sci. 1950, 91, 195–203. [Google Scholar]
- Jeffrey, C. Cucurbitaceae; University of Chicago Press: Chicago, IL, USA, 2000. [Google Scholar]
- Keraudren-Aymonin, M. Curcubitaceae: Flore du Cameroun, 6th ed.; Muséum National D’histoire Naturelle: Paris, France, 1967. [Google Scholar]
- De Wilde, W.J.; Duyfjes, B.E.; Phonsena, P.; van Der Ham, R.W. Miscellaneous South East Asian Cucurbit News IV. Thai For. Bull. Bot. 2014, 39, 1–22. [Google Scholar]
- Chakravorti, A.K. Cytology of Coccinia indica W. & A. with reference to the behaviour of its sex-chromosomes. Proc. Indian Acad. Sci. B 1948, 27, 74–86. [Google Scholar]
- Kreitschitz, A. Mucilage formation in selected taxa of the genus Artemisia L. (Asteraceae, Anthemideae). Seed Sci. Res. 2012, 22, 177–189. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Kovalev, A.; Gorb, S.N. Plant seed mucilage as a glue: Adhesive properties of hydrated and dried-in-contact seed mucilage of five plant species. Int. J. Mol. Sci. 2021, 22, 1443. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Kovalev, A.; Gorb, S.N. Slipping vs. sticking: Water-dependent adhesive and frictional properties of Linum usitatissimum L. seed mucilaginous envelope and its biological significance. Acta Biomater. 2015, 17, 152–159. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Kovalev, A.; Gorb, S.N. “Sticky invasion”—The physical properties of Plantago lanceolata L. seed mucilage. Beilstein J. Nanotechnol. 2016, 7, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Kendall, K.; Roberts, A.D. Surface energy and the contact of elastic solids. Proc. R. Soc. Lond. A 1971, 324, 301–313. [Google Scholar]
- Yang, X.; Baskin, J.M.; Baskin, C.C.; Huang, Z. More than just a coating: Ecological importance, taxonomic occurrence and phylogenetic relationships of seed coat mucilage. PPEES 2012, 14, 434–442. [Google Scholar] [CrossRef]
- Li, D.; Huson, M.G.; Graham, L.D. Proteinaceous adhesive secretions from insects, and in particular the egg attachment glue of Opodiphthera sp. moths. Arch. Insect Biochem. Physiol. 2008, 69, 85–105. [Google Scholar] [CrossRef]
- Beament, J.W.L.; Lal, R. Penetration through the egg-shell of Pieris brassicae (L.). Bull. Entomol. Res. 1957, 48, 109–125. [Google Scholar] [CrossRef]
- Riley, R.C.; Forgash, A.J. Drosophila melanogaster eggshell adhesive. J. Insect Physiol. 1967, 13, 509–517. [Google Scholar] [CrossRef]
- Burkhart, C.N.; Stankiewicz, B.A.; Pchalek, I.; Kruge, M.A.; Burkhart, C.G. Molecular composition of the louse sheath. J. Parasitol. 1999, 85, 559. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Y.; Jiang, Y.; Xu, M. Proteome analysis of the silkworm (Bombyx mori. L) colleterial gland during different development stages. Arch. Insect Biochem. Physiol. 2006, 61, 42–50. [Google Scholar] [CrossRef]
- Betz, O. Adhesive Exocrine Glands in Insects: Morphology, Ultrastructure, and Adhesive Secretion. In Biological Adhesive Systems—From Nature to Technical and Medical Application; von Byern, J., Grunwald, I., Eds.; Springer: Wien, Germany; New York, NY, USA, 2010; pp. 111–152. [Google Scholar]
- Burgess, I.F. Do nit removal formulations and other treatments loosen head louse eggs and nits from hair? Med. Vet. Entomol. 2010, 24, 55–61. [Google Scholar] [CrossRef]
- Graham, L.D. Biological Adhesives from Nature. In Encyclopedia of Biomaterials and Biomedical Engineering; Wnek, G.E., Bowlin, G.L., Eds.; Informa Healthcare: New York, NY, USA, 2008; pp. 236–253. [Google Scholar]
- Bedford, G.O. Biology and ecology of the Phasmatodea. Ann. Rev. Entomol. 1978, 23, 125–149. [Google Scholar] [CrossRef]
- Tihelka, E.; Cai, C.; Giacomelli, M.; Pisani, D.; Donoghue, P.C.J. Integrated phylogenomic and fossil evidence of stick and leaf insects (Phasmatodea) reveal a Permian-Triassic co-origination with insectivores. R. Soc. Open Sci. 2020, 7, 201689. [Google Scholar] [CrossRef] [PubMed]
- Bank, S.; Buckley, T.R.; Büscher, T.H.; Bresseel, J.; Constant, J.; de Haan, M.; Dittmar, D.; Dräger, H.; Kahar, R.S.; Kang, A.; et al. Reconstructing the nonadaptive radiation of an ancient lineage of ground-dwelling stick insects (Phasmatodea: Heteropterygidae). Syst. Entomol. 2021, 46, 487–507. [Google Scholar] [CrossRef]
- Bradler, S.; Buckley, T.R. Stick insect on unsafe ground: Does a fossil from the early Eocene of France really link Mesozoic taxa with the extant crown group of Phasmatodea? Syst. Entomol. 2011, 36, 218–222. [Google Scholar] [CrossRef]
- Engel, M.S.; Wang, B.; Alqarni, A.S. A thorny, ‘anareolate’ stick-insect (Phasmatidae s.l.) in Upper Cretaceous amber from Myanmar, with remarks on diversification times among Phasmatodea. Cretac. Res. 2016, 63, 45–53. [Google Scholar] [CrossRef]
- Ren, D.; Shih, C.K.; Gao, T.; Yao, Y. Rhythms of Insect Evolution: Evidence from the Jurassic and Cretaceous in Northern China; Wiley Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- Yang, H.; Shi, C.; Engel, M.S.; Zhao, Z.; Ren, D.; Gao, T. Early specializations for mimicry and defense in a Jurassic stick insect. Natl. Sci. Rev. 2021, 8, nwaa056. [Google Scholar] [CrossRef] [PubMed]
- Wedmann, S.; Bradler, S.; Rust, J. The first fossil leaf insect: 47 million years of specialized cryptic morphology and behavior. Proc. Natl. Acad. Sci. USA 2007, 104, 565–569. [Google Scholar] [CrossRef]
- Bank, S.; Cumming, R.T.; Li, Y.; Henze, K.; Le Tirant, S.; Bradler, S. A tree of leaves: Phylogeny and historical biogeography of the leaf insects (Phasmatodea: Phylliidae). Commun. Biol. 2021, 4, 932. [Google Scholar] [CrossRef]
- Cumming, R.T.; Le Tirant, S.; Teemsma, S.N.; Hennemann, F.H.; Willemse, L.; Büscher, T.H. Lost lovers linked at long last: Elusive female Nanophyllium mystery solved after a century of being placed in a different genus (Phasmatodea, Phylliidae). ZooKeys 2020, 969, 43–84. [Google Scholar] [CrossRef]
- Boisseau, R.P.; Büscher, T.H.; Klawitter, L.J.; Gorb, S.N.; Emlen, D.J.; Tobalske, B.W. Multi-modal locomotor costs favor smaller males in a sexually dimorphic leaf-mimicking insect. BMC Ecol. Evol. 2022, 22, 39. [Google Scholar] [CrossRef]
- Kimsey, L.; Dewhurst, C.; Nyaure, S. New species of egg parasites from the Oil Palm Stick Insect (Eurycantha insularis) in Papua New Guinea (Hymenoptera, Chrysididae, Phasmatodea, Phasmatidae). J. Hymenopt. Res. 2013, 30, 19–28. [Google Scholar] [CrossRef]
- Baker, E. An online taxonomic database of the stick insect (Phasmida) egg-parasitising subfamilies Amiseginae and Loboscelidiinae (Hymenoptera: Chrysididae). Biodivers. Data J. 2016, 4, e7441. [Google Scholar] [CrossRef]
- Goldberg, J.; Bresseel, J.; Constant, J.; Kneubühler, B.; Leubner, F.; Michalik, P.; Bradler, S. Extreme convergence in egg-laying strategy across insect orders. Sci. Rep. 2015, 5, 7825. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.A.; Bradler, S.; Whiting, M.F. Evolution of oviposition techniques in stick and leaf insects (Phasmatodea). Front. Ecol. Evol. 2018, 6, 125. [Google Scholar] [CrossRef]
- Hennemann, F.H.; Conle, O.V.; Gottardo, M.; Bresseel, J. On certain species of the genus Phyllium Illiger, 1798, with proposals for an intra-generic systematization and the descriptions of five new species from the Philippines and Palawan (Phasmatodea: Phylliidae: Phylliinae: Phylliini). Zootaxa 2009, 2322, 1–83. [Google Scholar] [CrossRef]
- Cumming, R.T.; Le Tirant, S.; Büscher, T.H. Resolving a century-old case of generic mistaken identity: Polyphyly of Chitoniscus sensu lato resolved with the description of the endemic New Caledonia Trolicaphyllium gen. nov. (Phasmatodea, Phylliidae). ZooKeys 2021, 1055, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Crispino, E.B.; Ghirotto, V.M.; Engelking, P.W. Contributions to the knowledge of Ceroys (Miroceroys) Piza, 1936 (Phasmatodea: Heteronemiidae): Two new mossy stick insects from the Atlantic Forest of Brazil. Zootaxa 2022, 5134, 34–60. [Google Scholar] [CrossRef] [PubMed]
- Bank, S.; Bradler, S. A second view on the evolution of flight in stick and leaf insects (Phasmatodea). BMC Ecol. Evol. 2022, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Whiting, M.F.; Bradler, S.; Maxwell, T. Loss and recovery of wings in stick insects. Nature 2003, 421, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Büscher, T.H.; Gorb, S.N. Complementary effect of attachment devices in stick insects (Phasmatodea). J. Exp. Biol. 2019, 222, jeb209833. [Google Scholar] [CrossRef]
- Büscher, T.H.; Becker, M.; Gorb, S.N. Attachment performance of stick insects (Phasmatodea) on convex substrates. J. Exp. Biol. 2020, 223, jeb226514. [Google Scholar] [CrossRef] [PubMed]
- Labonte, D.; Federle, W. Functionally different pads on the same foot allow control of attachment: Stick insects have load-sensitive “heel” pads for friction and shear-sensitive “toe” pads for adhesion. PLoS ONE 2013, 8, e81943. [Google Scholar] [CrossRef]
- Labonte, D.; Struecker, M.-Y.; Birn-Jeffery, A.; Federle, W. Shear-sensitive adhesion enables size-independent adhesive performance in stick insects. Proc. R. Soc. B 2019, 286, 20191327. [Google Scholar] [CrossRef] [PubMed]
- Pattrick, J.G.; Labonte, D.; Federle, W. Scaling of claw sharpness: Mechanical constraints reduce attachment performance in larger insects. J. Exp. Biol. 2018, 221, jeb188391. [Google Scholar] [CrossRef] [PubMed]
- Grubert, M. Studies on the distribution of myxospermy among seeds and fruits of angiospermae and its ecological importance. Acta Biol. Venez. 1974, 8, 315–551. [Google Scholar]
- Kreitschitz, A.; Valles, J. Achene morphology and slime structure in some taxa of Artemisia L. and Neopallasia L. (Asteraceae). Flora 2007, 202, 570–580. [Google Scholar] [CrossRef]
- Western, T.L. The sticky tale of seed coat mucilages: Production, genetics, and role in seed germination and dispersal. Seed Sci. Res. 2012, 22, 1–25. [Google Scholar] [CrossRef]
- Huang, Z.; Huc, Z.; Gutterman, Y. Structure and function of mucilaginous achenes of Artemisia monosperma inhabiting the Negev desert of Israel. Israel J. Plant Sci. 2000, 48, 255–266. [Google Scholar] [CrossRef]
- García-Fayos, P.; Bochet, E.; Cerdà, A. Seed removal susceptibility through soil erosion shapes vegetation composition. Plant Soil 2010, 334, 289–297. [Google Scholar] [CrossRef]
- Engelbrecht, M.; García-Fayos, P. Mucilage secretion by seeds doubles the chance to escape removal by ants. Plant Ecol. 2012, 213, 1167–1175. [Google Scholar] [CrossRef]
- Yu, L.; Yakubov, G.E.; Zeng, W.; Xing, X.; Stenson, J.; Bulone, V.; Stokes, J.R. Multi-layer mucilage of Plantago ovata seeds: Rheological differences arise from variations in arabinoxylan side chains. Carbohydr. Polym. 2017, 165, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.T. Seed Biology: Importance, Development, and Germination; Elsevier Science: Oxford, UK, 1972. [Google Scholar]
- Carlquist, S. The biota of long-distance dispersal. V. Plant dispersal to pacific islands. Bull. Torrey Bot. Club 1967, 94, 129–162. [Google Scholar] [CrossRef]
- Vazacová, K.; Münzbergová, Z. Simulation of seed digestion by birds: How does it reflect the real passage through a pigeon’s gut? Folia Geobot. 2013, 48, 257–269. [Google Scholar] [CrossRef]
- Elangovan, V.; Marimuthu, G.; Kunz, T.H. Temporal patterns of resource use by the short-nosed fruit bat, Cynopterus sphinx (Megachiroptera: Pteropodidae). J. Mammal. 2001, 82, 161–165. [Google Scholar] [CrossRef]
- Ruby, J.; Nathan, P.T.; Balasingh, J.; Kunz, T.H. Chemical composition of fruits and leaves eaten by short-nosed fruit bat, Cynopterus sphinx. J. Chem. Ecol. 2000, 26, 2825–2841. [Google Scholar] [CrossRef]
- Bhatt, D.; Kumar, A. Foraging ecology of red-vented bulbul Pycnonotus cafer in Haridwar, India. Forktail 2001, 16, 109–110. [Google Scholar]
- Voigt, J.O. Hortus Suburbanus Calcuttensis: A Catalogue of the Plants Which Have Been Cultivated in the Hon. East India Company’s Botanical Garden, Calcutta, and in the Serampore Botanical Garden, Genera; Bishop’s College Press: Calcutta, India, 1845. [Google Scholar]
- Zimmermann, A. Die Cucurbitaceen 1; Gustav Fischer: Jena, Germany, 1922. [Google Scholar]
- Mubalama, L. Population and distribution of elephants (Loxodonta africana africana) in the central sector of the Virunga National Park, eastern DRC. Pachyderm 2000, 28, 44–55. [Google Scholar]
- Kreitschitz, A.; Haase, E.; Gorb, S.N. The role of mucilage envelope in the endozoochory of selected plant taxa. Sci. Nat. 2020, 108, 2. [Google Scholar] [CrossRef]
- Suetsugu, K.; Funaki, S.; Takahashi, A.; Ito, K.; Yokoyama, T. Potential role of bird predation in the dispersal of otherwise flightless stick insects. Ecology 2018, 99, 1504–1506. [Google Scholar] [CrossRef]
- Shelomi, M. Phasmid eggs do not survive digestion by quails and chickens. J. Orthoptera Res. 2011, 20, 159–162. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Piersanti, S.; Büscher, T.H.; Gorb, E.V.; Gorb, S.N. Oviposition site selection and attachment ability of Propylea quatuordecimpunctata and Harmonia axyridis from the egg to the adult stage. Physiol. Entomol. 2021, 217, 20–37. [Google Scholar] [CrossRef]
- Al Bitar, L.; Gorb, S.N.; Zebitz, C.P.W.; Voigt, D. Egg adhesion of the codling moth Cydia pomonella L. (Lepidoptera, Tortricidae) to various substrates: I. Leaf surfaces of different apple cultivars. Arthropod-Plant Interact. 2012, 603, 471–488. [Google Scholar] [CrossRef]
- Al Bitar, L.; Gorb, S.N.; Zebitz, C.P.W.; Voigt, D. Egg adhesion of the codling moth Cydia pomonella L. (Lepidoptera, Tortricidae) to various substrates: II. Fruit surfaces of different apple cultivars. Arthropod-Plant Interact. 2014, 8, 57–77. [Google Scholar] [CrossRef]
- Cogley, T.P.; Anderson, J.R.; Weintraub, J. Ultrastructure and function of the attachment organ of warble fly eggs (Diptera: Oestridae: Hypodermatinae). Int. J. Insect Morphol. Embryol. 1981, 10, 7–18. [Google Scholar] [CrossRef]
- Gaino, E.; Rebora, M. Synthesis and function of the fibrous layers covering the eggs of Siphlonurus lacustris (Ephemeroptera, Siphlonuridae). Acta Zool. 2001, 82, 41–48. [Google Scholar] [CrossRef]
- Cogley, T.P.; Cogley, M.C. Morphology of the eggs of the human bot fly, Dermatobia hominis (L. Jr.) (Diptera: Cuterebridae) and their adherence to the transport carrier. Int. J. Insect Morphol. Embryol. 1989, 18, 239–248. [Google Scholar] [CrossRef]
- Gorb, S.N.; Gorb, E.V. Aquatic Insects as a Source for Biomimetics. In Aquatic Insects: Behavior and Ecology; Del-Claro, K., Guillermo, R., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Speck, O.; Speck, T. Functional morphology of plants—A key to biomimetic applications. New Phytol. 2021, 231, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Gorb, S.; Speck, T. Biological and biomimetic materials and surface. Beilstein J. Nanotechnol. 2017, 8, 403–407. [Google Scholar] [CrossRef]
- Masselter, T.; Scharf, U.; Speck, T. Plants and animals as concept generators for the development of biomimetic cable entry systems. J. Bionic Eng. 2008, 5, 167–173. [Google Scholar] [CrossRef]
- Schaber, C.F.; Kreitschitz, A.; Gorb, S.N. Friction-active surfaces based on free-standing anchored cellulose nanofibrils. ACS Appl. Mater. Interfaces 2018, 10, 37566–37574. [Google Scholar] [CrossRef]
- Jain, D.; Sahni, V.; Dhinojwala, A. Synthetic adhesive attachment discs inspired by spider’s pyriform silk architecture. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 553–560. [Google Scholar] [CrossRef]
- Wang, L.; Culha, U.; Iida, F. A dragline-forming mobile robot inspired by spiders. Bioinspir. Biomim. 2014, 9, 16006. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.O. Structural Effects of Glue Application in Spiders—What Can We Learn from Silk Anchors? In Bio-Inspired Structured Adhesives—Biological Prototypes, Fabrication, Tribological Properties, Contact Mechanics and Novel Concepts; Heepe, L., Xue, L., Gorb, S.N., Eds.; Springer International Publishing: Basel, Switzerland, 2017; pp. 63–80. [Google Scholar]
- Gorb, S.N. Biological attachment devices: Exploring nature’s diversity for biomimetics. Philos. Trans. R. Soc. Lond. A 2008, 366, 1557–1574. [Google Scholar] [CrossRef] [PubMed]
- Scherge, M.; Gorb, S.N. Biological Micro- and Nanotribology; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Hennemann, O.-D. Kleben von Kunststoffen. Anwendung, Ausbildung, Trend. Kunststoffe 2000, 90, 184–188. [Google Scholar]
- Lei, Y.; Guo, K.; Zhang, Y.; Zhang, X.; Qin, L.; Wang, X.; Zhu, H.; Guo, Y.; Yang, W.; Li, B.; et al. Adhesive property and mechanism of silkworm egg glue protein. Acta Biomater. 2021, 134, 499–512. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büscher, T.H.; Gorb, S.N. Convergent Evolution of Adhesive Properties in Leaf Insect Eggs and Plant Seeds: Cross-Kingdom Bioinspiration. Biomimetics 2022, 7, 173. https://doi.org/10.3390/biomimetics7040173
Büscher TH, Gorb SN. Convergent Evolution of Adhesive Properties in Leaf Insect Eggs and Plant Seeds: Cross-Kingdom Bioinspiration. Biomimetics. 2022; 7(4):173. https://doi.org/10.3390/biomimetics7040173
Chicago/Turabian StyleBüscher, Thies H., and Stanislav N. Gorb. 2022. "Convergent Evolution of Adhesive Properties in Leaf Insect Eggs and Plant Seeds: Cross-Kingdom Bioinspiration" Biomimetics 7, no. 4: 173. https://doi.org/10.3390/biomimetics7040173
APA StyleBüscher, T. H., & Gorb, S. N. (2022). Convergent Evolution of Adhesive Properties in Leaf Insect Eggs and Plant Seeds: Cross-Kingdom Bioinspiration. Biomimetics, 7(4), 173. https://doi.org/10.3390/biomimetics7040173