Evaluating the pH of Various Commercially Available Beverages in Pakistan: Impact of Highly Acidic Beverages on the Surface Hardness and Weight Loss of Human Teeth
Abstract
1. Introduction
2. Materials and Methods
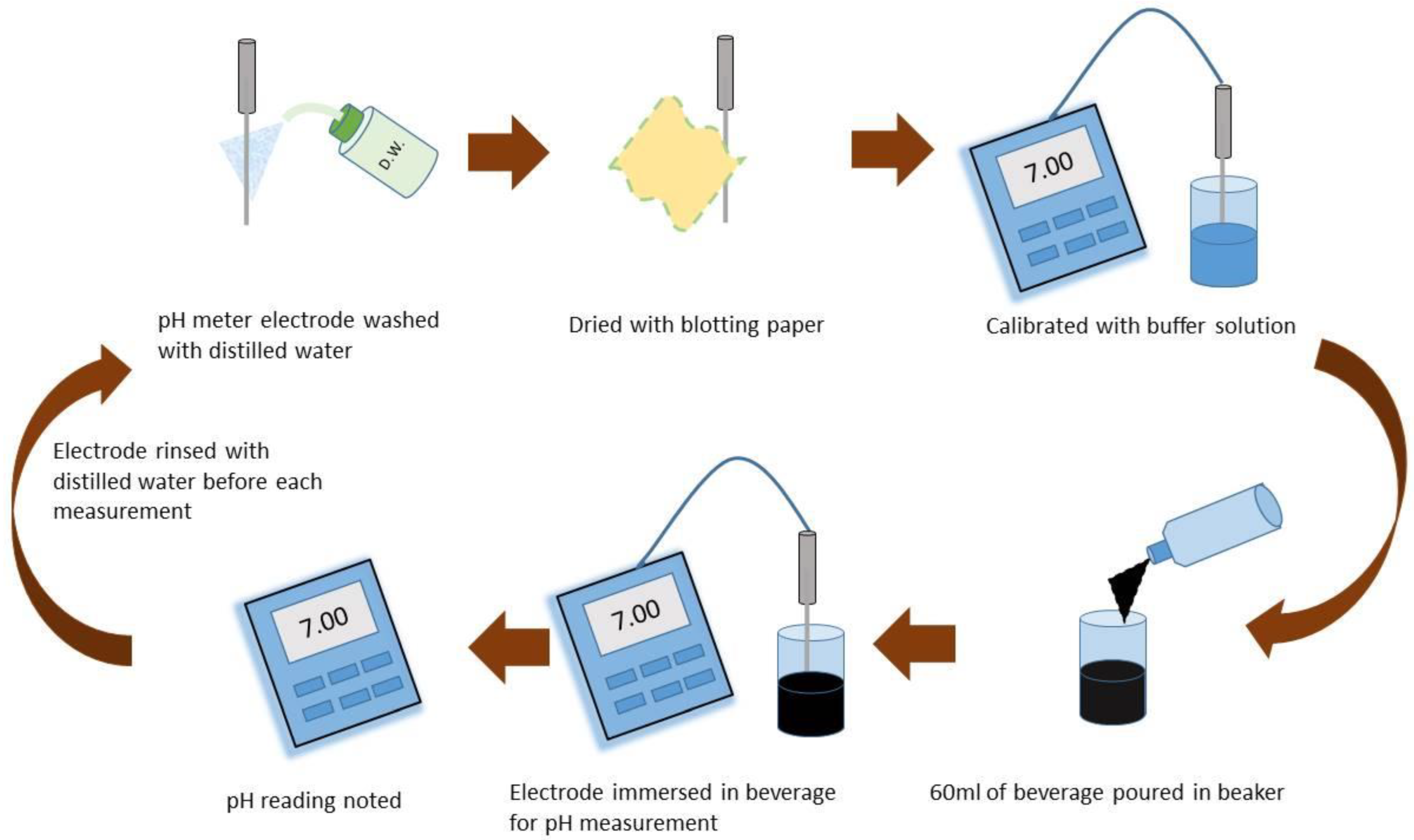
2.1. Evaluation of pH of Beverages
2.2. Specimen Preparation
2.3. Weight Analysis of Specimens
2.4. Surface Hardness Testing
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmer, S.; Kirchner, G.; Bizhang, M.; Benedix, M. Influence of various acidic beverages on tooth erosion. Evaluation by a new method. PLoS ONE. 2015, 10, e0129462. [Google Scholar] [CrossRef]
- Schlueter, N.; Luka, B. Erosive tooth wear. A review on global prevalence and on its prevalence in risk groups. Br. Dent. J. 2018, 224, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Ganss, C. Definition of erosion and links to tooth wear. In Dental Erosion; Karger Publishers: London, UK, 2006; Volume 20, pp. 9–16. [Google Scholar]
- Jensdottir, T.; Arnadottir, I.; Thorsdottir, I.; Bardow, A.; Gudmundsson, K.; Theodors, A. Relationship between dental erosion, soft drink consumption, and gastroesophageal reflux among Icelanders. Clin. Oral Investig. 2004, 8, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.R. Dental Erosion: Etiology, Diagnosis and Prevention. ADA Acad. Dent. Ther. Stomatol. 2011, 31, 75–84. [Google Scholar]
- Quartarone, E.; Mustarelli, P.; Poggio, J.C.; Lombardini, M. Surface kinetic roughening caused by dental erosion: An atomic force microscopy study. J. Appl. Phys. 2008, 103, 104702. [Google Scholar] [CrossRef]
- Ehlen, L.A.; Marshall, T.A.; Qian, F.; Wefel, J.S.; Warren, J.J. Acidic beverages increase the risk of in vitro tooth erosion. Food Nutr. Res. 2008, 28, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Hefferren, J. Why is there and should there be more attention paid to dental erosion? Compend. Contin. Educ. Dent. 2004, 25, 4–8. [Google Scholar]
- Bagde, N.I.; Tumane, P. Studies on microbial flora of fruit juices and cold drinks. Asiat. J. Biotechnol. 2011, 2, 454–460. [Google Scholar]
- De Almeida, L.d.F.D.; Abílio, G.M.F.; Cavalcante, M.T.; Castro, R.D.; Cavalcanti, A.L. Cariogenic and erosive potential of industrialized fruit juices available in Brazil. Braz. J. Oral Sci. 2017, 9, 351–357. [Google Scholar]
- Shellis, R.P.; Addy, M. Interaction between attrition, abrasion and erosion in tooth wear. Monogr. Oral Sci. 2014, 25, 32–45. [Google Scholar]
- Larsen, M.J.; Richards, A. Flouride is unable to reduce dental erosion from soft drinks. Caries Res. 2002, 36, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Cairns, A.M.; Watson, M.; Creanor, S.L.; Foye, R.H. The pH and titratable acidity of a range of diluting drinks and their potential effect on Dental Erosion. J. Dent. 2002, 30, 313–317. [Google Scholar] [CrossRef]
- Joshi, M.; Joshi, N.; Kathariya, R.; Angadi, P.; Raikar, S. Technique to Evaluate Dental Erosion: A systematic Review of Literature. J. Clin. Diagn. Res. 2016, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Chang, H.H.; Chang, Y.C.; Lu, Y.C. Effects of fluoride and epigallocatechin gallate on soft-drink-induced dental erosion of enamel and root dentin. J. Formos. Med. Assoc. 2018, 117, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Chang, C.C.; Chi, C.W.; Chang, H.H.; Chiang, Y.C.; Chuang, Y.C.; Chang, H.H.; Huang, G.F.; Liao, Y.S.; Lin, C.P. Erosive potential of soft drinks on human enamel: An in vitro study. J. Formos. Med. Assoc. 2014, 113, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.; Casamassimo, P.S.; Hayes, J.R. Relationship between sports drinks and dental erosion in 304 university athletes in Columbus, Ohio, USA. Caries Res. 2002, 36, 281–287. [Google Scholar] [CrossRef]
- Harding, M.; Whelton, H.; O’ Mullane, D.; Cronin, M. Dental erosion in 5-year-old Irish school children and associated factors: A pilot study. Community Dent. Health 2003, 20, 165–170. [Google Scholar]
- Coombes, J.S. Sports drinks and dental erosion. Am. J. Dent. 2005, 18, 101–104. [Google Scholar]
- Bello, L.; Al-Hammad, N. Pattern of fluid consumption in a sample of Saudi Arabian adolescents aged 12–13 years. Int. J. Paediatr. Dent. 2006, 16, 168–173. [Google Scholar] [CrossRef]
- Seow, W.; Thong, K. Erosive effects of common beverages on extracted premolar teeth. Aust. Dent. J. 2005, 50, 173–178. [Google Scholar] [CrossRef]
- Sirimaharaj, V.; Messer, L.B.; Morgan, M. Acidic diet and dental erosion among athletes. Aust. Dent. J. 2002, 47, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Dugmore, C.R.; Rock, W.P. The prevalence of tooth erosion in 12-year-old children. Br. Dent. J. 2004, 196, 279–282. [Google Scholar] [CrossRef] [PubMed]
- El Aidi, H.; Bronkhorst, E.M.; Truin, G.J. A longitudinal study of tooth erosion in adolescents. Dent. Res. 2008, 87, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, U.; Quadir, F.; Hosein, T. Determination of Prevalence of Dental Erosion in 12–14 Years School Children and Its Relationship with Dietary Habits. J. Coll. Physicians Surg. 2016, 26, 553–556. [Google Scholar]
- Khan, M.S.; Nisar, N.; Naqvi, S.A.A.; Nawab, F. Caffeine Consumption and Academic Performance among Medical Students of Dow University of Health Sciences (DUHS), Karachi, Pakistan. Annals 2017, 22, 81–87. [Google Scholar]
- Tschammler, C.; Simon, A.; Brockmann, K.; Röbl, M.; Wiegand, A. Erosive tooth wear and caries experience in children and adolescents with obesity. J. Dent. 2019, 83, 77–86. [Google Scholar] [CrossRef]
- Larsen, M.J.; Nyvad, B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999, 33, 81–87. [Google Scholar] [CrossRef]
- Amaya-Pajares, S.P.; Koi, K.; Watanabe, H.; da Costa, J.B.; Ferracane, J.L. Development and maintenance of surface gloss of dental composites after polishing and brushing: Review of the literature. J. Esthet. Restor. Dent. 2022, 34, 15–41. [Google Scholar] [CrossRef]
- Zipperian, D.C. Metallographic Specimen Preparation Basics. Available online: www.metallographic.com (accessed on 16 August 2021).
- Haq, M.W.; Batool, M.; Ahsan, S.H.; Lone, M.A.; Islam, T. Dental Erosion; Influencing Factors & pH Analysis. Can. J. Appl. Sci. 2012, 2, 222–232. [Google Scholar]
- Kazmi, S.; Mughal, A.; Habib, M.; Ayaz, M.; Tariq, H.; Khan, A. Effects on the enamel due to the carbonated drinks-a SEM study. Pak. Oral Dent. J. 2016, 36, 221–225. [Google Scholar]
- Cochrane, N.; Yuan, Y.; Walker, G.; Shen, P.; Chang, C.; Reynolds, C. Erosive potential of sports beverages. Aust. Dent. J. 2012, 57, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Jensdottir, T.; Holbrook, P.; Nauntofte, B.; Buchwald, C.; Bardow, A. Immediate erosive potential of cola drinks and orange juices. J. Dent. Res. 2006, 85, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Norris, D.F.; Momeni, S.S.; Waldo, B.; Ruby, J.D. The pH of beverages in the United States. J. Am. Dent. Assoc. 2013, 147, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.T.; Carvalho, J.C.; Zero, D.T. Causes of dental erosion: Extrinsic factors. In Dental Erosion and Its Clinical Management; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 69–96. [Google Scholar]
- Wang, X.; Lussi, A. Assessment and management of dental erosion. Dent. Clin. N. Am. 2010, 54, 565–578. [Google Scholar] [CrossRef]
- Zero, D.T.; Lussi, A. Erosion—Chemical and biological factors of importance to the dental practitioner. Int. Dent. J. 2005, 55, 285–290. [Google Scholar] [CrossRef]
- Moynihan, P.J. Dietary advice in dental practice. Br. Dent. J. 2002, 193, 563–568. [Google Scholar] [CrossRef]
- Lussi, A.; Jaeggi, T. Etiology and risk assessment. In Dental Erosion: Diagnosis, Risk Assessment, Prevention, Treatment; Lussi, A., Jaeggi, T., Eds.; Quintessence: London, UK, 2011; pp. 37–53. [Google Scholar]
- Ganess, C.; Klimek, J.; Schwartz, N. A comparative profilometric in vitro study of the susceptibility of polished and natural human enamel and dentine surface to erosive demineralization. Arch. Oral Biol. 2000, 45, 897–902. [Google Scholar] [CrossRef]
- Jeong, M.J.; Jeong, S.J.; Son, J.H.; Chung, S.K.; Kim, A.; Kang, E.J.; Kim, E.J.; Kim, H.I.; Jang, K.E.; Cho, M.H.; et al. A study on the enamel erosion caused by energy drinks. J. Dent. Hyg. 2014, 14, 597–609. [Google Scholar] [CrossRef][Green Version]
- Sauro, S.; Mannocci, F.; Piemontese, M.; Mongiorgi, R. In situ enamel morphology evaluation after acidic soft drink consumption: Protection factor of contemporary toothpaste. Int. J. Dent. Hyg. 2008, 6, 188–192. [Google Scholar] [CrossRef]
- Von Fraunhofer, J.A.; Rogers, M.M. Dissolution of dental enamel in soft drinks. Gen. Dent. 2004, 52, 308–312. [Google Scholar]
- Mathew, S.; Luke, A.M.; Walia, T.; Masri, A.G.; Jamal, H.; Pawar, A.M. Effect of fruit juices and other beverages on loss of tooth structure. Pesq. Bras. Odontoped. Clin. Integr. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Bitri, E.; Petcu, L.; Mocanu, G.; Balaban, D.P. In vitro assessment of erosive effects of some common soft drinks on dental hard tissues. Balk. J. Dent. Med. 2019, 23, 132–140. [Google Scholar] [CrossRef]


| S.No. | Sports Drinks/Energy Drinks | pH (Standard Deviation) | Batch No. |
|---|---|---|---|
| Erosive | |||
| 1 | Holsten Black Grapes Flavor | 3.04 (0.00) | 132A13 |
| 2 | Bavaria Holland Peach | 3.18 (0.00) | CLFB40612R |
| 3 | Bavaria Holland Pomegranate | 3.22 (0.00) | CLFB40612R |
| 4 | Bavaria Holland Mango Passion | 3.25 (0.00) | CLFB40612R |
| 5 | Bavaria Holland Strawberry | 3.30 (0.00) | CLFB 406 12R |
| 6 | Bavaria Holland Apple | 3.31 (0.00) | CLFB 406 12R |
| 7 | Walkers’ Ginger Beverage | 3.33 (0.00) | ** |
| 8 | Red Bull | 3.65 (0.00) | 8L01B07C |
| 9 | Activade Grapes | 3.83 (0.00) | ACT34 |
| 10 | Gatrorade Blue Bolt | 3.98 (0.01) | 14:20 P 071120 |
| Minimally Erosive | |||
| 11 | ** Malt Beverage Barbican | 4.01(0.00) | ** |
| 12 | Activade Berry Blue | 4.06 (0.00) | ACT32 |
| 13 | Activade Lemon Lime | 4.07 (0.00) | ACT31 |
| 14 | Gatrorade Tropical Fruit | 4.09 (0.00) | |
| 15 | Activade Orange | 4.14 (0.00) | |
| 16 | Gatrorade White Lightning | 4.15 (0.00) | B 13:16 P 081020 |
| 17 | Activade Fruit Punch | 4.15 (0.00) | ACT33 |
| 18 | Sting Energy Berry Blast | 4.17 (0.00) | P281120G58 |
| 19 | Sting Gold Rush | 4.20 (0.00) | P101120638 |
| 20 | **Malt 79 Murree Brewery | 4.42 (0.00) | 33H1 09:59 |
| 21 | Three Horses | 4.58 (0.00) | MRP17202 |
| pH of Waters | |||
|---|---|---|---|
| Waters | pH (Standard Deviation) | Batch No. | |
| Non Erosive | |||
| 22 | Aquafina | 7.0 (0.00) | 15:46 |
| 23 | Masafi Water | 7.1 (0.00) | ** |
| 24 | Mai Dubai | 7.33 (0.00) | ** |
| 25 | Nestle Pure Life Active Water | 7.58 (0.00) | 010215801A |
| 26 | Nestle Pure Life | 7.63 (0.00) | 028330621D |
| pH of Fruit Juices and Drinks | |||
|---|---|---|---|
| Fruit Juices/Drinks | pH (Standard Deviation) | Batch No. | |
| Erosive | |||
| 27 | Tang Mosambi | 3.15 (0.01) | OTG 6301341 |
| 28 | Tang Orange Flavor | 3.16 (0.01) | |
| 29 | Tang Lemon and Pepper | 3.19 (0.00) | |
| 30 | Smart Choice Pineapple with Pulp | 3.49 (0.00) | ** |
| 31 | Lemonade (Active Foods) Mint Lemonade | 3.52 (0.00) | LIM 33 |
| 32 | Limonade (Active Foods) | 3.56 (0.00) | LIM02 |
| 33 | Smart Choice Peach with Pulp | 3.66 (0.00) | ** |
| 34 | Smart Choice Apple with Pulp Drink | 3.70 (0.00) | ** |
| 35 | Fruiti-O Guava Nectar | 3.91 (0.00) | 21004376L2 |
| 36 | Smile Lychee Flavor | 3.94 (0.00) | 21091291 L2 |
| 37 | Must Mango Fruit Drink | 3.96 (0.00) | 1008(16:02:07) |
| 38 | Fruiti-O Peach Fruit Drink | 4.00 (0.00) | 20031102009735368001 L2 |
| Minimally Erosive | |||
| 39 | Smile Apple | 4.04 (0.00) | ** |
| 40 | Smart Choice Red Grape | 4.12 (0.00) | ** |
| 41 | Fruitien Red Grapes Fruit Drink | 4.18 (0.00) | 20075:6L3E |
| 42 | Hemani Peach Drink with Basil Seeds | 4.20 (0.00) | EX007R2309 |
| 43 | Nestle Fruita Vitals Red Grapes | 4.24 (0.00) | 028015801L |
| 44 | Slice Mango Fruit Drink | 4.24 (0.00) | PX12B21:53 |
| 45 | Fruitien Pomegranate Nectar | 4.29 (0.00) | 0024826L |
| 46 | Nestle Fruita Vitals Pineapple | 4.50 (0.00) | 2.5E + 08 |
| 47 | Fruitien Joy Mango Fruit Drink | 4.52 (0.00) | 202892132L4 |
| 48 | Fruitien Pineapple Nectar | 4.54 (0.00) | ** |
| 49 | Hemani Cocktail Drink with Basil Seeds | 4.57 (0.00) | EX007R0103 |
| 50 | Nestle Fruita Vitals Peach Fruit Drink | 4.60 (0.00) | 018215801Z |
| 51 | Hemani Lychee Drink with Basil Seeds | 4.63 (0.00) | EX007R1202 |
| 52 | Anytime Green Apple Fruit Nectar | 4.66 (0.00) | 124(05:4248) |
| 53 | Hemani White Grapes with Basil Seeds | 4.77 (0.00) | ** |
| 54 | Nestle Fruita Vitals Apple Nectar | 4.82 (0.00) | 0309158010(13:59) |
| 55 | Nestle Fruita Vitals Kinnow Nectar | 4.95(0.00) | 031715803H(05:21) |
| 56 | Fruitien Chaunsa Mango Nectar | 5.02 (0.00) | 20276411L3E |
| 57 | Nestle Fruita Vitals Chaunsa Mango Nectar | 5.03 (0.00) | 031515802G |
| 58 | Nestle Fruita Vitals Guava Nectar | 5.10 (0.00) | 2.5E + 08 |
| 59 | Nestle Fruita Vitals Royal Mangoes | 5.22 (0.00) | 3.1E + 08 |
| pH of Sodas | |||
|---|---|---|---|
| Soda | pH (Standard Deviation) | Batch No. | |
| Erosive | |||
| 60 | Pepsi | 3.40 (0.00) | P301120638 04:17 |
| 61 | Forest Club Soda | 3.47 (0.00) | ** |
| 62 | Pepsi Diet | 3.52 (0.00) | P061120GA 03:46 |
| 63 | Coca Cola Original Taste | 3.54 (0.00) | 1319L3 |
| 64 | Pakola (Lychee) | 3.55 (0.00) | ** |
| 65 | Pakola Lemon Lime | 3.61 (0.00) | 22106 |
| 66 | Pepsi Cola | 3.62 (0.00) | |
| 67 | Apple Sidra | 3.73 (0.00) | |
| 68 | Mirinda Orange Flavor | 3.78 (0.00) | P241120638 |
| 69 | Fanta Orange Flavor | 3.84 (0.00) | 0205M6PP36 |
| 70 | Mountain Dew | 3.87 (0.00) | P281020058 |
| 71 | Vimto Sparkling | 3.91 (0.01) | 661JLY21 |
| 72 | 7Up | 3.95 (0.00) | |
| 73 | Sprite | 3.96 (0.00) | |
| Minimally Erosive | |||
| 74 | 7Up Free | 4.06 (0.00) | P26102003A |
| 75 | Pakola Cream Soda | 4.59 (0.00) | 21APR217UMD |
| pH of Teas and Coffees | |||
|---|---|---|---|
| Teas and Coffees | pH (Standard Deviation) | Batch No. | |
| Minimally Erosive | |||
| 76 | Lipton Tea Yellow Label | 5.08 (0.01) | 5 |
| 77 | Tea Supreme | 5.11 (0.02) | 5 |
| 78 | Tapal Family Mixture | 5.31 (0.00) | 509234 |
| 79 | Tapal Danedar | 5.46 (0.00) | 513252 |
| 80 | Green Tea Lipton | 6.65 (0.01) | |
| 81 | Nescafe Chilled Latte | 6.70 (0.00) | 3.1E + 08 |
| 82 | Nescafe Coffee | 6.73 (0.01) | |
| 83 | Nescafe Chilled Mocha | 6.88 (0.00) | 029715801d |
| pH of Milks and Flavored Milks | |||
|---|---|---|---|
| Milks | pH (Standard Deviation) | Batch No. | |
| Minimally Erosive | |||
| 84 | Go Fresh Coconut Milk Drink plus Coconut Water with Nata De CoCo Rose Flavour | 6.27 (0.00) | RA189 2205J1626 |
| 85 | Go Fresh Coconut Milk Drink plus Coconut Water with Melon Flavor | 6.29(0.00) | RA189 2205J1809 |
| 86 | Day Fresh Milk Full Cream | 6.51 (0.00) | 0333P1B7 |
| 87 | Nurpur Full Cream Milk | 6.52 (0.00) | |
| 88 | Day Fresh Flavored Milk Banana | 6.53 (0.00) | 0186B1C4 |
| 89 | Day Fresh Flavored Milk Strawberry | 6.58 (0.00) | 0294S1B6 |
| 90 | Olpers Full Cream Milk | 6.60 (0.00) | |
| 91 | Oolala Flavored Milk Strawberry Shakarganj | 6.61 (0.00) | 13B(13:36:12) |
| 92 | Olpers Chocolate Flavored Milk | 6.65 (0.00) | 20(13:38:59) |
| 93 | Tarang (liquid tea whitener) | 6.65 (0.00) | 173(04:28:38) |
| 94 | Nestle Milk Pak Full Cream | 6.69 (0.00) | 032315801C |
| 95 | Day Fresh Flavored Milk Mango | 6.70 (0.00) | 0256M1C7 |
| 96 | Oolala Flavored Milk Badam and Zaffran | 6.71(0.00) | 125LFM(05:35:33) |
| 97 | Olpers Pro Cal Low Fat Milk | 6.72 (0.00) | D0233(19:26:43) |
| 98 | Day Fresh Flavored Milk Pista Zaffran | 6.73(0.00) | 0292Z1b7 |
| 99 | Nestle Nesvita | 6.77 (0.00) | 030915801U |
| 100 | Nurpur Flavored Milk Badam and Zaffran | 6.84 (0.00) | 32(06:41:56) |
| 101 | Pakola Chocolate Flavored Milk | 6.90 (0.00) | GS 12:44 B05 |
| 102 | Go Fresh Coconut Milk Drink with Chocolate | 6.93 (0.00) | |
| 103 | Pakola Flavored Milk Strawberry | 6.94 (0.00) | ** |
| 104 | Oolala Chocolate Flavored Milk Shakarganj | 6.97 (0.00) | 011(12:29:05) |
| Non Erosive | |||
| 105 | Pakola Double Delight Strawberry plus Vanilla | 7.07 (0.00) | ** |
| 106 | Milo Nestle | 7.87 (0.00) | 028515801U |
| Beverage Type | Red Bull | Pepsi | Apple Cidra | Tang Mosambi | Tang Orange |
|---|---|---|---|---|---|
| Pre-immersion weight (grams) | 0.18 (±0.00) | 0.08 (±0.00) | 0.10 (±0.00) | 0.12 (±0.00) | 0.16 (±0.01) |
| Post-immersion Weight (grams) | 0.11 (±0.00) | 0.04 (±0.00) | 0.05 (±0.00) | 0.07 (±0.00) | 0.11 (±0.00) |
| Weight Reduction in Specimens After Immersion (%) | 38.89 | 50.00 | 50.00 | 41.67 | 31.25 |
| p Value | p = 0.000 | p = 0.001 | p = 0.000 | p = 0.000 | p = 0.003 |
| Beverages Type | Red Bull | Pepsi | Apple Cidra | Tang Mosambi | Tang Orange |
|---|---|---|---|---|---|
| Pre-immersion Hardness (VHN) | 598 (±50) | 553 (±13) | 686 (±9) | 669 (±17) | 654 (±40) |
| Post-immersion Hardness (VHN) | 473 (±20) | 457 (±16) | 606 (±21) | 566 (±21) | 347 (±46) |
| Surface Hardness Reduction in Specimens After Immersion (%) | 21 | 17 | 12 | 15 | 47 |
| p Value | p = 0.001 | p = 0.001 | p = 0.003 | p = 0.003 | p = 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.; Amin, F.; Hashem, D.; Khan, S.; Zaidi, H.; Rahman, S.; Farhan, T.; Mahmood, S.J.; Asghar, M.A.; Zafar, M.S. Evaluating the pH of Various Commercially Available Beverages in Pakistan: Impact of Highly Acidic Beverages on the Surface Hardness and Weight Loss of Human Teeth. Biomimetics 2022, 7, 102. https://doi.org/10.3390/biomimetics7030102
Kumar N, Amin F, Hashem D, Khan S, Zaidi H, Rahman S, Farhan T, Mahmood SJ, Asghar MA, Zafar MS. Evaluating the pH of Various Commercially Available Beverages in Pakistan: Impact of Highly Acidic Beverages on the Surface Hardness and Weight Loss of Human Teeth. Biomimetics. 2022; 7(3):102. https://doi.org/10.3390/biomimetics7030102
Chicago/Turabian StyleKumar, Naresh, Faiza Amin, Danya Hashem, Sara Khan, Huma Zaidi, Sehrish Rahman, Tooba Farhan, Syed Junaid Mahmood, Muhammad Asif Asghar, and Muhammad Sohail Zafar. 2022. "Evaluating the pH of Various Commercially Available Beverages in Pakistan: Impact of Highly Acidic Beverages on the Surface Hardness and Weight Loss of Human Teeth" Biomimetics 7, no. 3: 102. https://doi.org/10.3390/biomimetics7030102
APA StyleKumar, N., Amin, F., Hashem, D., Khan, S., Zaidi, H., Rahman, S., Farhan, T., Mahmood, S. J., Asghar, M. A., & Zafar, M. S. (2022). Evaluating the pH of Various Commercially Available Beverages in Pakistan: Impact of Highly Acidic Beverages on the Surface Hardness and Weight Loss of Human Teeth. Biomimetics, 7(3), 102. https://doi.org/10.3390/biomimetics7030102








