The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay
Abstract
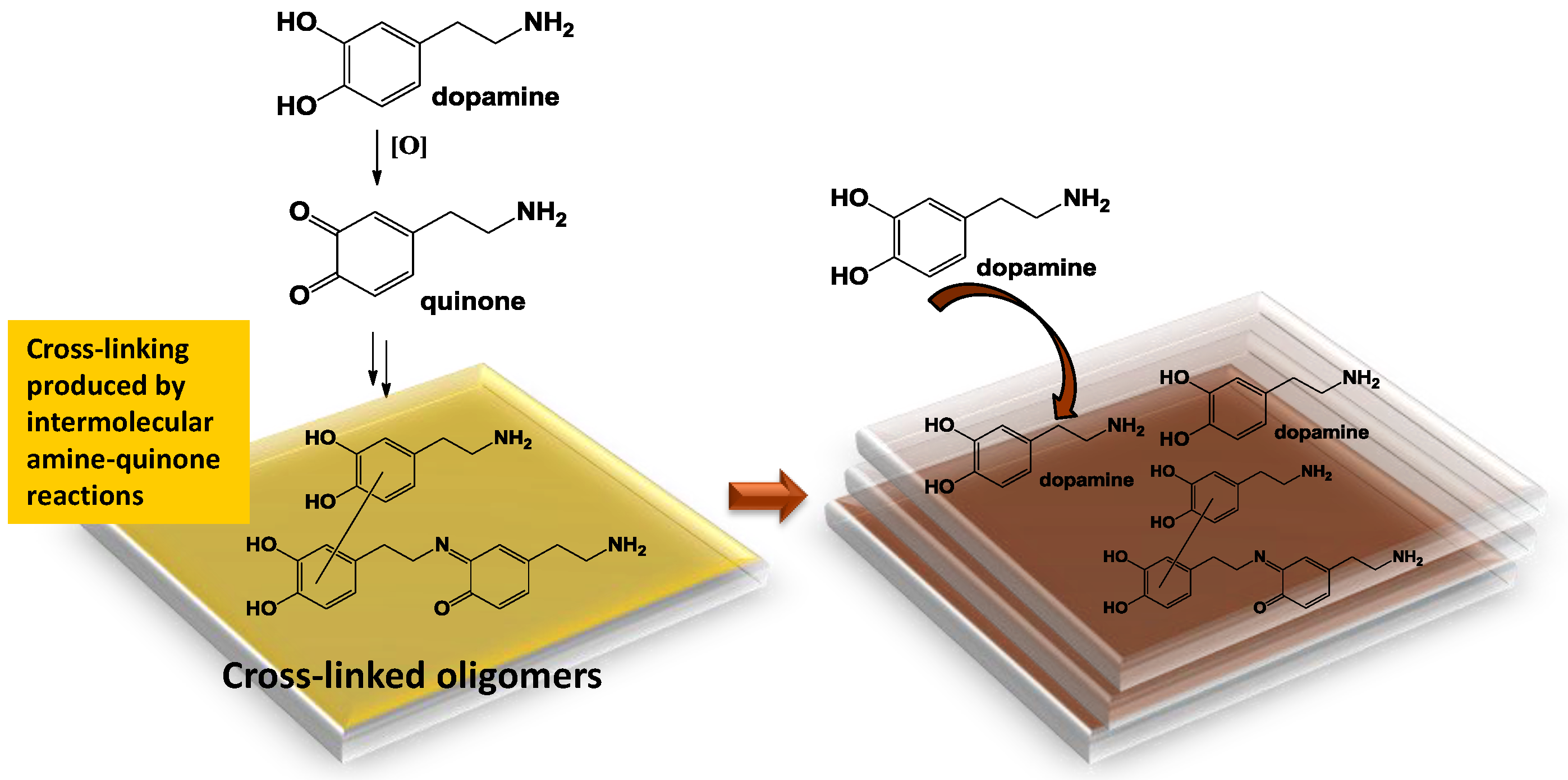
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PDA and General Procedure for Substrate Coating
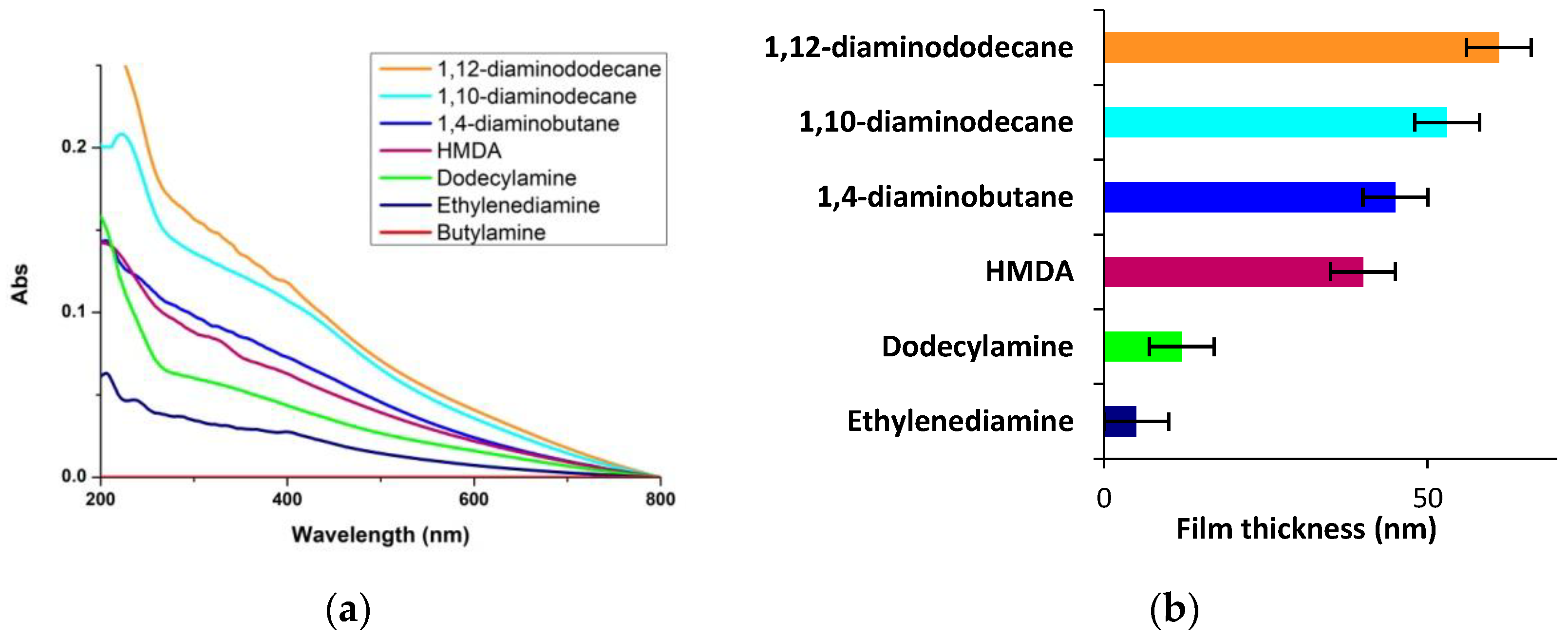
2.3. Synthesis of PDA-Amines Films
2.4. Synthesis of PDA-Periodate Films
2.5. Film Growth
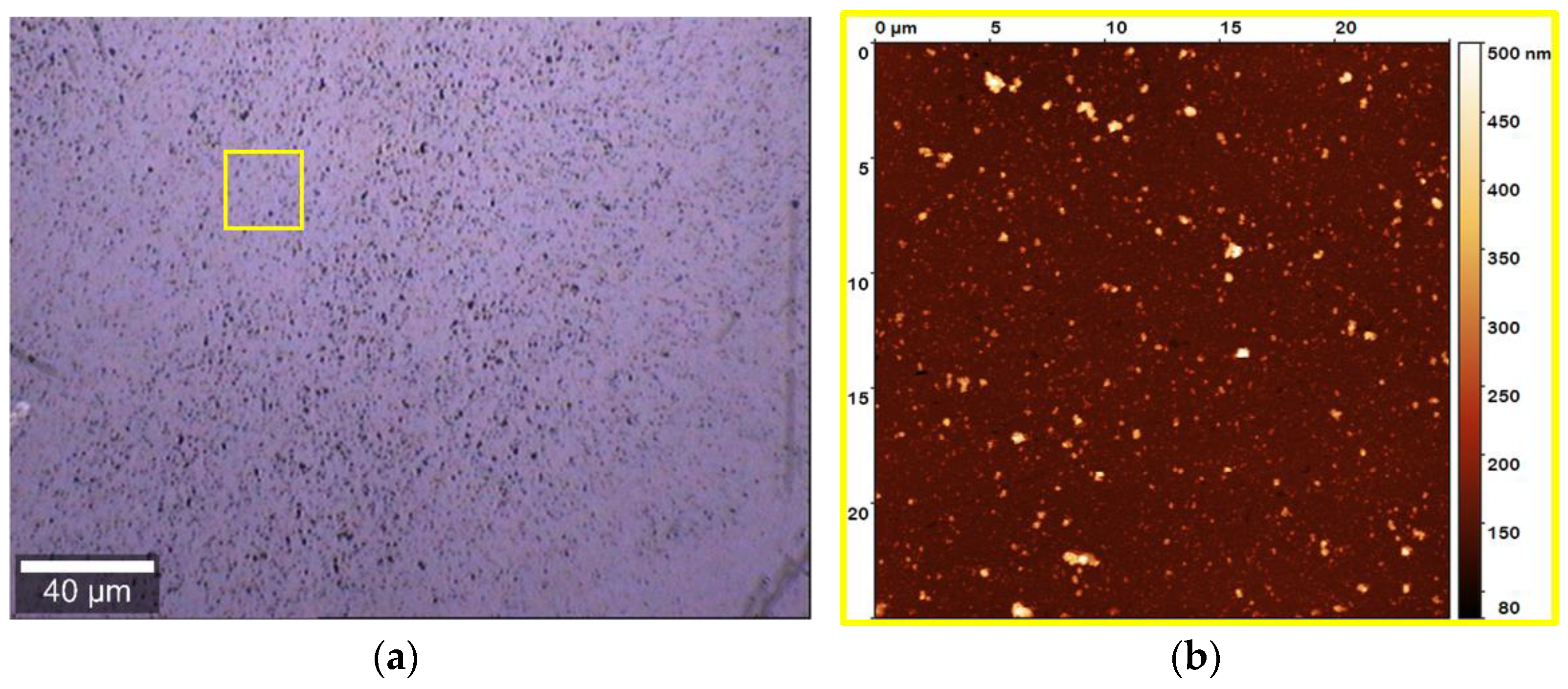
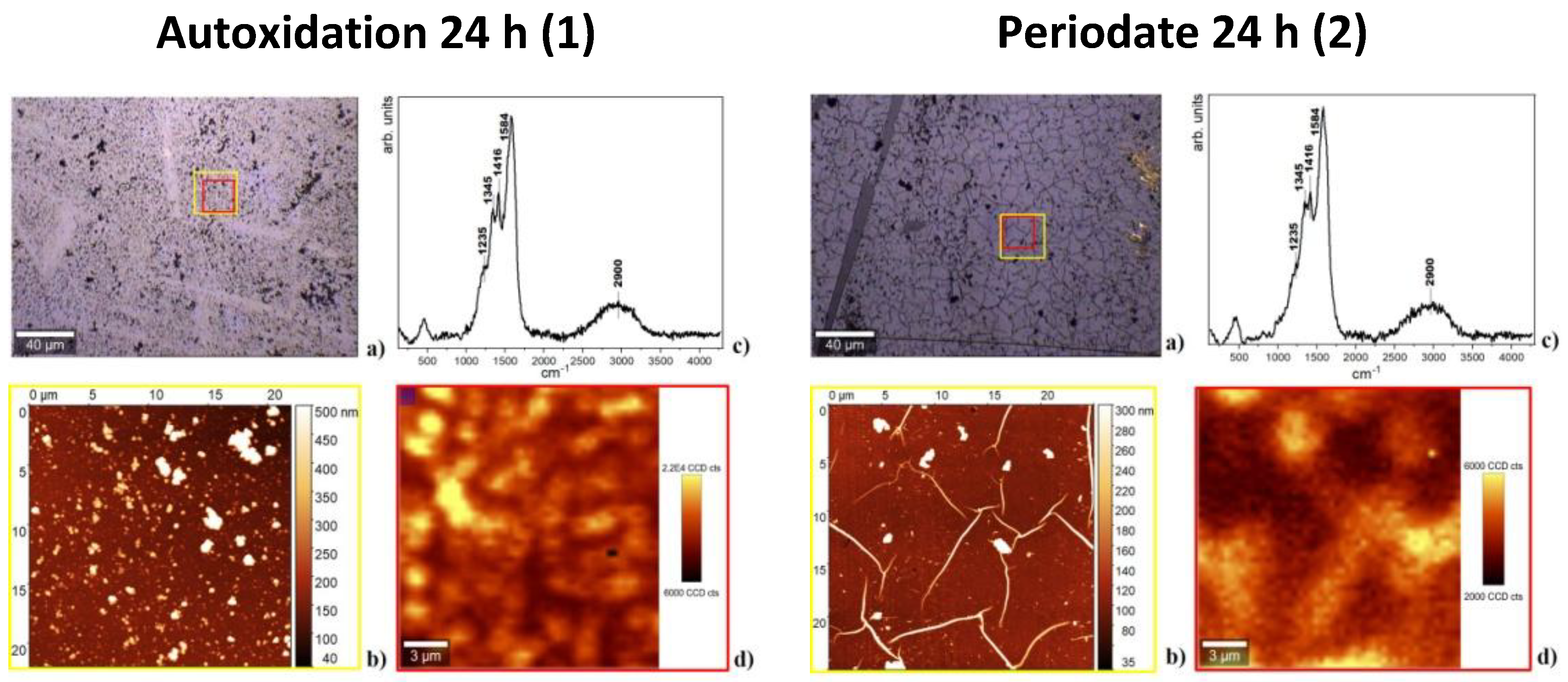
2.6. Atomic Force Microscopy and Micro-Raman Analysis
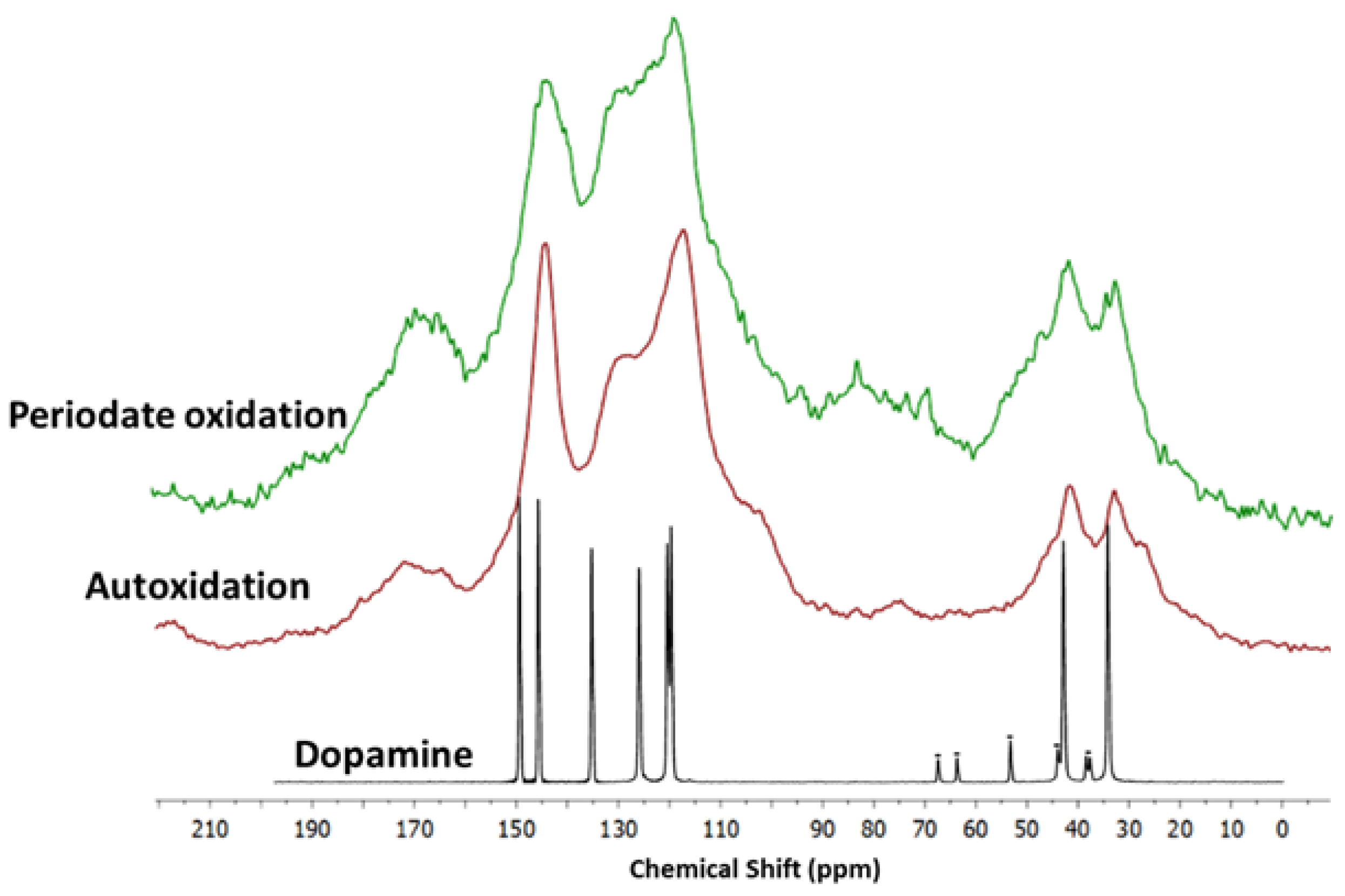
2.7. Solid State Nuclear Magnetic Resonance
3. Results
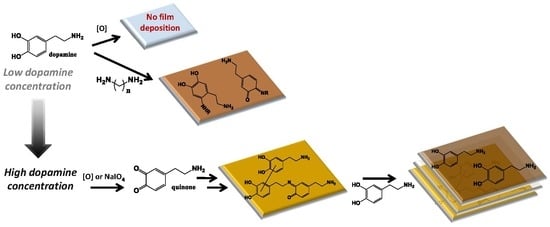
3.1. Concentration Dependence
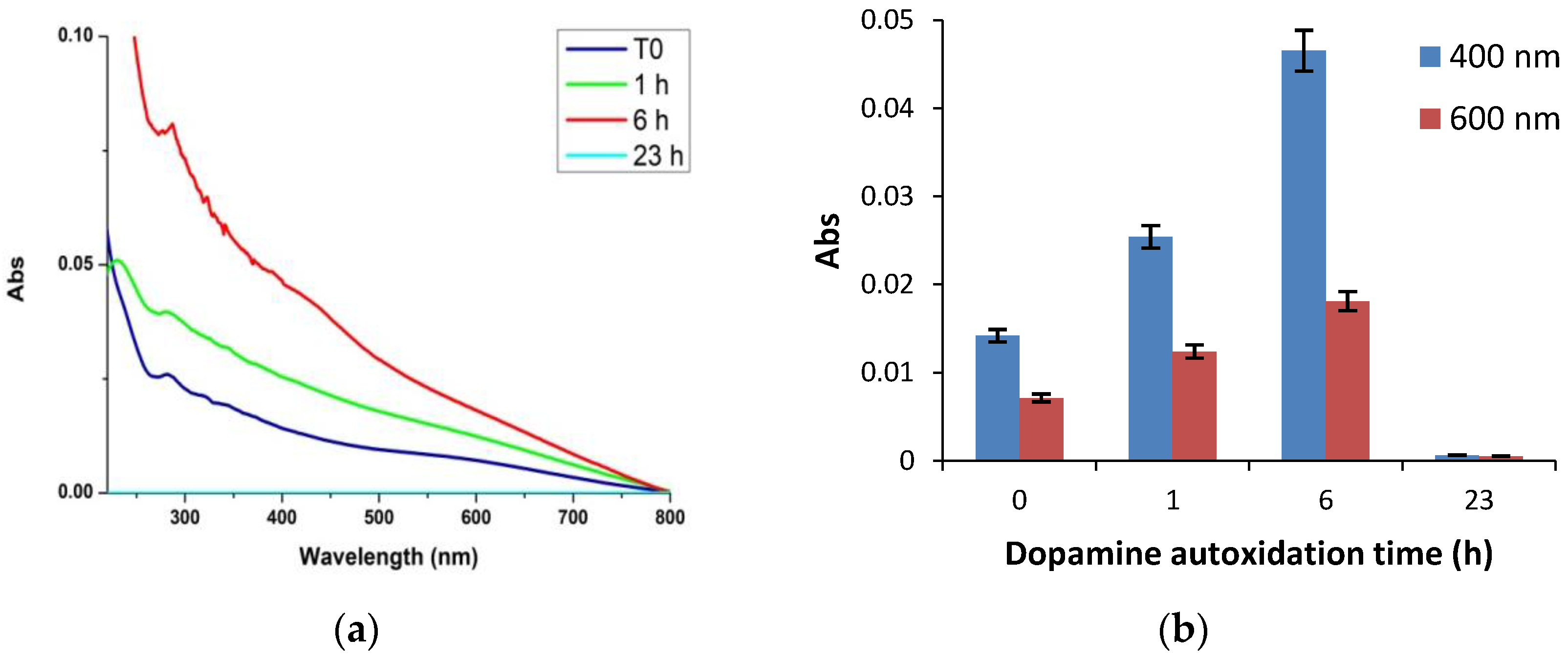
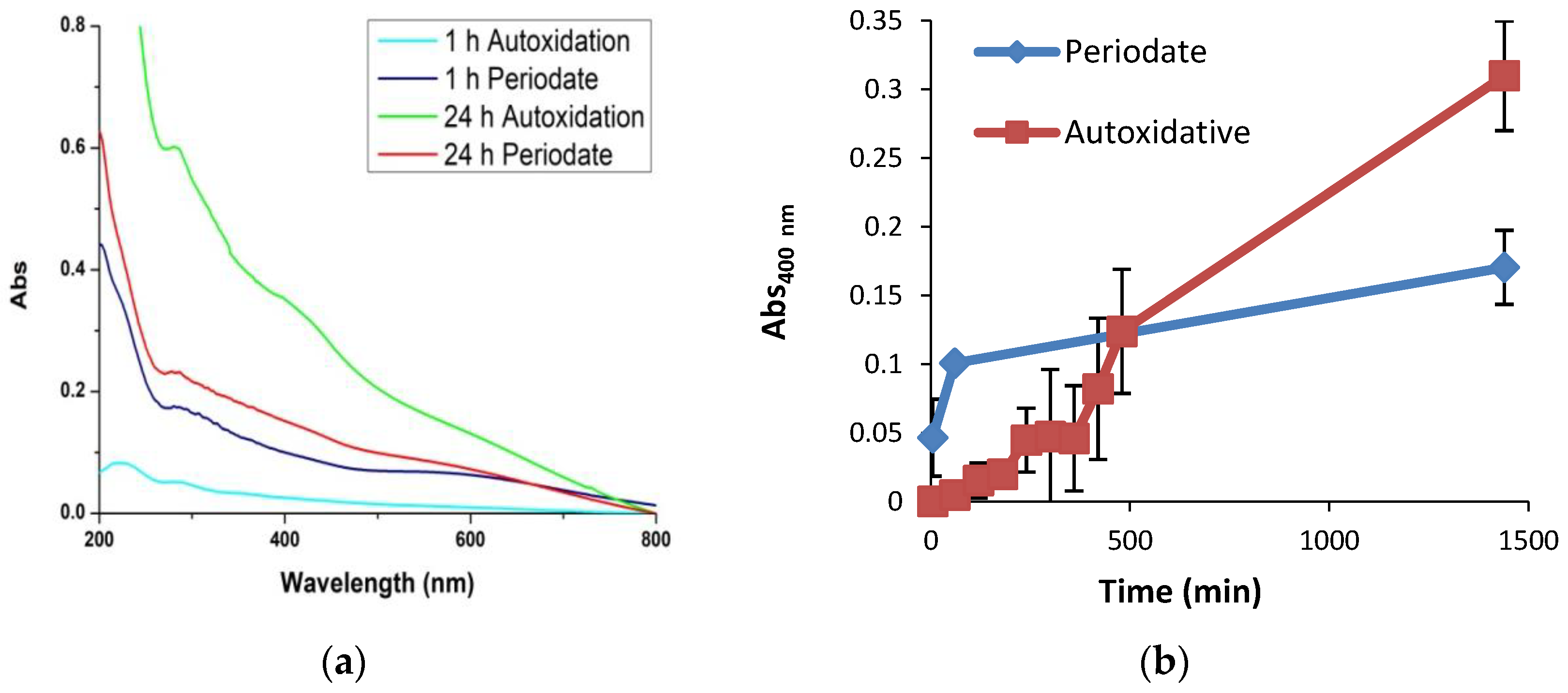
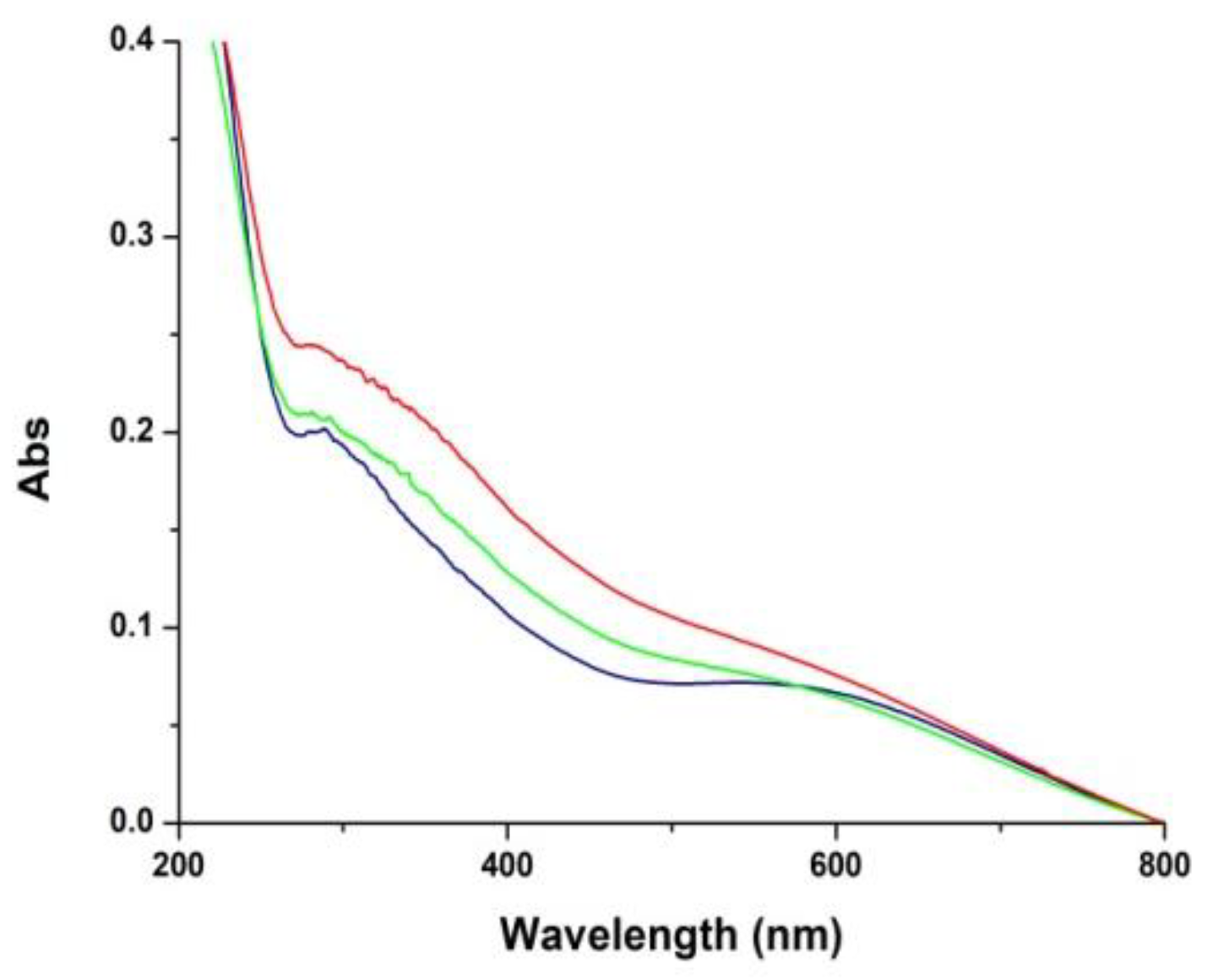
3.2. Temporal Evolution of Film Forming Properties and the Role of Dopamine Quinone
3.3. Film Growth Mechanisms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of polydopamine: A never-ending story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Lim, C.; Huang, J.; Kim, S.; Lee, H.; Zeng, H.; Hwang, D.S. Nanomechanics of poly(catecholamine) coatings in aqueous solutions. Angew. Chem. Int. Ed. 2016, 55, 3342–3346. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Molina, D.; Saiz Poseu, J.; Busque, F.; Nador, F.; Mancebo, J. The chemistry behind catechol-based adhesion. Angew. Chem. Int. Ed. 2018, in press. [Google Scholar] [CrossRef]
- Della Vecchia, N.F.; Avolio, R.; Alfè, M.; Errico, M.E.; Napolitano, A.; d’Ischia, M. Building-block diversity in polydopamine underpins a multifunctional eumelanin-type platform tunable through a quinone control point. Adv. Funct. Mater. 2013, 23, 1331–1340. [Google Scholar] [CrossRef]
- Yang, Y.; Qi, P.; Ding, Y.; Maitz, M.F.; Yang, Z.; Tu, Q.; Xiong, K.; Leng, Y.; Huang, N. A biocompatible and functional adhesive amine-rich coating based on dopamine polymerization. J. Mater. Chem. B 2015, 3, 72–81. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Yang, Z.; Zhou, S.; Luo, R.; Maitz, M.F.; Zhao, Y.; Wang, J.; Xiong, K.; Huang, N. A simple one-step modification of various materials for introducing effective multi-functional groups. Colloids Surf. B Biointerfaces 2014, 113, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, M.; Paez, J.I.; Avolio, R.; Carpentieri, A.; Panzella, L.; Falco, G.; Pizzo, E.; Errico, M.E.; Napolitano, A.; del Campo, A.; et al. Multifunctional thin films and coatings from caffeic acid and a cross-linking diamine. Langmuir 2017, 33, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Suárez-García, S.; Sedó, J.; Saiz-Poseu, J.; Ruiz-Molina, D. Copolymerization of a catechol and a diamine as a versatile polydopamine-like platform for surface functionalization: The case of a hydrophobic coating. Biomimetics 2017, 2, 22. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Micillo, R.; Panzella, L.; Crescenzi, O.; Oscurato, S.L.; Maddalena, P.; Napolitano, A.; Ball, V.; d’Ischia, M. Structural basis of polydopamine film formation: Probing 5,6-dihydroxyindole-based eumelanin type units and the porphyrin issue. ACS Appl. Mater. Interfaces 2018, 10, 7670–7680. [Google Scholar] [CrossRef] [PubMed]
- Ball, V.; Del Frari, D.; Toniazzo, V.; Ruch, D. Kinetics of polydopamine film deposition as a function of pH and dopamine concentration: Insights in the polydopamine deposition mechanism. J. Colloid Interface Sci. 2012, 386, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Weng, L.-T.; Yang, M.; Yang, Z.; Lu, X.; Huang, N.; Leng, Y. Insights into the aggregation/deposition and structure of a polydopamine film. Langmuir 2014, 30, 12258–12269. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; McCloskey, B.D.; Choi, T.H.; Lee, C.; Kim, M.J.; Freeman, B.D.; Park, H.B. Oxygen concentration control of dopamine-induced high uniformity surface coating chemistry. ACS Appl. Mater. Interfaces 2013, 5, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ou, Y.; Lei, W.-X.; Wan, L.-S.; Ji, J.; Xu, Z.-K. CuSO4/H2O2-Induced rapid deposition of polydopamine coatings with high uniformity and enhanced stability. Angew. Chem. 2016, 128, 3106–3109. [Google Scholar] [CrossRef]
- Salomäki, M.; Marttila, L.; Kivelä, H.; Ouvinen, T.; Lukkari, J.O. Effect of pH and oxidant on the first steps of polydopamine formation: A thermodynamic approach. J. Phys. Chem. B 2018, 122, 6314–6327. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, F.; Barthès, J.; Bour, J.; Michel, M.; Bertani, P.; Hemmerlé, J.; d’Ischia, M.; Ball, V. Oxidant control of polydopamine surface chemistry in acids: A mechanism-based entry to superhydrophilic- superoleophobic coatings. Chem. Mater. 2016, 28, 4697–4705. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Yang, J.; Saggiomo, V.; Velders, A.H.; Stuart, M.A.C.; Kamperman, M. Reaction pathways in catechol/primary amine mixtures: A window on crosslinking chemistry. PLoS ONE 2016, 11, e0166490. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, M.L.; Panzella, L.; Oscurato, S.L.; Salvatore, M.; Avolio, R.; Errico, M.E.; Maddalena, P.; Napolitano, A.; D’Ischia, M. The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics 2018, 3, 26. https://doi.org/10.3390/biomimetics3030026
Alfieri ML, Panzella L, Oscurato SL, Salvatore M, Avolio R, Errico ME, Maddalena P, Napolitano A, D’Ischia M. The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics. 2018; 3(3):26. https://doi.org/10.3390/biomimetics3030026
Chicago/Turabian StyleAlfieri, Maria Laura, Lucia Panzella, Stefano Luigi Oscurato, Marcella Salvatore, Roberto Avolio, Maria Emanuela Errico, Pasqualino Maddalena, Alessandra Napolitano, and Marco D’Ischia. 2018. "The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay" Biomimetics 3, no. 3: 26. https://doi.org/10.3390/biomimetics3030026
APA StyleAlfieri, M. L., Panzella, L., Oscurato, S. L., Salvatore, M., Avolio, R., Errico, M. E., Maddalena, P., Napolitano, A., & D’Ischia, M. (2018). The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics, 3(3), 26. https://doi.org/10.3390/biomimetics3030026