Fast and Fractionated: Correlation of Dose Attenuation and the Response of Human Cancer Cells in a New Anthropomorphic Brain Phantom
Abstract
1. Introduction
2. Materials and Methods
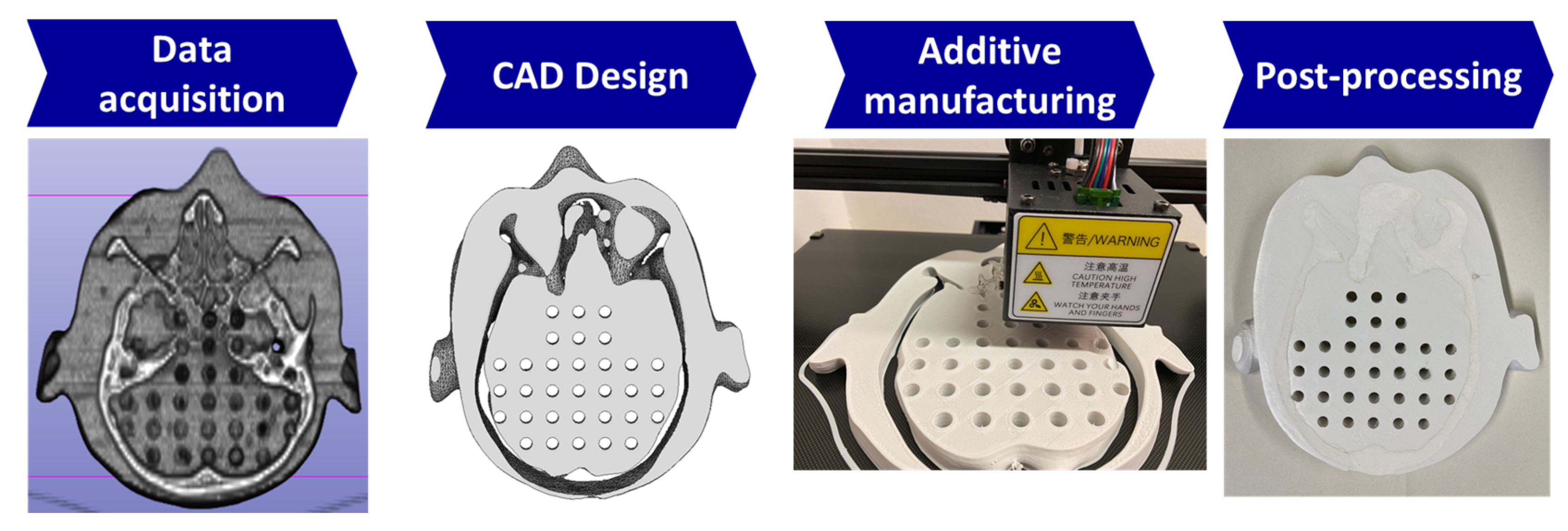
2.1. Producing the Biomimetic Tool
2.2. Cancer Cell Cultures
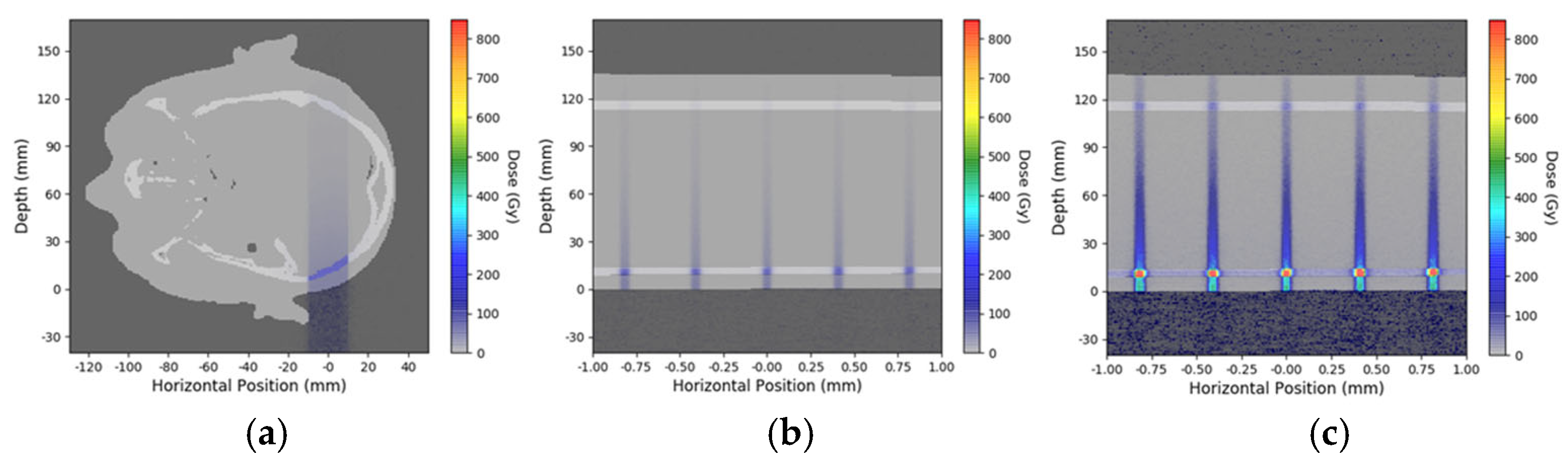
2.3. Simulation for Depth-Dependent Dose Deposition After MRT
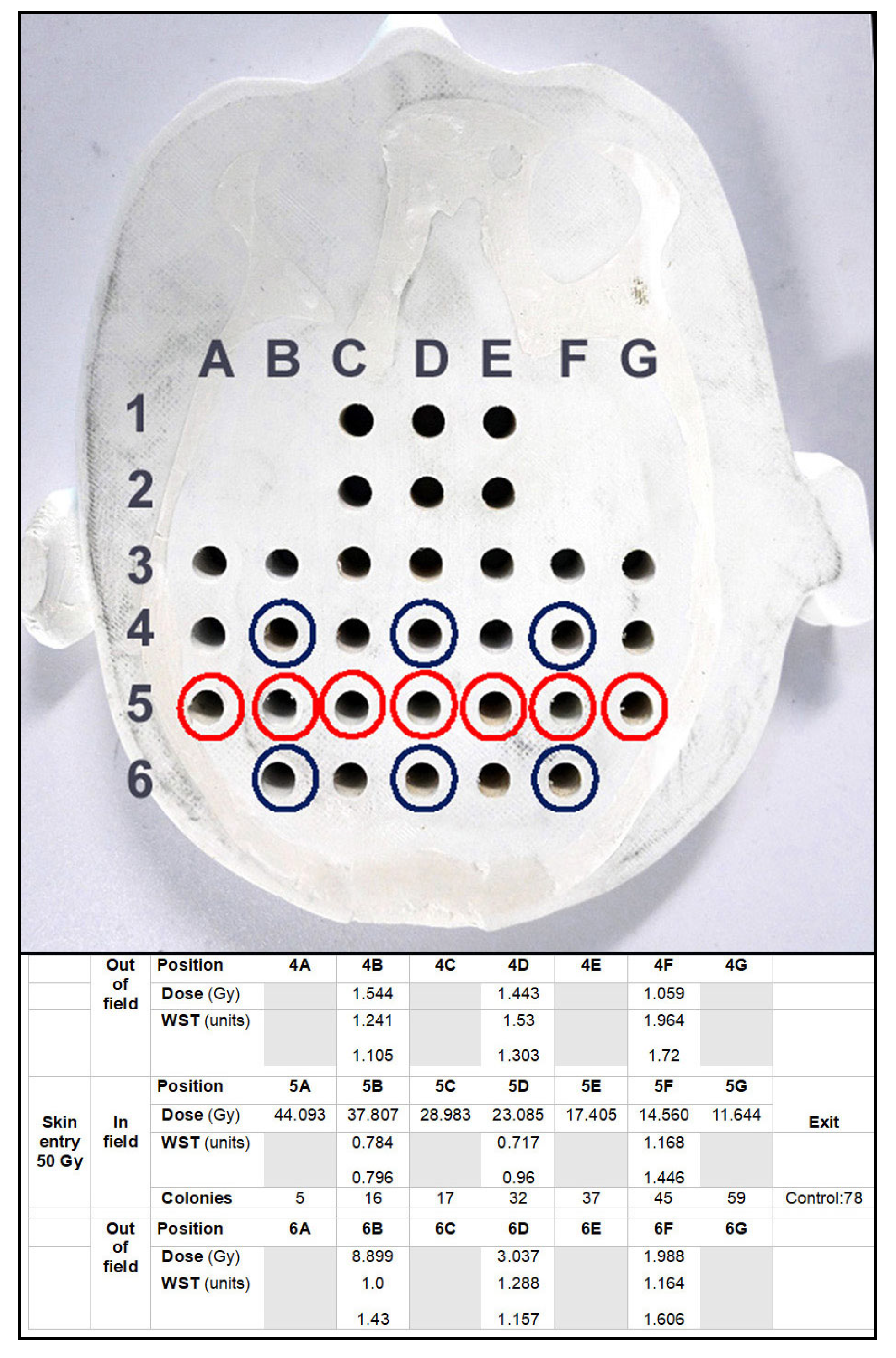
2.4. Dosimetry and Microbeam Irradiation at the Synchrotron
2.5. Colony Counts and WST Test
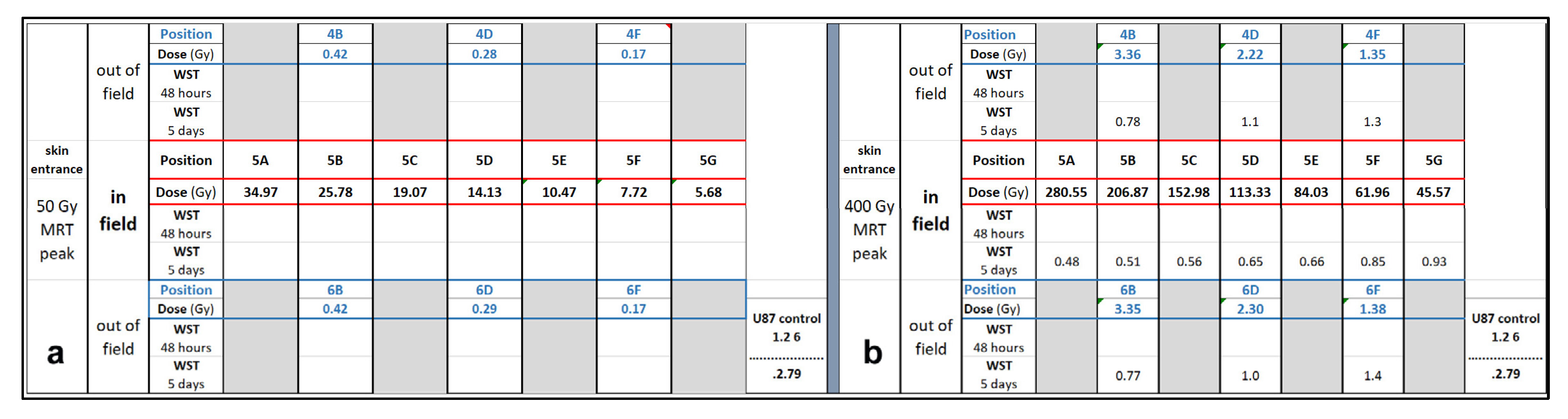
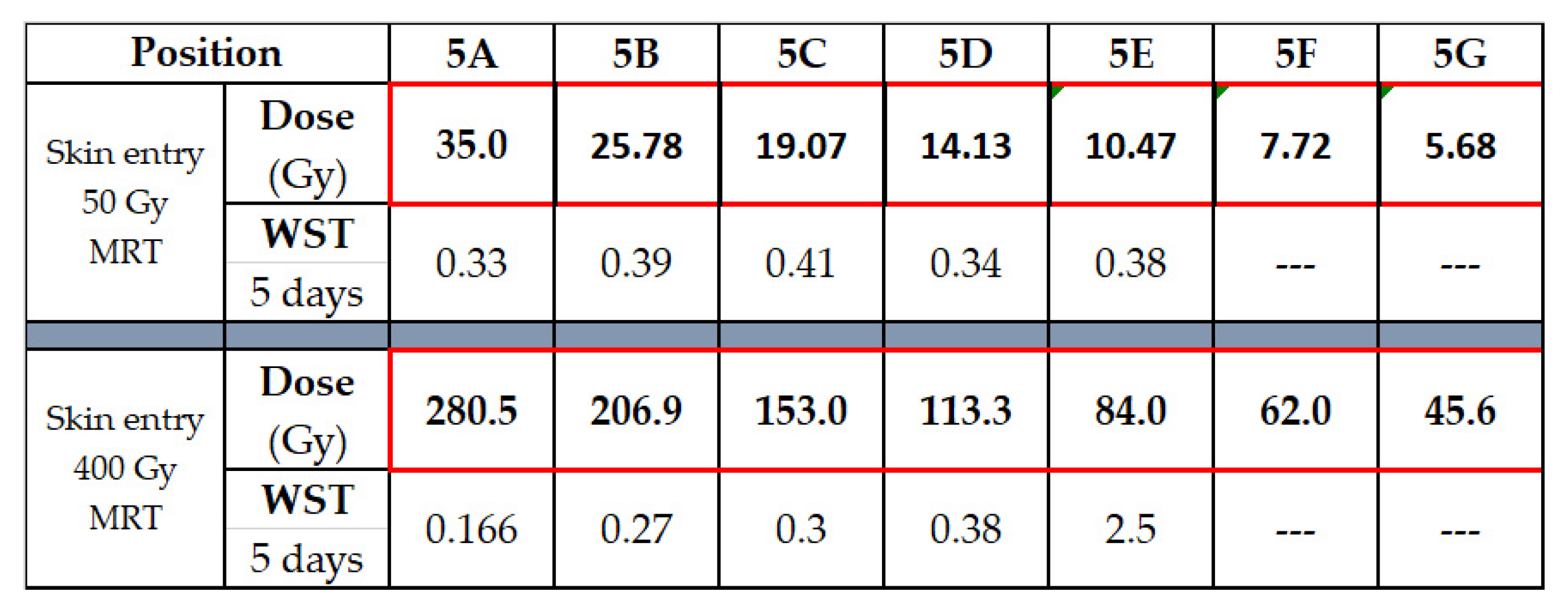
3. Results
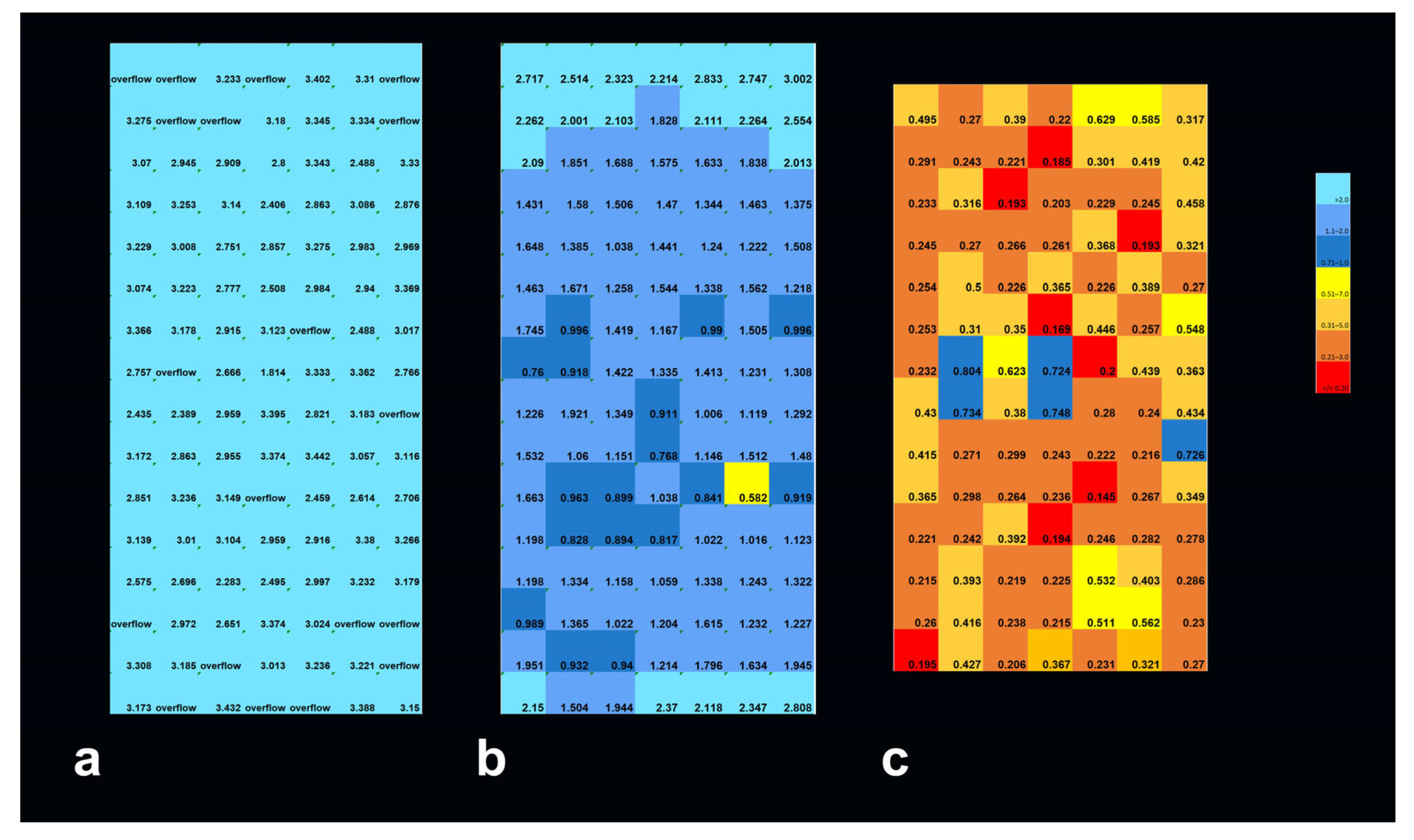
3.1. Depth-Dependent Dose Decrease After High-Dose-Rate Broad-Beam Irradiation, Correlated to Clonogenic Potential
3.2. Depth-Dependency of Metabolic Activity of Cancer Cells After MRT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Australian Synchrotron |
| ESRF | European Synchrotron Radiation Facility in Grenoble, France |
| FDM | Fused Deposition Modeling |
| IMBL | Imaging and Biomedical Beamline |
| kV | kilo Volt |
| LINAC | Linear Accelerator |
| MRT | Microbeam Radiation Therapy |
| MV | Megavolt |
| PLA | Polylactic Acid |
References
- Owen, D.; Harmsen, W.S.; Ahmed, S.K.; Petersen, I.A.; Haddock, M.G.; Ma, D.J.; Pulsipher, S.; Corbin, K.S.; Lester, S.C.; Park, S.S.; et al. Highs and Lows of Spatially Fractionated Radiation Therapy: Dosimetry and Clinical Outcomes. Pract. Radiat. Oncol. 2024, 15, e388–e395. [Google Scholar] [CrossRef]
- Studer, G.; Jeller, D.; Streller, T.; Huebner, D.; Glanzmann, C. Time-Related Outcome Following Palliative Spatially Fractionated Stereotactic Radiation Therapy (Lattice) of Large Tumors—A Case Series. Adv. Radiat. Oncol. 2024, 9, 101566. [Google Scholar] [CrossRef]
- Eling, L.; Kefs, S.; Keshmiri, S.; Balosso, J.; Calvet, S.; Chamel, G.; Drevon-Gaud, R.; Flandin, I.; Gaudin, M.; Giraud, L.; et al. Neuro-Oncologic Veterinary Trial for the Clinical Transfer of Microbeam Radiation Therapy: Acute to Subacute Radiotolerance after Brain Tumor Irradiation in Pet Dogs. Cancers 2024, 16, 2701. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.-F.; Balosso, J.; Bayat, S.; Berkvens, P.; Berruyer, G.; Bräuer-Krisch, E.; Brochard, T.; Chamel, G.; Desagneaux, A.; Drevon-Gaud, R.; et al. Toward Neuro-Oncologic Clinical Trials of High-Dose-Rate Synchrotron Microbeam Radiation Therapy: First Treatment of a Spontaneous Canine Brain Tumor. Int. J. Radiat. Oncol. 2022, 113, 967–973. [Google Scholar] [CrossRef]
- Ondrejová, B.; Rajťúková, V.; Šavrtková, K.; Galajdová, A.; Živčák, J.; Hudák, R. Analysis of MRI Artifacts Induced by Cranial Implants in Phantom Models. Healthcare 2025, 13, 803. [Google Scholar] [CrossRef] [PubMed]
- Scheidt, K.; Kropla, F.; Winkler, D.; Möbius, R.; Vychopen, M.; Wach, J.; Güresir, E.; Grunert, R. 3D-printed skull model for enhancing training in external ventricular drainage within medical education. 3D Print Med. 2025, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Schültke, E.; Fiedler, S.; Mewes, C.; Gargioni, E.; Klingenberg, J.; Abreu Faria, G.; Lerch, M.; Petasecca, M.; Prehn, F.; Wegner, M.; et al. The Microbeam Insert at the White Beam Beamline P61A at the Synchrotron PETRA III/DESY: A New Tool for High Dose Rate Irradiation Research. Cancers 2022, 14, 5137. [Google Scholar] [CrossRef]
- The Alderson Radiation Therapy Phantom—Radiology Support Devices Inc. Available online: https://rsdphantoms.com/product/the-alderson-radiation-therapy-phantom/ (accessed on 24 April 2025).
- Available online: www.cirsinc.com/wp-content/uploads/2019/05/701-706-ATOM-PB-120418.pdfv (accessed on 3 May 2025).
- Available online: www.slicer.org (accessed on 3 May 2023).
- Wegner, M.; Hinrichsen, N.; Rosendahl, M.; Krause, D.; Gargioni, E. Development of an additively manufactured head and neck phantom for computed tomography studies. Trans. Addit. Manuf. Meets Med. 2025, 7, 2057. [Google Scholar] [CrossRef]
- Ahmed, A.M.M.; Buschmann, M.; Breyer, L.; Kuntner, C.; Homolka, P. Tailoring the Mass Density of 3D Printing Materials for Accurate X-ray Imaging Simulation by Controlled Underfilling for Radiographic Phantoms. Polymers 2024, 16, 1116. [Google Scholar] [CrossRef]
- World Statistics Lung Cancer. Available online: www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 24 April 2025).
- Patallo, I.S.; Carter, R.; Maughan, D.; Nisbet, A.; Schettino, G.; Subiel, A. Evaluation of a micro ionization chamber for dosimetric measurements in image-guided preclinical irradiation platforms. Phys. Med. Biol. 2021, 66, 245012. [Google Scholar] [CrossRef]
- Bräuer-Krisch, E.; Adam, J.-F.; Alagoz, E.; Bartzsch, S.; Crosbie, J.; DeWagter, C.; Dipuglia, A.; Donzelli, M.; Doran, S.; Fournier, P.; et al. Medical physics aspects of the synchrotron radiation therapies: Microbeam radiation therapy (MRT) and synchrotron stereotactic radiotherapy (SSRT). Phys. Med. 2015, 31, 568–583. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Petasecca, M.; Cullen, A.; Paino, J.R.; Archer, J.; Rosenfeld, A.B.; Lerch, M.L.F. X-Tream dosimetry of synchrotron radiation with the PTW microDiamond. J. Instrum. 2019, 14, P10037. [Google Scholar] [CrossRef]
- Livingstone, J.; Stevenson, A.W.; Butler, D.J.; Häusermann, D.; Adam, J.F. Characterization of a synthetic single crystal diamond detector for dosimetry in spatially fractionated synchrotron X-ray fields. Med. Phys. 2016, 43, 4283–4293. [Google Scholar] [CrossRef]
- Paino, J.; Cameron, M.; Large, M.; Barnes, M.; Engels, E.; Vogel, S.; Tehei, M.; Corde, S.; Guatelli, S. DoseMRT: A Software Package for Individualised Monte Carlo Dose Calculations of Synchrotron-Generated Microbeam Radiation Therapy. Radiation 2023, 3, 123–137. [Google Scholar] [CrossRef]
- Breslin, T.; Paino, J.; Wegner, M.; Engels, E.; Fiedler, S.; Forrester, H.; Rennau, H.; Bustillo, J.; Cameron, M.; Häusermann, D.; et al. A Novel Anthropomorphic Phantom Composed of Tissue-Equivalent Materials for Use in Experimental Radiotherapy: Design, Dosimetry and Biological Pilot Study. Biomimetics 2023, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Bräuer-Krisch, E.; Requardt, H.; Brochard, T.; Berruyer, G.; Renier, M.; Laissue, J.A.; Bravin, A. New technology enables high precision multislit collimators for microbeam radiation therapy. Rev. Sci. Instrum. 2009, 80, 74301. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, J.C.; Rogers, P.A.; Stevenson, A.W.; Hall, C.J.; Lye, J.E.; Nordström, T.; Midgley, S.M.; Lewis, R.A. Reference dosimetry at the Australian Synchrotron’s imaging and medical beamline using free-air ionization chamber measurements and theoretical predictions of air kerma rate and half value layer. Med. Phys. 2013, 40, 062103. [Google Scholar] [CrossRef]
- Laissue, J.A.; Blattmann, H.; Slatkin, D.N. Alban Köhler (1874–1947): Inventor of grid therapy. Z. Med. Phys. 2012, 22, 90–99. [Google Scholar] [CrossRef]
- Steel, H.; Brüningk, S.C.; Box, C.; Oelfke, U.; Bartzsch, S.H. Quantification of Differential Response of Tumour and Normal Cells to Microbeam Radiation in the Absence of FLASH Effects. Cancers 2021, 13, 3238. [Google Scholar] [CrossRef]
- McGarrigle, J.M.; Long, K.R.; Prezado, Y. On the significance of the different geometrical and dosimetric parameters in microbeam and minibeam radiation therapy a retrospective evaluation. Front. Oncol. 2024, 14, 1449293. [Google Scholar] [CrossRef]
- Bouchet, A.; Bräuer-Krisch, E.; Prezado, Y.; El Atifi, M.; Rogalev, L.; Le Clec’h, C.; Laissue, J.A.; Pelletier, L.; Le Duc, G. Better Efficacy of Synchrotron Spatially Microfractionated Radiation Therapy Than Uniform Radiation Therapy on Glioma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1485–1494. [Google Scholar] [CrossRef]
- Bolt, M.; Clark, C.H.; Nisbet, A.; Chen, T. Quantification of the uncertainties within the radiotherapy dosimetry chain and their impact on tumour control. Phys. Imaging Radiat. Oncol. 2021, 19, 33–38. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Zhang, D.Y.; Ming, G.L.; Song, H. Glioblastoma modeling with 3D organoids: Progress and challenges. Oxf. Open Neurosci. 2023, 2, kvad008. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, J.; Wang, S.; Guo, P.; Liao, K.; Shi, Z.; Zhao, J.; Lin, S.; Yang, M.; Cai, G.; et al. Generation and banking of patient-derived glioblastoma organoid and its application in cancer neuroscience. Am. J. Cancer Res. 2024, 14, 5000–5010. [Google Scholar] [CrossRef]
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell 2020, 180, 188–204.e22. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frerker, B.; Engels, E.; Paino, J.; Rover, V.d.; Bustillo, J.P.; Wegner, M.; Cameron, M.; Fiedler, S.; Häusermann, D.; Hildebrandt, G.; et al. Fast and Fractionated: Correlation of Dose Attenuation and the Response of Human Cancer Cells in a New Anthropomorphic Brain Phantom. Biomimetics 2025, 10, 440. https://doi.org/10.3390/biomimetics10070440
Frerker B, Engels E, Paino J, Rover Vd, Bustillo JP, Wegner M, Cameron M, Fiedler S, Häusermann D, Hildebrandt G, et al. Fast and Fractionated: Correlation of Dose Attenuation and the Response of Human Cancer Cells in a New Anthropomorphic Brain Phantom. Biomimetics. 2025; 10(7):440. https://doi.org/10.3390/biomimetics10070440
Chicago/Turabian StyleFrerker, Bernd, Elette Engels, Jason Paino, Vincent de Rover, John Paul Bustillo, Marie Wegner, Matthew Cameron, Stefan Fiedler, Daniel Häusermann, Guido Hildebrandt, and et al. 2025. "Fast and Fractionated: Correlation of Dose Attenuation and the Response of Human Cancer Cells in a New Anthropomorphic Brain Phantom" Biomimetics 10, no. 7: 440. https://doi.org/10.3390/biomimetics10070440
APA StyleFrerker, B., Engels, E., Paino, J., Rover, V. d., Bustillo, J. P., Wegner, M., Cameron, M., Fiedler, S., Häusermann, D., Hildebrandt, G., Lerch, M., & Schültke, E. (2025). Fast and Fractionated: Correlation of Dose Attenuation and the Response of Human Cancer Cells in a New Anthropomorphic Brain Phantom. Biomimetics, 10(7), 440. https://doi.org/10.3390/biomimetics10070440








