Biometric Strategies to Improve Vaccine Immunogenicity and Effectiveness
Abstract
1. Introduction
2. Immunogenicity and Vaccine Response
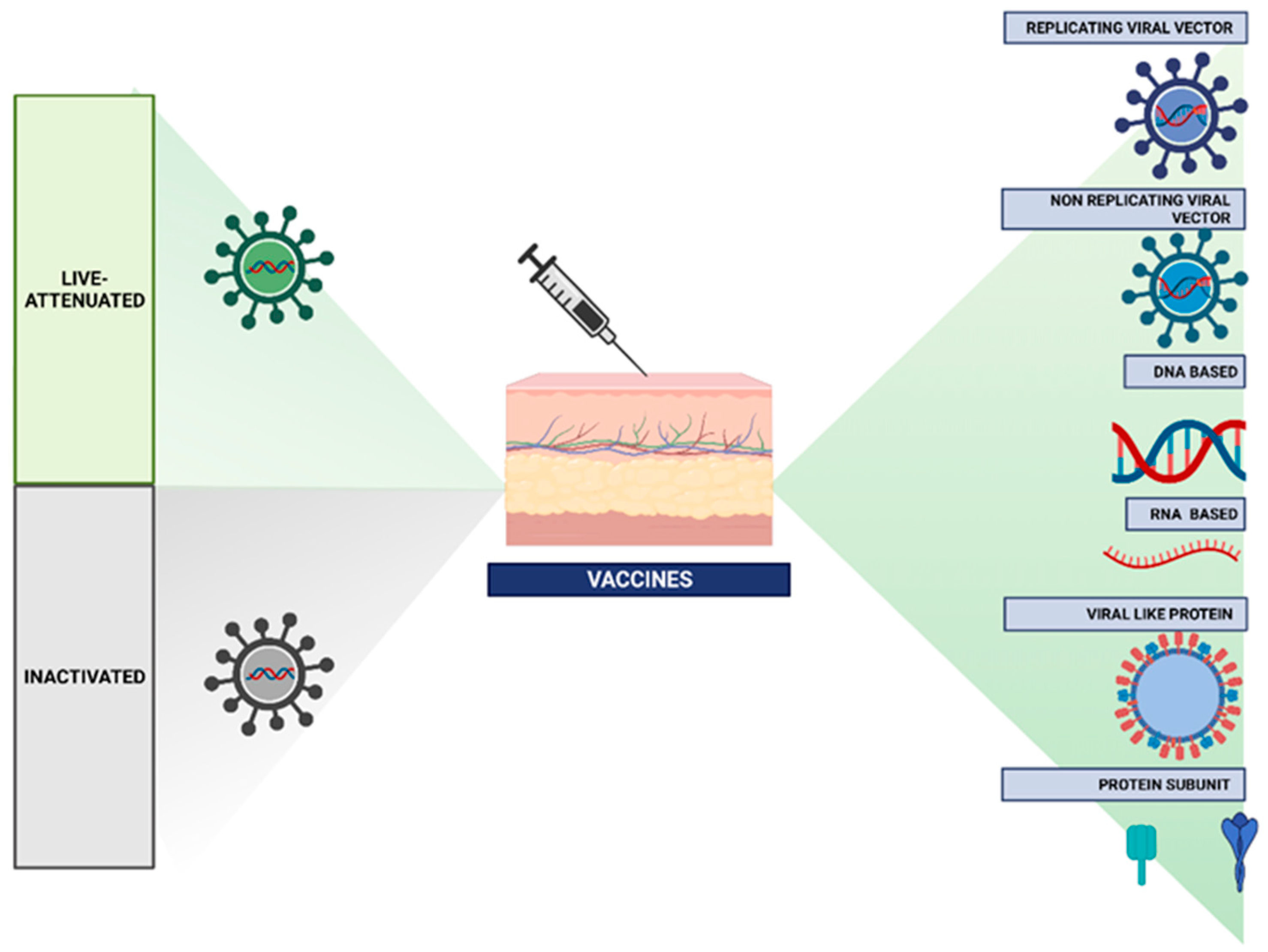
2.1. Types of Vaccines
Vaccines, Action, and Reaction
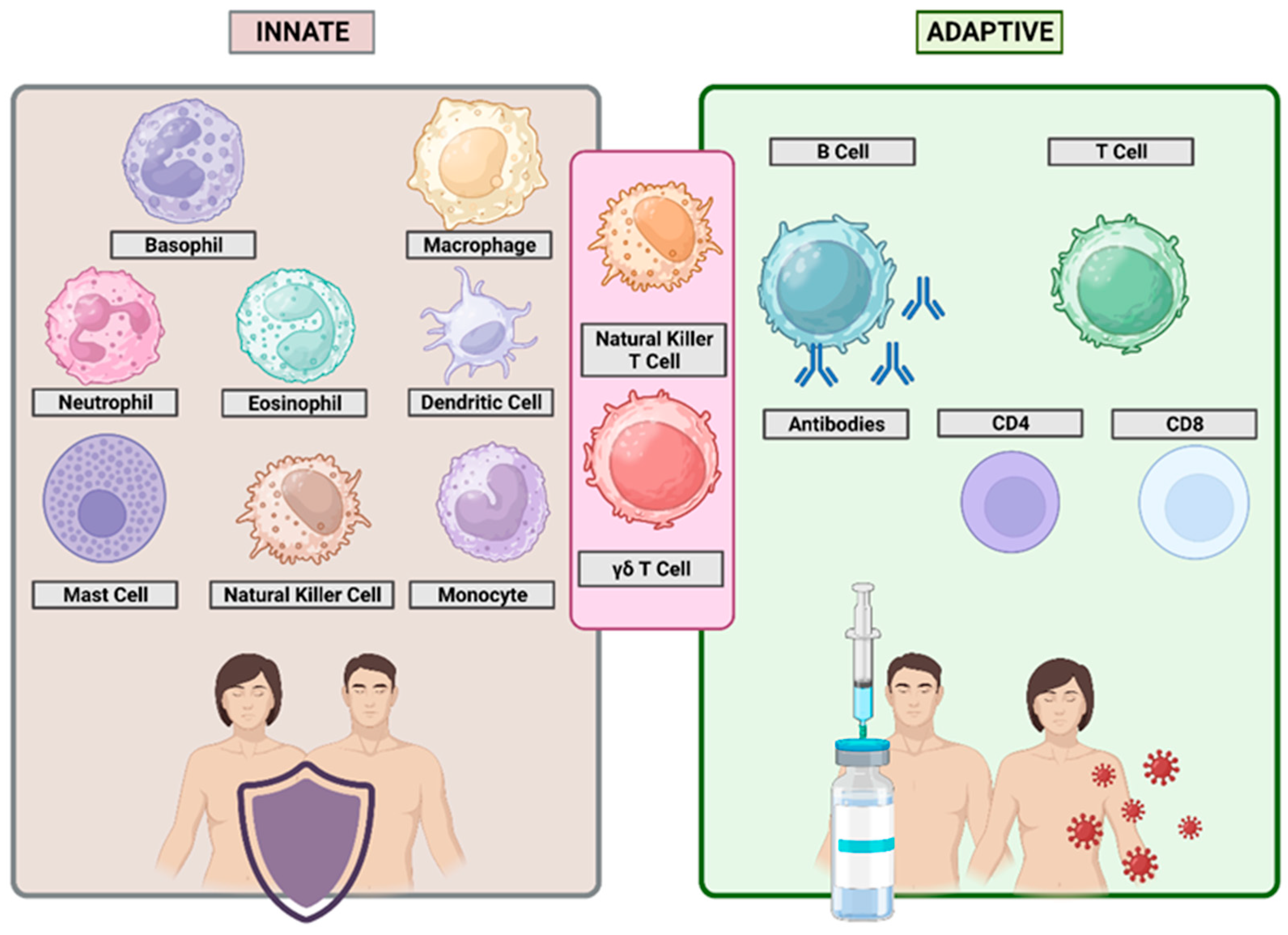
3. Immune Response Mechanisms
3.1. Immune System Cells
3.2. Innate and Adaptive Immune Response
4. Duration of Vaccine-Induced Immunity
4.1. Characteristics of the Pathogen
4.2. Formulation of the Vaccine
4.3. Individual Response
4.4. Waning Immunity over Time
- Personalized booster schedules should be developed based on age, immunological status, and exposure risk, moving beyond the one-size-fits-all approach.
- Surveillance-guided updates of vaccine antigens should be prioritized to match circulating variants, particularly for mutable viruses.
- Mucosal and microbiota-informed boosters offer a promising frontier and should be further explored for their potential to enhance both systemic and local immunity.
- Global equity in booster access must be addressed, ensuring that low- and middle-income countries are not left behind in periodic immunization campaigns.
5. Variability in Vaccine Responses
The Impact of the Infant Gastrointestinal Tract Microbiome on Immunity and Vaccination
6. Impact of Vaccination on Transmission
Real World Scenarios
7. Vaccine Effectiveness Against Emerging Variants
8. Immunosenescence and Vaccination in Older Adults
9. Maternal Vaccination and Neonatal Immunity
10. Impact of Co-Administration of Vaccines
11. Factors Influencing Vaccine Failure
12. Immunomodulatory Adjuvants and Vaccine Enhancement
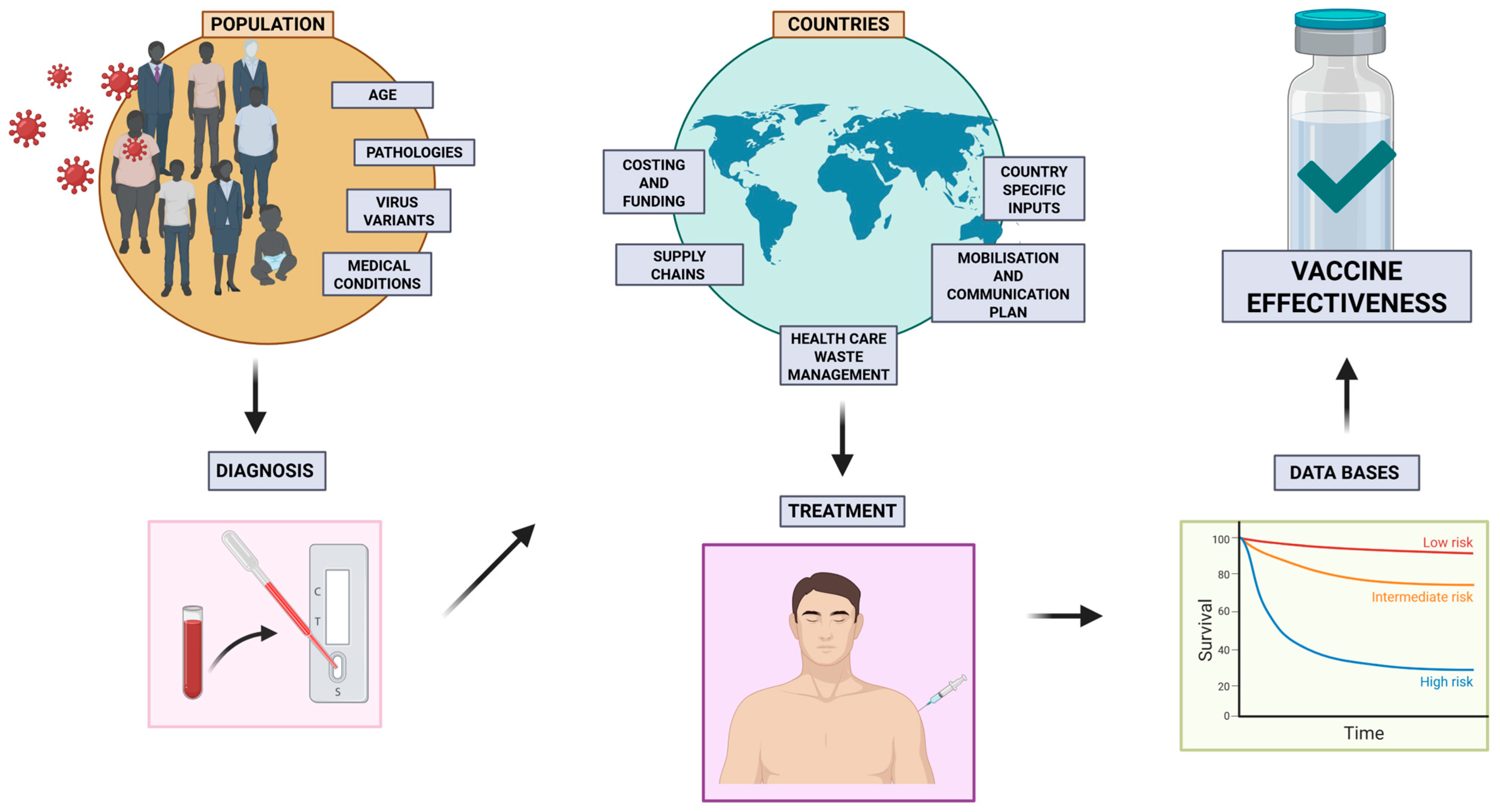
13. Assessing Vaccine Effectiveness in Real-World Settings
13.1. Limitations and Challenges
13.2. Regulatory and Translational Perspectives
14. Future Perspectives
15. Biomimetic Innovations in Vaccine Design: Future Directions
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clemente-Suárez, V.J.; Hormeño-Holgado, A.; Jiménez, M.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Perez-Palencia, N.; Maestre-Serrano, R.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic. Vaccines 2020, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Mintz, M.A.; Cyster, J.G. T Follicular Helper Cells in Germinal Center B Cell Selection and Lymphomagenesis. Immunol. Rev. 2020, 296, 48–61. [Google Scholar] [CrossRef]
- Poland, G.A.; Kennedy, R.B.; Ovsyannikova, I.G. Vaccinomics and Personalized Vaccinology: Is Science Leading Us toward a New Path of Directed Vaccine Development and Discovery? PLoS Pathog. 2011, 7, e1002344. [Google Scholar] [CrossRef]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated Transmissibility and Impact of SARS-CoV-2 Lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef]
- Greenough, A. Respiratory Syncytial Virus Infection: Clinical Features, Management, and Prophylaxis. Curr. Opin. Pulm. Med. 2002, 8, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Bynum, S.A.; Brandt, H.M.; Sharpe, P.A.; Williams, M.S.; Kerr, J.C. Working to Close the Gap: Identifying Predictors of HPV Vaccine Uptake among Young African American Women. J. Health Care Poor Underserved 2011, 22, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G. Unmet Needs in Modern Vaccinology: Adjuvants to Improve the Immune Response. Vaccine 2010, 28 (Suppl. 3), C25–C36. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Navarro-Jiménez, E.; Ruisoto, P.; Dalamitros, A.A.; Beltran-Velasco, A.I.; Hormeño-Holgado, A.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Performance of Fuzzy Multi-Criteria Decision Analysis of Emergency System in COVID-19 Pandemic. An Extensive Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 5208. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Navarro-Jiménez, E.; Moreno-Luna, L.; Saavedra-Serrano, M.C.; Jimenez, M.; Simón, J.A.; Tornero-Aguilera, J.F. The Impact of the COVID-19 Pandemic on Social, Health, and Economy. Sustainability 2021, 13, 6314. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Navarro-Jiménez, E.; Jimenez, M.; Hormeño-Holgado, A.; Martinez-Gonzalez, M.B.; Benitez-Agudelo, J.C.; Perez-Palencia, N.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Impact of COVID-19 Pandemic in Public Mental Health: An Extensive Narrative Review. Sustainability 2021, 13, 3221. [Google Scholar] [CrossRef]
- Rodriguez-Besteiro, S.; Valencia-Zapata, G.; Beltrán de la Rosa, E.; Clemente-Suárez, V.J. Food Consumption and COVID-19 Risk Perception of University Students. Sustainability 2022, 14, 1625. [Google Scholar] [CrossRef]
- World Health Organization. How Do Vaccines Work? Available online: https://www.who.int/news-room/feature-stories/detail/how-do-vaccines-work (accessed on 5 May 2025).
- Knezevic, I.; Kang, H.N.; Thorpe, R. Immunogenicity assessment of monoclonal antibody products: A simulated case study correlating antibody induction with clinical outcomes. Biologicals 2015, 43, 307–317. [Google Scholar] [CrossRef]
- Van Sleen, Y.; van der Geest, K.S.; Huckriede, A.L.; van Baarle, D.; Brouwer, E. Effect of DMARDs on the immunogenicity of vaccines. Nat. Rev. Rheumatol. 2023, 19, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Baxter, D. Active and Passive Immunization for Cancer. Hum. Vaccin. Immunother. 2014, 10, 2123–2129. [Google Scholar] [CrossRef]
- Plotkin, S.A. Updates on Immunologic Correlates of Vaccine-Induced Protection. Vaccine 2020, 38, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.B.; Schneerson, R.; Szu, S.C.; Fattom, A.; Yang, Y.; Lagergard, T.; Chu, C.; Sørensen, U.S. Prevention of Invasive Bacterial Diseases by Immunization with Polysaccharide-Protein Conjugates. Curr. Top. Microbiol. Immunol. 1989, 146, 169–180. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Ishii, K.J.; Coban, C.; Akira, S. Innate immune response to viral infection. Cytokine 2008, 43, 336–341. [Google Scholar] [CrossRef]
- Przedpelski, A.; Tepp, W.H.; Pellett, S.; Johnson, E.A.; Barbieri, J.T. A Novel High-Potency Tetanus Vaccine. mBio 2020, 11, e01668-20. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The Use of Viral Vectors in Vaccine Development. NPJ Vaccines 2022, 7, 75. [Google Scholar] [CrossRef]
- Sell, S. How Vaccines Work: Immune Effector Mechanisms and Designer Vaccines. Expert Rev. Vaccines 2019, 18, 993–1015. [Google Scholar] [CrossRef]
- Kaku, C.I.; Starr, T.N.; Zhou, P.; Dugan, H.L.; Khalifé, P.; Song, G.; Champney, E.R.; Mielcarz, D.W.; Geoghegan, J.C.; Burton, D.R.; et al. Evolution of Antibody Immunity Following Omicron BA.1 Breakthrough Infection. Nat. Commun. 2023, 14, 2751. [Google Scholar] [CrossRef] [PubMed]
- Forthal, D.N. Functions of Antibodies. Microbiol. Spectr. 2014, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Medetgul-Ernar, K.; Davis, M.M. Standing on the Shoulders of Mice. Immunity 2022, 55, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Wik, J.A.; Skålhegg, B.S. T Cell Metabolism in Infection. Front. Immunol. 2022, 13, 840610. [Google Scholar] [CrossRef]
- De Martino, M.; Chiappini, E.; Galli, L. Vaccines and Autoimmunity. Int. J. Immunopathol. Pharmacol. Engl. 2013, 26, 283–290. [Google Scholar] [CrossRef]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef]
- Ollila, J.; Vihinen, M. B Cells. Int. J. Biochem. Cell Biol. 2005, 37, 518–523. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Zafar, H.; Yousefiasl, S.; Raza, F. T-cell membrane-functionalized nanosystems for viral infectious diseases. Mater. Chem. Horiz. 2023, 2, 41–48. [Google Scholar]
- Guimarães, L.E.; Baker, B.; Perricone, C.; Shoenfeld, Y. Vaccines, Adjuvants and Autoimmunity. Pharmacol. Res. 2015, 100, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Molina, V.; Shoenfeld, Y. Infection, Vaccines and Other Environmental Triggers of Autoimmunity. Autoimmunity 2005, 38, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012, 23, viii6–viii9. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the Immune Response. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S3–S23. [Google Scholar] [CrossRef]
- Golubovskaya, V.; Wu, L. Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef]
- Chaui-Berlinck, J.G.; Barbuto, J.A.M.; Monteiro, L.H.A. Conditions for Pathogen Elimination by Immune Systems. Theory Biosci. 2004, 123, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, A.P.; Guttman, J.A.; Finlay, B.B. Manipulation of Host-Cell Pathways by Bacterial Pathogens. Nature 2007, 449, 827–834. [Google Scholar] [CrossRef]
- Fabbri, M.; Smart, C.; Pardi, R. T Lymphocytes. Int. J. Biochem. Cell Biol. 2003, 35, 1004–1008. [Google Scholar] [CrossRef]
- Netea, M.G.; Schlitzer, A.; Placek, K.; Joosten, L.A.B.; Schultze, J.L. Innate and Adaptive Immune Memory: An Evolutionary Continuum in the Host’s Response to Pathogens. Cell Host Microbe 2019, 25, 13–26. [Google Scholar] [CrossRef]
- Balato, A.; Unutmaz, D.; Gaspari, A.A. Natural Killer T Cells: An Unconventional T-Cell Subset with Diverse Effector and Regulatory Functions. J. Investig. Dermatol. 2009, 129, 1628–1642. [Google Scholar] [CrossRef]
- Marshall, K.M.; Loeliger, J.; Nolte, L.; Kelaart, A.; Kiss, N.K. Prevalence of Malnutrition and Impact on Clinical Outcomes in Cancer Services: A Comparison of Two Time Points. Clin. Nutr. 2019, 38, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Influence of Microbes on Neutrophil Life and Death. Front. Cell. Infect. Microbiol. 2017, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in Inflammation. Curr. Drug Targets. Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An Introduction to Immunology and Immunopathology. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2018, 14 (Suppl. 2), 49. [Google Scholar] [CrossRef]
- Okeke, E.B.; Uzonna, J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef]
- Kanneganti, T.-D. Intracellular Innate Immune Receptors: Life inside the Cell. Immunol. Rev. 2020, 297, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Regulation of Adaptive Immunity by the Innate Immune System. Science 2010, 327, 291–295. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Hori, T.; Nakashima, T.; Take, S.; Kaizuka, Y.; Mori, T.; Katafuchi, T. Immune Cytokines and Regulation of Body Temperature, Food Intake and Cellular Immunity. Brain Res. Bull. 1991, 27, 309–313. [Google Scholar] [CrossRef]
- Conigliaro, P.; Triggianese, P.; Ballanti, E.; Perricone, C.; Perricone, R.; Chimenti, M.S. Complement, Infection, and Autoimmunity. Curr. Opin. Rheumatol. 2019, 31, 532–541. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Brummelman, J.; Pilipow, K.; Lugli, E. The Single-Cell Phenotypic Identity of Human CD8(+) and CD4(+) T Cells. Int. Rev. Cell Mol. Biol. 2018, 341, 63–124. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T Cell Receptor (TCR) Signaling in Health and Disease. Signal Transduct. Target. Ther. 2021, 6, 412. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Hammad, H.; Lambrecht, B.N. Professional and “Amateur” Antigen-Presenting Cells In Type 2 Immunity. Trends Immunol. 2019, 40, 22–34. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef]
- Bonilla, F.A.; Oettgen, H.C. Adaptive Immunity. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S33–S40. [Google Scholar] [CrossRef]
- Sigal, L.J. Activation of CD8 T Lymphocytes during Viral Infections. Encycl. Immunobiol. 2016, 286–290. [Google Scholar] [CrossRef]
- Samji, T.; Khanna, K.M. Understanding Memory CD8(+) T Cells. Immunol. Lett. 2017, 185, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Rabb, H. The T Cell as a Bridge between Innate and Adaptive Immune Systems: Implications for the Kidney. Kidney Int. 2002, 61, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- de Vries, N.L.; van de Haar, J.; Veninga, V.; Chalabi, M.; Ijsselsteijn, M.E.; van der Ploeg, M.; van den Bulk, J.; Ruano, D.; van den Berg, J.G.; Haanen, J.B.; et al. Γδ T Cells Are Effectors of Immunotherapy in Cancers with HLA Class I Defects. Nature 2023, 613, 743–750. [Google Scholar] [CrossRef]
- Hoffman, W.; Lakkis, F.G.; Chalasani, G. B Cells, Antibodies, and More. Clin. J. Am. Soc. Nephrol. 2016, 11, 137–154. [Google Scholar] [CrossRef]
- Cooper, M.A.; Colonna, M.; Yokoyama, W.M. Hidden Talents of Natural Killers: NK Cells in Innate and Adaptive Immunity. EMBO Rep. 2009, 10, 1103–1110. [Google Scholar] [CrossRef]
- Yokoyama, W.M. Mistaken Notions about Natural Killer Cells. Nat. Immunol. 2008, 9, 481–485. [Google Scholar] [CrossRef]
- Segal, Y.; Shoenfeld, Y. Vaccine-Induced Autoimmunity: The Role of Molecular Mimicry and Immune Crossreaction. Cell. Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-Y.; Zeng, D.; Gilbert, P.B. Evaluating the Long-Term Efficacy of Coronavirus Disease 2019 (COVID-19) Vaccines. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, 1927–1939. [Google Scholar] [CrossRef]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-Term Effectiveness of COVID-19 Vaccines against Infections, Hospitalisations, and Mortality in Adults: Findings from a Rapid Living Systematic Evidence Synthesis and Meta-Analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Sillanpaa, M.; Rezakhani, Y.; Wang, C. Review of Concerned SARS-CoV-2 Variants like Alpha (B. 1.1. 7), Beta (B. 1.351), Gamma (P. 1), Delta (B. 1.617. 2), and Omicron (B. 1.1. 529), as Well as Novel Methods for Reducing and Inactivating SARS-CoV-2 Mutants in Wastewater Treatment Facilities. J. Hazard. Mater. Adv. 2022, 7, 100140. [Google Scholar]
- Siegrist, C.-A. Vaccine Immunology. Vaccines 2008, 5, 17–36. [Google Scholar]
- Finlay, B.B.; McFadden, G. Anti-Immunology: Evasion of the Host Immune System by Bacterial and Viral Pathogens. Cell 2006, 124, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Andrés, J.; van Crevel, R.; Divangahi, M.; Netea, M.G. Designing the Next Generation of Vaccines: Relevance for Future Pandemics. mBio 2020, 11, e02616-20. [Google Scholar] [CrossRef] [PubMed]
- Tlaxca, J.L.; Ellis, S.; Remmele, R.L.J. Live Attenuated and Inactivated Viral Vaccine Formulation and Nasal Delivery: Potential and Challenges. Adv. Drug Deliv. Rev. 2015, 93, 56–78. [Google Scholar] [CrossRef]
- Zepp, F. Principles of Vaccine Design-Lessons from Nature. Vaccine 2010, 28 (Suppl. 3), C14–C24. [Google Scholar] [CrossRef]
- Wang, N.; Shang, J.; Jiang, S.; Du, L. Subunit Vaccines Against Emerging Pathogenic Human Coronaviruses. Front. Microbiol. 2020, 11, 298. [Google Scholar] [CrossRef]
- Chavda, V.P.; Hossain, M.K.; Beladiya, J.; Apostolopoulos, V. Nucleic Acid Vaccines for COVID-19: A Paradigm Shift in the Vaccine Development Arena. Biologics 2021, 1, 337–356. [Google Scholar] [CrossRef]
- Tsang, J.S.; Dobaño, C.; VanDamme, P.; Moncunill, G.; Marchant, A.; Othman, R.B.; Sadarangani, M.; Koff, W.C.; Kollmann, T.R. Improving Vaccine-Induced Immunity: Can Baseline Predict Outcome? Trends Immunol. 2020, 41, 457–465. [Google Scholar] [CrossRef]
- Sobh, A.; Bonilla, F.A. Vaccination in Primary Immunodeficiency Disorders. J. Allergy Clin. Immunol. Pract. 2016, 4, 1066–1075. [Google Scholar] [CrossRef]
- Kimman, T.G.; Vandebriel, R.J.; Hoebee, B. Genetic Variation in the Response to Vaccination. Community Genet. 2007, 10, 201–217. [Google Scholar] [CrossRef]
- Posteraro, B.; Pastorino, R.; Di Giannantonio, P.; Ianuale, C.; Amore, R.; Ricciardi, W.; Boccia, S. The Link between Genetic Variation and Variability in Vaccine Responses: Systematic Review and Meta-Analyses. Vaccine 2014, 32, 1661–1669. [Google Scholar] [CrossRef]
- Gray, D. A Role for Antigen in the Maintenance of Immunological Memory. Nat. Rev. Immunol. 2002, 2, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Younas, M.; Carrat, F.; Desaint, C.; Launay, O.; Corbeau, P. Immune Activation, Smoking, and Vaccine Response. AIDS 2017, 31, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Chakrabarti, A.K.; Dutta, S. Current Challenges: From the Path of “Original Antigenic Sin” towards the Development of Universal Flu Vaccines. Int. Rev. Immunol. 2020, 39, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Bosco, N.; Noti, M. The Aging Gut Microbiome and Its Impact on Host Immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The Influence of the Intestinal Microbiome on Vaccine Responses. Vaccine 2018, 36, 4433–4439. [Google Scholar] [CrossRef]
- Kumar, M.; James, M.M.; Kumawat, M.; Nabi, B.; Sharma, P.; Pal, N.; Shubham, S.; Tiwari, R.R.; Sarma, D.K.; Nagpal, R. Aging and Microbiome in the Modulation of Vaccine Efficacy. Biomedicines 2022, 10, 1545. [Google Scholar] [CrossRef]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium Longum Subspecies Infantis: Champion Colonizer of the Infant Gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Hrncir, T.; Stepankova, R.; Kozakova, H.; Hudcovic, T.; Tlaskalova-Hogenova, H. Gut Microbiota and Lipopolysaccharide Content of the Diet Influence Development of Regulatory T Cells: Studies in Germ-Free Mice. BMC Immunol. 2008, 9, 65. [Google Scholar] [CrossRef]
- Coombes, J.L.; Powrie, F. Dendritic Cells in Intestinal Immune Regulation. Nat. Rev. Immunol. 2008, 8, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.S.; Schumacher, A. The T Helper Type 17/Regulatory T Cell Paradigm in Pregnancy. Immunology 2016, 148, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial Short-Chain Fatty Acids Modulate CD8(+) T Cell Responses and Improve Adoptive Immunotherapy for Cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Parker, E.P.K.; Praharaj, I.; Zekavati, A.; Lazarus, R.P.; Giri, S.; Operario, D.J.; Liu, J.; Houpt, E.; Iturriza-Gómara, M.; Kampmann, B.; et al. Influence of the Intestinal Microbiota on the Immunogenicity of Oral Rotavirus Vaccine given to Infants in South India. Vaccine 2018, 36, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Fix, J.; Chandrashekhar, K.; Perez, J.; Bucardo, F.; Hudgens, M.G.; Yuan, L.; Twitchell, E.; Azcarate-Peril, M.A.; Vilchez, S.; Becker-Dreps, S. Association between Gut Microbiome Composition and Rotavirus Vaccine Response among Nicaraguan Infants. Am. J. Trop. Med. Hyg. 2020, 102, 213–219. [Google Scholar] [CrossRef]
- Carrow, H.C.; Batachari, L.E.; Chu, H. Strain Diversity in the Microbiome: Lessons from Bacteroides Fragilis. PLoS Pathog. 2020, 16, e1009056. [Google Scholar] [CrossRef]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia 2017, 19, 848–855. [Google Scholar] [CrossRef]
- Wilky, B.A. Immune Checkpoint Inhibitors: The Linchpins of Modern Immunotherapy. Immunol. Rev. 2019, 290, 6–23. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Yadegar, A.; Asadzadeh Aghdaei, H.; Reza Zali, M. Modulatory Effects of Gut Microbiome in Cancer Immunotherapy: A Novel Paradigm for Blockade of Immune Checkpoint Inhibitors. Cancer Med. 2021, 10, 1141–1154. [Google Scholar] [CrossRef]
- Xie, J.; Li, Q.; Haesebrouck, F.; Van Hoecke, L.; Vandenbroucke, R.E. The Tremendous Biomedical Potential of Bacterial Extracellular Vesicles. Trends Biotechnol. 2022, 40, 1173–1194. [Google Scholar] [CrossRef]
- De Jong, M.C.; Kimman, T.G. Experimental Quantification of Vaccine-Induced Reduction in Virus Transmission. Vaccine 1994, 12, 761–766. [Google Scholar] [CrossRef]
- Woolthuis, R.G.; Wallinga, J.; van Boven, M. Variation in Loss of Immunity Shapes Influenza Epidemics and the Impact of Vaccination. BMC Infect. Dis. 2017, 17, 632. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Pourbohloul, B.; Meyers, L.A. A Comparative Analysis of Influenza Vaccination Programs. PLoS Med. 2006, 3, e387. [Google Scholar] [CrossRef]
- Lee, B.Y.; Brown, S.T.; Cooley, P.; Grefenstette, J.J.; Zimmerman, R.K.; Zimmer, S.M.; Potter, M.A.; Rosenfeld, R.; Wheaton, W.D.; Wiringa, A.E.; et al. Vaccination Deep into a Pandemic Wave Potential Mechanisms for a “Third Wave” and the Impact of Vaccination. Am. J. Prev. Med. 2010, 39, e21–e29. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-H. Age Groups and Spread of Influenza: Implications for Vaccination Strategy. BMC Infect. Dis. 2010, 10, 106. [Google Scholar] [CrossRef]
- Pitman, R.J.; White, L.J.; Sculpher, M. Estimating the Clinical Impact of Introducing Paediatric Influenza Vaccination in England and Wales. Vaccine 2012, 30, 1208–1224. [Google Scholar] [CrossRef] [PubMed]
- Arinaminpathy, N.; Kim, I.K.; Gargiullo, P.; Haber, M.; Foppa, I.M.; Gambhir, M.; Bresee, J. Estimating Direct and Indirect Protective Effect of Influenza Vaccination in the United States. Am. J. Epidemiol. 2017, 186, 92–100. [Google Scholar] [CrossRef]
- Lyngse, F.P.; Mølbak, K.; Denwood, M.; Christiansen, L.E.; Møller, C.H.; Rasmussen, M.; Cohen, A.S.; Stegger, M.; Fonager, J.; Sieber, R.N.; et al. Effect of Vaccination on Household Transmission of SARS-CoV-2 Delta Variant of Concern. Nat. Commun. 2022, 13, 3764. [Google Scholar] [CrossRef]
- Allen, H.; Tessier, E.; Turner, C.; Anderson, C.; Blomquist, P.; Simons, D.; Løchen, A.; Jarvis, C.I.; Groves, N.; Capelastegui, F.; et al. Comparative Transmission of SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) Variants and the Impact of Vaccination: National Cohort Study, England. Epidemiol. Infect. 2023, 151, e58. [Google Scholar] [CrossRef]
- Haque, A.; Pant, A.B. Mitigating Covid-19 in the Face of Emerging Virus Variants, Breakthrough Infections and Vaccine Hesitancy. J. Autoimmun. 2022, 127, 102792. [Google Scholar] [CrossRef]
- Holmes, E.C. RNA Virus Genomics: A World of Possibilities. J. Clin. Investig. 2009, 119, 2488–2495. [Google Scholar] [CrossRef]
- Qiu, Z.; Feng, Z. Transmission Dynamics of an Influenza Model with Vaccination and Antiviral Treatment. Bull. Math. Biol. 2010, 72, 1–33. [Google Scholar] [CrossRef]
- Debbink, K.; McCrone, J.T.; Petrie, J.G.; Truscon, R.; Johnson, E.; Mantlo, E.K.; Monto, A.S.; Lauring, A.S. Vaccination Has Minimal Impact on the Intrahost Diversity of H3N2 Influenza Viruses. PLoS Pathog. 2017, 13, e1006194. [Google Scholar] [CrossRef]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, Á. Transmission of SARS-CoV-2 Lineage, B. 1.1. 7 in England: Insights from Linking Epidemiological and Genetic Data. medRxiv 2021, 2012–2020. [Google Scholar] [CrossRef]
- Zhou, D.; Dejnirattisai, W.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Tuekprakhon, A.; Nutalai, R.; et al. Evidence of Escape of SARS-CoV-2 Variant B.1.351 from Natural and Vaccine-Induced Sera. Cell 2021, 184, 2348–2361.e6. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.-D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Effect of Delta Variant on Viral Burden and Vaccine Effectiveness against New SARS-CoV-2 Infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Novavax Vaccine Efficacy Is 86% against UK Variant and 60% against South African Variant. BMJ 2021, 372, n296. [Google Scholar] [CrossRef]
- Bruxvoort, K.J.; Sy, L.S.; Qian, L.; Ackerson, B.K.; Luo, Y.; Lee, G.S.; Tian, Y.; Florea, A.; Aragones, M.; Tubert, J.E.; et al. Effectiveness of MRNA-1273 against Delta, Mu, and Other Emerging Variants of SARS-CoV-2: Test Negative Case-Control Study. BMJ 2021, 375, e068848. [Google Scholar] [CrossRef]
- Ranzani, O.T.; dos Santos Leite, R.; Castilho, L.D.; Maymone Gonçalves, C.C.; Resende, G.; de Melo, R.L.; Croda, J. Vaccine Effectiveness of Ad26. COV2. S against Symptomatic COVID-19 and Clinical Outcomes in Brazil: A Test-Negative Study Design. medRxiv 2021, 2010–2021. [Google Scholar] [CrossRef]
- Vadrevu, K.M.; Ganneru, B.; Reddy, S.; Jogdand, H.; Raju, D.; Sapkal, G.; Yadav, P.; Reddy, P.; Verma, S.; Singh, C.; et al. Persistence of Immunity and Impact of Third Dose of Inactivated COVID-19 Vaccine against Emerging Variants. Sci. Rep. 2022, 12, 12038. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Setayeshgar, S.; Zou, M.; Prystajecky, N.; Tyson, J.R.; Galanis, E.; Naus, M.; Patrick, D.M.; Sbihi, H.; El Adam, S.; et al. Single-Dose MRNA Vaccine Effectiveness Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Including Alpha and Gamma Variants: A Test-Negative Design in Adults 70 Years and Older in British Columbia, Canada. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 74, 1158–1165. [Google Scholar] [CrossRef]
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Hoffmann, M.; Arora, P.; Groß, R.; Seidel, A.; Hörnich, B.F.; Hahn, A.S.; Krüger, N.; Graichen, L.; Hofmann-Winkler, H.; Kempf, A.; et al. SARS-CoV-2 Variants B.1.351 and P.1 Escape from Neutralizing Antibodies. Cell 2021, 184, 2384–2393.e12. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 Variants Escape Neutralization by Vaccine-Induced Humoral Immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J. Reduced Sensitivity of Infectious SARS-CoV-2 Variant B. 1.617. 2 to Monoclonal Antibodies and Sera from Convalescent and Vaccinated Individuals. bioRxiv 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Nikolich-Žugich, J. The Twilight of Immunity: Emerging Concepts in Aging of the Immune System. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef]
- Alonso-Fernández, P.; De la Fuente, M. Role of the Immune System in Aging and Longevity. Curr. Aging Sci. 2011, 4, 78–100. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of High-Dose versus Standard-Dose Influenza Vaccine in Older Adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- Turley, J.L.; Lavelle, E.C. Resolving Adjuvant Mode of Action to Enhance Vaccine Efficacy. Curr. Opin. Immunol. 2022, 77, 102229. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B.; Paganelli, R. Aging, Obesity, and Inflammatory Age-Related Diseases. Front. Immunol. 2017, 8, 1745. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Fuentes, M.; Alarcón, M.; Palomo, I. Immune System Dysfunction in the Elderly. An. Acad. Bras. Cienc. 2017, 89, 285–299. [Google Scholar] [CrossRef]
- Pera, A.; Campos, C.; López, N.; Hassouneh, F.; Alonso, C.; Tarazona, R.; Solana, R. Immunosenescence: Implications for Response to Infection and Vaccination in Older People. Maturitas 2015, 82, 50–55. [Google Scholar] [CrossRef]
- Dube, S.; Errazuriz, I.; Cobelli, C.; Basu, R.; Basu, A. Assessment of Insulin Action on Carbohydrate Metabolism: Physiological and Non-Physiological Methods. Diabet. Med. 2013, 30, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Leask, J.; Wolff, B.; Hickler, B.; Balaban, V.; Hosein, E.; Habersaat, K. The WHO Tailoring Immunization Programmes (TIP) Approach: Review of Implementation to Date. Vaccine 2018, 36, 1509–1515. [Google Scholar] [CrossRef]
- Rappuoli, R.; Mandl, C.W.; Black, S.; De Gregorio, E. Vaccines for the Twenty-First Century Society. Nat. Rev. Immunol. 2011, 11, 865–872. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Maternal Immunization and Antenatal Care Situation Analysis Report of the MIACSA Project 2016–2019. Available online: https://www.who.int/publications/i/item/9789240004016 (accessed on 5 May 2025).
- Englund, J.A. The Influence of Maternal Immunization on Infant Immune Responses. J. Comp. Pathol. 2007, 137 (Suppl. 1), S16–S19. [Google Scholar] [CrossRef]
- Thwaites, C.L.; Beeching, N.J.; Newton, C.R. Maternal and Neonatal Tetanus. Lancet 2015, 385, 362–370. [Google Scholar] [CrossRef]
- World Health Organization. Maternal and Neonatal Tetanus Elimination. Available online: https://www.who.int/initiatives/maternal-and-neonatal-tetanus-elimination-(mnte) (accessed on 25 May 2025).
- Wang, X.; Kulkarni, D.; Dozier, M.; Hartnup, K.; Paget, J.; Campbell, H.; Nair, H. Influenza Vaccination Strategies for 2020-21 in the Context of COVID-19. J. Glob. Health 2020, 10, 21102. [Google Scholar] [CrossRef]
- Blanchard-Rohner, G.; Eberhardt, C. Review of Maternal Immunisation during Pregnancy: Focus on Pertussis and Influenza. Swiss Med. Wkly. 2017, 147, w14526. [Google Scholar] [CrossRef]
- Sawyer, M.; Liang, J.L.; Messonnier, N.; Clark, T.A. Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccine (Tdap) in Pregnant Women—Advisory Committee on Immunization Practices (ACIP), 2012. Morb. Mortal. Wkly. Rep. 2013, 62, 131. [Google Scholar]
- Swamy, G.K.; Heine, R.P. Vaccinations for Pregnant Women. Obstet. Gynecol. 2015, 125, 212–226. [Google Scholar] [CrossRef]
- Gall, S.A.; Myers, J.; Pichichero, M. Maternal Immunization with Tetanus-Diphtheria-Pertussis Vaccine: Effect on Maternal and Neonatal Serum Antibody Levels. Am. J. Obstet. Gynecol. 2011, 204, 334.e1–334.e5. [Google Scholar] [CrossRef] [PubMed]
- Donegan, K.; King, B.; Bryan, P. Safety of Pertussis Vaccination in Pregnant Women in UK: Observational Study. BMJ 2014, 349, g4219. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, G. Strategies to Control Pertussis in Infants. Arch. Dis. Child. 2013, 98, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, G.; Andrews, N.; Campbell, H.; Ribeiro, S.; Kara, E.; Donegan, K.; Fry, N.K.; Miller, E.; Ramsay, M. Effectiveness of maternal pertussis vaccination in England: An observational study. Lancet 2014, 384, 1521–1528. [Google Scholar] [CrossRef]
- Munoz, F.M.; Bond, N.H.; Maccato, M.; Pinell, P.; Hammill, H.A.; Swamy, G.K.; Walter, E.B.; Jackson, L.A.; Englund, J.A.; Edwards, M.S.; et al. Safety and Immunogenicity of Tetanus Diphtheria and Acellular Pertussis (Tdap) Immunization during Pregnancy in Mothers and Infants: A Randomized Clinical Trial. JAMA 2014, 311, 1760–1769. [Google Scholar] [CrossRef]
- Mast, E.E.; Weinbaum, C.M.; Fiore, A.E.; Alter, M.J.; Bell, B.P.; Finelli, L.; Rodewald, L.E.; Douglas, J.M.J.; Janssen, R.S.; Ward, J.W. A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of Adults. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2006, 55, 1–33. [Google Scholar]
- O’Dempsey, T.J.; McArdle, T.; Ceesay, S.J.; Secka, O.; Demba, E.; Banya, W.A.; Francis, N.; Greenwood, B.M. Meningococcal Antibody Titres in Infants of Women Immunised with Meningococcal Polysaccharide Vaccine during Pregnancy. Arch. Dis. Child. Fetal Neonatal Ed. 1996, 74, F43–F46. [Google Scholar] [CrossRef]
- Makris, M.C.; Polyzos, K.A.; Mavros, M.N.; Athanasiou, S.; Rafailidis, P.I.; Falagas, M.E. Safety of Hepatitis B, Pneumococcal Polysaccharide and Meningococcal Polysaccharide Vaccines in Pregnancy: A Systematic Review. Drug Saf. 2012, 35, 1–14. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19), Vaccination and Pregnancy: Guidance and FAQs; Royal College of Obstetricians and Gynaecologists: London, UK, 2020; Available online: https://www.rcog.org.uk/guidance/coronavirus-covid-19-pregnancy-and-women-s-health/ (accessed on 1 June 2025).
- Verani, J.R.; McGee, L.; Schrag, S.J. Prevention of Perinatal Group B Streptococcal Disease--Revised Guidelines from CDC, 2010. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2010, 59, 1–36. [Google Scholar]
- Jordan, H.T.; Farley, M.M.; Craig, A.; Mohle-Boetani, J.; Harrison, L.H.; Petit, S.; Lynfield, R.; Thomas, A.; Zansky, S.; Gershman, K.; et al. Revisiting the Need for Vaccine Prevention of Late-Onset Neonatal Group B Streptococcal Disease: A Multistate, Population-Based Analysis. Pediatr. Infect. Dis. J. 2008, 27, 1057–1064. [Google Scholar] [CrossRef]
- Pfizer. Trial to Evaluate the Safety, Tolerability, and Immunogenicity of a Multivalent Group B Streptococcus Vaccine in Healthy Nonpregnant Women and Pregnant Women and Their Infants. 2023. Available online: https://clinicaltrials.gov/study/NCT03765073?term=NCT03765073&rank=1 (accessed on 1 June 2025).
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Pérez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef]
- Kroger, A.T.; Duchin, J.S.; Vázquez, M. General Best Practice Guidelines for Immunization: Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). 1917. Available online: https://health.hawaii.gov/docd/files/2019/08/HAR11-157_EXHIBIT_B.pdf (accessed on 1 June 2025).
- Domnich, A.; Orsi, A.; Trombetta, C.-S.; Guarona, G.; Panatto, D.; Icardi, G. COVID-19 and Seasonal Influenza Vaccination: Cross-Protection, Co-Administration, Combination Vaccines, and Hesitancy. Pharmaceuticals 2022, 15, 322. [Google Scholar] [CrossRef]
- Aaby, P.; Andersen, A.; Ravn, H.; Zaman, K. Co-Administration of BCG and Diphtheria-Tetanus-Pertussis (DTP) Vaccinations May Reduce Infant Mortality More Than the WHO-Schedule of BCG First and Then DTP. A Re-Analysis of Demographic Surveillance Data From Rural Bangladesh. EBioMedicine 2017, 22, 173–180. [Google Scholar] [CrossRef]
- Hirve, S.; Bavdekar, A.; Juvekar, S.; Benn, C.S.; Nielsen, J.; Aaby, P. Non-Specific and Sex-Differential Effects of Vaccinations on Child Survival in Rural Western India. Vaccine 2012, 30, 7300–7308. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Nielsen, J.; Benn, C.S.; Trape, J.-F. Sex-Differential and Non-Specific Effects of Routine Vaccinations in a Rural Area with Low Vaccination Coverage: An Observational Study from Senegal. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.J.; Larsen, N.; Biering-Sørensen, S.; Andersen, A.; Eriksen, H.B.; Monteiro, I.; Hougaard, D.; Aaby, P.; Netea, M.G.; Flanagan, K.L.; et al. Heterologous Immunological Effects of Early BCG Vaccination in Low-Birth-Weight Infants in Guinea-Bissau: A Randomized-Controlled Trial. J. Infect. Dis. 2015, 211, 956–967. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin Induces NOD2-Dependent Nonspecific Protection from Reinfection via Epigenetic Reprogramming of Monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Vesikari, T.; Karvonen, A.; Bianco, V.; Van der Wielen, M.; Miller, J. Tetravalent Meningococcal Serogroups A, C, W-135 and Y Conjugate Vaccine Is Well Tolerated and Immunogenic When Co-Administered with Measles-Mumps-Rubella-Varicella Vaccine during the Second Year of Life: An Open, Randomized Controlled Trial. Vaccine 2011, 29, 4274–4284. [Google Scholar] [CrossRef]
- Klein, N.P.; Shepard, J.; Bedell, L.; Odrljin, T.; Dull, P. Immunogenicity and Safety of a Quadrivalent Meningococcal Conjugate Vaccine Administered Concomitantly with Measles, Mumps, Rubella, Varicella Vaccine in Healthy Toddlers. Vaccine 2012, 30, 3929–3936. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Nissen, M.D.; Naz, A.; Shepard, J.; Bedell, L.; Hohenboken, M.; Odrljin, T.; Dull, P.M. Immunogenicity and Safety of a CRM-Conjugated Meningococcal ACWY Vaccine Administered Concomitantly with Routine Vaccines Starting at 2 Months of Age. Hum. Vaccin. Immunother. 2014, 10, 280–289. [Google Scholar] [CrossRef]
- Scott, L.J. Measles-Mumps-Rubella-Varicella Combination Vaccine (ProQuad): A Guide to Its Use in Children in the E.U. Paediatr. Drugs 2015, 17, 167–174. [Google Scholar] [CrossRef]
- Durando, P.; Esposito, S.; Bona, G.; Cuccia, M.; Desole, M.G.; Ferrera, G.; Gabutti, G.; Pellegrino, A.; Salvini, F.; Henry, O.; et al. The Immunogenicity and Safety of a Tetravalent Measles-Mumps-Rubella-Varicella Vaccine When Co-Administered with Conjugated Meningococcal C Vaccine to Healthy Children: A Phase IIIb, Randomized, Multi-Center Study in Italy. Vaccine 2016, 34, 4278–4284. [Google Scholar] [CrossRef] [PubMed]
- Aplasca-De Los Reyes, M.R.; Dimaano, E.; Macalalad, N.; Dbaibo, G.; Bianco, V.; Baine, Y.; Miller, J. The Investigational Meningococcal Serogroups A, C, W-135, Y Tetanus Toxoid Conjugate Vaccine (ACWY-TT) and the Seasonal Influenza Virus Vaccine Are Immunogenic and Well-Tolerated When Co-Administered in Adults. Hum. Vaccin. Immunother. 2012, 8, 881–887. [Google Scholar] [CrossRef]
- Østergaard, L.; Silfverdal, S.-A.; Berglund, J.; Flodmark, C.-E.; West, C.; Bianco, V.; Baine, Y.; Miller, J.M. A Tetravalent Meningococcal Serogroups A, C, W-135, and Y Tetanus Toxoid Conjugate Vaccine Is Immunogenic and Well-Tolerated When Co-Administered with Twinrix(®) in Subjects Aged 11-17 Years: An Open, Randomised, Controlled Trial. Vaccine 2012, 30, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Knuf, M.; Pantazi-Chatzikonstantinou, A.; Pfletschinger, U.; Tichmann-Schumann, I.; Maurer, H.; Maurer, L.; Fischbach, T.; Zinke, H.; Pankow-Culot, H.; Papaevangelou, V.; et al. An Investigational Tetravalent Meningococcal Serogroups A, C, W-135 and Y-Tetanus Toxoid Conjugate Vaccine Co-Administered with InfanrixTM Hexa Is Immunogenic, with an Acceptable Safety Profile in 12-23-Month-Old Children. Vaccine 2011, 29, 4264–4273. [Google Scholar] [CrossRef]
- Skibinski, D.A.G.; Baudner, B.C.; Singh, M.; T O’Hagan, D. Combination Vaccines. J. Glob. Infect. Dis. 2011, 3, 63. [Google Scholar] [CrossRef]
- Fletcher, M.A.; Fabre, P.; Debois, H.; Saliou, P. Vaccines Administered Simultaneously: Directions for New Combination Vaccines Based on an Historical Review of the Literature. Int. J. Infect. Dis. 2004, 8, 328–338. [Google Scholar] [CrossRef]
- Dodd, D. Benefits of Combination Vaccines: Effective Vaccination on a Simplified Schedule. Am. J. Manag. Care 2003, 9 (Suppl. 1), S6–S12. [Google Scholar]
- Gilchrist, S.A.N.; Nanni, A.; Levine, O. Benefits and Effectiveness of Administering Pneumococcal Polysaccharide Vaccine with Seasonal Influenza Vaccine: An Approach for Policymakers. Am. J. Public Health 2012, 102, 596–605. [Google Scholar] [CrossRef]
- Bonanni, P.; Boccalini, S.; Bechini, A.; Varone, O.; Matteo, G.; Sandri, F.; Gabutti, G. Co-Administration of Vaccines: A Focus on Tetravalent Measles-Mumps-Rubella-Varicella (MMRV) and Meningococcal C Conjugate Vaccines. Hum. Vaccin. Immunother. 2020, 16, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Saadi, N.; Chi, Y.; Ghosh, S.; Eggo, R.M.; McCarthy, C.V.; Quaife, M.; Dawa, J.; Jit, M.; Vassall, A. Models of COVID-19 Vaccine Prioritisation: A Systematic Literature Search and Narrative Review. BMC Med. 2021, 19, 318. [Google Scholar] [CrossRef] [PubMed]
- Morales, K.F.; Brown, D.W.; Dumolard, L.; Steulet, C.; Vilajeliu, A.; Ropero Alvarez, A.M.; Moen, A.; Friede, M.; Lambach, P. Seasonal Influenza Vaccination Policies in the 194 WHO Member States: The Evolution of Global Influenza Pandemic Preparedness and the Challenge of Sustaining Equitable Vaccine Access. Vaccine X 2021, 8, 100097. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coadministration of Seasonal Inactivated Influenza and COVID-19 Vaccines. 2023. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-coadministration-influenza-vaccines (accessed on 14 April 2023).
- European Centre for Disease Prevention and Control. Interim Public Health Considerations for COVID-19 Vaccination. Available online: https://www.ecdc.europa.eu/en/publications-data/interim-public-health-considerations-covid-19-vaccination-roll-out-during-2023 (accessed on 14 April 2025).
- Amato, M.; Werba, J.P.; Frigerio, B.; Coggi, D.; Sansaro, D.; Ravani, A.; Ferrante, P.; Veglia, F.; Tremoli, E.; Baldassarre, D. Relationship between Influenza Vaccination Coverage Rate and COVID-19 Outbreak: An Italian Ecological Study. Vaccines 2020, 8, 535. [Google Scholar] [CrossRef]
- Marín-Hernández, D.; Schwartz, R.E.; Nixon, D.F. Epidemiological Evidence for Association between Higher Influenza Vaccine Uptake in the Elderly and Lower COVID-19 Deaths in Italy. J. Med. Virol. 2021, 93, 64–65. [Google Scholar] [CrossRef]
- Domnich, A.; Orsi, A.; Sticchi, L.; Panatto, D.; Dini, G.; Ferrari, A.; Ogliastro, M.; Boccotti, S.; De Pace, V.; Ricucci, V.; et al. Effect of the 2020/21 Season Influenza Vaccine on SARS-CoV-2 Infection in a Cohort of Italian Healthcare Workers. Vaccine 2022, 40, 1755–1760. [Google Scholar] [CrossRef]
- Conlon, A.; Ashur, C.; Washer, L.; Eagle, K.A.; Hofmann Bowman, M.A. Impact of the Influenza Vaccine on COVID-19 Infection Rates and Severity. Am. J. Infect. Control 2021, 49, 694–700. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Islam, N.; Dambha-Miller, H. Association between Influenza Vaccination and Hospitalisation or All-Cause Mortality in People with COVID-19: A Retrospective Cohort Study. BMJ Open Respir. Res. 2021, 8, e000857. [Google Scholar] [CrossRef]
- Martínez-Baz, I.; Trobajo-Sanmartín, C.; Arregui, I.; Navascués, A.; Adelantado, M.; Indurain, J.; Fresán, U.; Ezpeleta, C.; Castilla, J. Influenza Vaccination and Risk of SARS-CoV-2 Infection in a Cohort of Health Workers. Vaccines 2020, 8, 611. [Google Scholar] [CrossRef]
- Pedote, P.D.; Termite, S.; Gigliobianco, A.; Lopalco, P.L.; Bianchi, F.P. Influenza Vaccination and Health Outcomes in COVID-19 Patients: A Retrospective Cohort Study. Vaccines 2021, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, M.; Liu, J. The Association between Influenza Vaccination and COVID-19 and Its Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Vaccines 2021, 9, 529. [Google Scholar] [CrossRef]
- Izikson, R.; Brune, D.; Bolduc, J.-S.; Bourron, P.; Fournier, M.; Moore, T.M.; Pandey, A.; Perez, L.; Sater, N.; Shrestha, A.; et al. Safety and Immunogenicity of a High-Dose Quadrivalent Influenza Vaccine Administered Concomitantly with a Third Dose of the MRNA-1273 SARS-CoV-2 Vaccine in Adults Aged ≥65 Years: A Phase 2, Randomised, Open-Label Study. Lancet Respir. Med. 2022, 10, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Pambudi, N.A.; Sarifudin, A.; Gandidi, I.M.; Romadhon, R. Vaccine Cold Chain Management and Cold Storage Technology to Address the Challenges of Vaccination Programs. Energy Rep. 2022, 8, 955–972. [Google Scholar] [CrossRef]
- United Nations Environmental Programme. Why Optimized Cold-Chains Could Save a Billion COVID Vaccines. 2020. Available online: https://www.unep.org/news-and-stories/story/why-optimized-cold-chains-could-save-billion-covid-vaccines (accessed on 20 April 2023).
- Zipursky, S.; Boualam, L.; Cheikh, D.O.; Fournier-Caruana, J.; Hamid, D.; Janssen, M.; Kartoglu, U.; Waeterloos, G.; Ronveaux, O. Assessing the Potency of Oral Polio Vaccine Kept Outside of the Cold Chain during a National Immunization Campaign in Chad. Vaccine 2011, 29, 5652–5656. [Google Scholar] [CrossRef]
- Patterson, J.; Kagina, B.M.; Gold, M.; Hussey, G.D.; Muloiwa, R. Comparison of Adverse Events Following Immunisation with Acellular and Whole-Cell Pertussis Vaccines: A Systematic Review. Vaccine 2018, 36, 6007–6016. [Google Scholar] [CrossRef]
- Larson, H.J.; Wilson, R.; Hanley, S.; Parys, A.; Paterson, P. Tracking the Global Spread of Vaccine Sentiments: The Global Response to Japan’s Suspension of Its HPV Vaccine Recommendation. Hum. Vaccin. Immunother. 2014, 10, 2543–2550. [Google Scholar] [CrossRef]
- Opel, D.J.; Heritage, J.; Taylor, J.A.; Mangione-Smith, R.; Salas, H.S.; Devere, V.; Zhou, C.; Robinson, J.D. The Architecture of Provider-Parent Vaccine Discussions at Health Supervision Visits. Pediatrics 2013, 132, 1037–1046. [Google Scholar] [CrossRef]
- Patel, A.R.; Nowalk, M.P. Expanding Immunization Coverage in Rural India: A Review of Evidence for the Role of Community Health Workers. Vaccine 2010, 28, 604–613. [Google Scholar] [CrossRef]
- Leask, J.; Kinnersley, P.; Jackson, C.; Cheater, F.; Bedford, H.; Rowles, G. Communicating with Parents about Vaccination: A Framework for Health Professionals. BMC Pediatr. 2012, 12, 154. [Google Scholar] [CrossRef]
- Basak, S.; Das, T.K. Liposome-Based Drug Delivery Systems: From Laboratory Research to Industrial Production—Instruments and Challenges. ChemEngineering 2025, 9, 56. [Google Scholar] [CrossRef]
- Moon, J.J.; Suh, H.; Bershteyn, A.; Stephan, M.T.; Liu, H.; Huang, B.; Sohail, M.; Luo, S.; Um, S.H.; Khant, H.; et al. Interbilayer-Crosslinked Multilamellar Vesicles as Synthetic Vaccines for Potent Humoral and Cellular Immune Responses. Nat. Mater. 2011, 10, 243–251. [Google Scholar] [CrossRef]
- Li, W.; Meng, J.; Ma, X.; Lin, J.; Lu, X. Advanced Materials for the Delivery of Vaccines for Infectious Diseases. Biosaf. Health 2022, 4, 95–104. [Google Scholar] [CrossRef]
- Pulendran, B.; SArunachalam, P.; O’Hagan, D.T. Emerging Concepts in the Science of Vaccine Adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, Y.; Cen, X.; Nandakumar, K.S.; Liu, S.; Cheng, K. Targeting Pattern-Recognition Receptors to Discover New Small Molecule Immune Modulators. Eur. J. Med. Chem. 2018, 144, 82–92. [Google Scholar] [CrossRef] [PubMed]
- den Haan, J.M.M.; Arens, R.; van Zelm, M.C. The Activation of the Adaptive Immune System: Cross-Talk between Antigen-Presenting Cells, T Cells and B Cells. Immunol. Lett. 2014, 162 Pt B, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, N.; Shang, Y.; Liu, X.; Wu, T.; Zhou, Y.; Liu, X.; Huang, J.; Liao, X.; Wu, L. The Novel Toll-Like Receptor 2 Agonist SUP3 Enhances Antigen Presentation and T Cell Activation by Dendritic Cells. Front. Immunol. 2017, 8, 158. [Google Scholar] [CrossRef]
- Bankoti, R.; Gupta, K.; Levchenko, A.; Stäger, S. Marginal Zone B Cells Regulate Antigen-Specific T Cell Responses during Infection. J. Immunol. 2012, 188, 3961–3971. [Google Scholar] [CrossRef]
- Weilhammer, D.; Dunkle, A.D.; Blanchette, C.D.; Fischer, N.O.; Corzett, M.; Lehmann, D.; Boone, T.; Hoeprich, P.; Driks, A.; Rasley, A. Enhancement of Antigen-Specific CD4(+) and CD8(+) T Cell Responses Using a Self-Assembled Biologic Nanolipoprotein Particle Vaccine. Vaccine 2017, 35, 1475–1481. [Google Scholar] [CrossRef]
- Taylor, J.J.; Jenkins, M.K.; Pape, K.A. Heterogeneity in the Differentiation and Function of Memory B Cells. Trends Immunol. 2012, 33, 590–597. [Google Scholar] [CrossRef]
- Feng, X.; Xu, W.; Li, Z.; Song, W.; Ding, J.; Chen, X. Immunomodulatory Nanosystems. Adv. Sci. 2019, 6, 1900101. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Sambi, M.; Bagheri, L.; Szewczuk, M.R. Current Challenges in Cancer Immunotherapy: Multimodal Approaches to Improve Efficacy and Patient Response Rates. J. Oncol. 2019, 2019, 4508794. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-World Effectiveness of COVID-19 Vaccines: A Literature Review and Meta-Analysis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Anderegg, N.; Althaus, C.L.; Colin, S.; Hauser, A.; Laube, A.; Mäusezahl, M.; Wagner, M.; Zaffora, B.; Riou, J. Assessing Real-World Vaccine Effectiveness against Severe Forms of SARS-CoV-2 Infection: An Observational Study from Routine Surveillance Data in Switzerland. Swiss Med. Wkly. 2022, 152, w30163. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). How Flu Vaccine Effectiveness and Efficacy Are Measured: Questions & Answers; CDC: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/flu/professionals/vaccination (accessed on 1 June 2025).
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 MRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Moore, S.; Hill, E.M.; Tildesley, M.J.; Dyson, L.; Keeling, M.J. Vaccination and Non-Pharmaceutical Interventions for COVID-19: A Mathematical Modelling Study. Lancet Infect. Dis. 2021, 21, 793–802. [Google Scholar] [CrossRef]
- Teerawattananon, Y.; Anothaisintawee, T.; Pheerapanyawaranun, C.; Botwright, S.; Akksilp, K.; Sirichumroonwit, N.; Budtarad, N.; Isaranuwatchai, W. A Systematic Review of Methodological Approaches for Evaluating Real-World Effectiveness of COVID-19 Vaccines: Advising Resource-Constrained Settings. PLoS ONE 2022, 17, e0261930. [Google Scholar] [CrossRef]
- Blonde, L.; Khunti, K.; Harris, S.B.; Meizinger, C.; Skolnik, N.S. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv. Ther. 2018, 35, 1763–1774. [Google Scholar] [CrossRef]
- Rapaka, R.R.; Hammershaimb, E.A.; Neuzil, K.M. Are Some COVID-19 Vaccines Better Than Others? Interpreting and Comparing Estimates of Efficacy in Vaccine Trials. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 74, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Loong, D.; Pham, B.; Amiri, M.; Saunders, H.; Mishra, S.; Radhakrishnan, A.; Rodrigues, M.; Yeung, M.W.; Muller, M.P.; Straus, S.E.; et al. Systematic Review on the Cost-Effectiveness of Seasonal Influenza Vaccines in Older Adults. Value Health J. Int. Soc. Pharmacoecon. Outcomes Res. 2022, 25, 1439–1458. [Google Scholar] [CrossRef] [PubMed]
- Mamdani, M.; Sykora, K.; Li, P.; Normand, S.-L.T.; Streiner, D.L.; Austin, P.C.; Rochon, P.A.; Anderson, G.M. Reader’s Guide to Critical Appraisal of Cohort Studies: 2. Assessing Potential for Confounding. BMJ 2005, 330, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Rochon, P.A.; Gurwitz, J.H.; Sykora, K.; Mamdani, M.; Streiner, D.L.; Garfinkel, S.; Normand, S.-L.T.; Anderson, G.M. Reader’s Guide to Critical Appraisal of Cohort Studies: 1. Role and Design. BMJ 2005, 330, 895–897. [Google Scholar] [CrossRef]
- Gupta, K.; O’Brien, W.J.; Bellino, P.; Linsenmeyer, K.; Doshi, S.J.; Sprague, R.S.; Charness, M.E. Incidence of SARS-CoV-2 Infection in Health Care Workers After a Single Dose of MRNA-1273 Vaccine. JAMA Netw. Open 2021, 4, e2116416. [Google Scholar] [CrossRef]
- Walsh, J.; Skally, M.; Traynor, L.; de Barra, E.; Dhuthaigh, A.N.; Hayes, B.; Fitzpatrick, F. Impact of First Dose of BNT162b2 Vaccine on COVID-19 Infection among Healthcare Workers in an Irish Hospital. Ir. J. Med. Sci. 2022, 191, 961–962. [Google Scholar] [CrossRef]
- Jaiswal, A.; Subbaraj, V.; Vivian Thangaraj, J.W.; Murhekar, M.V.; Muliyil, J. COVID-19 Vaccine Effectiveness in Preventing Deaths among High-Risk Sgroups in Tamil Nadu, India. Indian J. Med. Res. 2021, 153, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Pilishvili, T.; Fleming-Dutra, K.E.; Farrar, J.L.; Gierke, R.; Mohr, N.M.; Talan, D.A.; Krishnadasan, A.; Harland, K.K.; Smithline, H.A.; Hou, P.C.; et al. Interim Estimates of Vaccine Effectiveness of Pfizer-BioNTech and Moderna COVID-19 Vaccines Among Health Care Personnel—33 U.S. Sites, January-March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 753–758. [Google Scholar] [CrossRef]
- Doll, M.K.; Pettigrew, S.M.; Ma, J.; Verma, A. Effects of Confounding Bias in Coronavirus Disease 2019 (COVID-19) and Influenza Vaccine Effectiveness Test-Negative Designs Due to Correlated Influenza and COVID-19 Vaccination Behaviors. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 75, e564–e571. [Google Scholar] [CrossRef]
- Garvey, M.I.; Wilkinson, M.A.C.; Holden, E.; Shields, A.; Robertson, A.; Richter, A.; Ball, S. Early Observations on the Impact of a Healthcare Worker COVID-19 Vaccination Programme at a Major UK Tertiary Centre. J. Infect. 2021, 83, 119–145. [Google Scholar] [CrossRef]
- Burnett, E.; Parashar, U.D.; Tate, J.E. Real-World Effectiveness of Rotavirus Vaccines, 2006–2019: A Literature Review and Meta-Analysis. Lancet Glob. Health 2020, 8, e1195–e1202. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, A.; Ozawa, S.; Grewal, S.; Norman, B.A.; Rajgopal, J.; Gorham, K.M.; Haidari, L.A.; Brown, S.T.; Lee, B.Y. Costs of Vaccine Programs across 94 Low- and Middle-Income Countries. Vaccine 2015, 33 (Suppl. 1), A99–A108. [Google Scholar] [CrossRef] [PubMed]
- Eshun-Wilson, I.; Mody, A.; Tram, K.H.; Bradley, C.; Sheve, A.; Fox, B.; Thompson, V.; Geng, E.H. Preferences for COVID-19 Vaccine Distribution Strategies in the US: A Discrete Choice Survey. PLoS ONE 2021, 16, e0256394. [Google Scholar] [CrossRef] [PubMed]
- Guignard, A.; Praet, N.; Jusot, V.; Bakker, M.; Baril, L. Introducing New Vaccines in Low- and Middle-Income Countries: Challenges and Approaches. Expert Rev. Vaccines 2019, 18, 119–131. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- McNeil, M.M.; Gee, J.; Weintraub, E.S.; Belongia, E.A.; Lee, G.M.; Glanz, J.M.; Nordin, J.D.; Klein, N.P.; Baxter, R.; Naleway, A.L.; et al. The Vaccine Safety Datalink: Successes and Challenges Monitoring Vaccine Safety. Vaccine 2014, 32, 5390–5398. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Siliciano, R.F. In Vivo Dynamics of the Latent Reservoir for HIV-1: New Insights and Implications for Cure. Annu. Rev. Pathol. 2022, 17, 271–294. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive Immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Krause, P.R.; Fleming, T.R.; Longini, I.M.; Peto, R.; Briand, S.; Heymann, D.L.; Beral, V.; Snape, M.D.; Rees, H.; Ropero, A.-M.; et al. SARS-CoV-2 Variants and Vaccines. N. Engl. J. Med. 2021, 385, 179–186. [Google Scholar] [CrossRef]
- Wenham, C.; Katz, R.; Birungi, C.; Boden, L.; Eccleston-Turner, M.; Gostin, L.; Guinto, R.; Hellowell, M.; Onarheim, K.H.; Hutton, J.; et al. Global Health Security and Universal Health Coverage: From a Marriage of Convenience to a Strategic, Effective Partnership. BMJ Glob. Health 2019, 4, e001145. [Google Scholar] [CrossRef]
- Khalil, N.; Bernstein, D.I. Influenza Vaccines: Where We Are, Where We Are Going. Curr. Opin. Pediatr. 2022, 34, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.E.; Shay, D.K.; Broder, K.; Iskander, J.K.; Uyeki, T.M.; Mootrey, G.; Bresee, J.S.; Cox, N.J. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2009, 58, 1–52. [Google Scholar]
- Shefer, A.; Strikas, R.; Bridges, C.B. Updated Recommendations of the Advisory Committee on Immunization Practices for Healthcare Personnel Vaccination: A Necessary Foundation for the Essential Work That Remains to Build Successful Programs. Infect. Control. Hosp. Epidemiol. 2012, 33, 71–74. [Google Scholar] [CrossRef]
- Kunimasa, K.; Goto, T. Immunosurveillance and Immunoediting of Lung Cancer: Current Perspectives and Challenges. Int. J. Mol. Sci. 2020, 21, 597. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.-H.; Ambrosino, D.M.; Andersen, S.R.; Baric, R.S.; Black, S.B.; Chen, R.T.; Dekker, C.L.; Didierlaurent, A.M.; Graham, B.S.; Martin, S.D.; et al. Consensus Summary Report for CEPI/BC March 12-13, 2020 Meeting: Assessment of Risk of Disease Enhancement with COVID-19 Vaccines. Vaccine 2020, 38, 4783–4791. [Google Scholar] [CrossRef]
- Olusanya, O.A.; White, B.; Melton, C.A.; Shaban-Nejad, A. Examining the Implementation of Digital Health to Strengthen the COVID-19 Pandemic Response and Recovery and Scale up Equitable Vaccine Access in African Countries. JMIR Form. Res. 2022, 6, e34363. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Rodriguez-Besteiro, S.; Cabello-Eras, J.J.; Bustamante-Sanchez, A.; Navarro-Jiménez, E.; Donoso-Gonzalez, M.; Beltrán-Velasco, A.I.; Tornero-Aguilera, J.F. Sustainable Development Goals in the COVID-19 Pandemic: A Narrative Review. Sustainability 2022, 14, 7726. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martínez-González, M.B.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Beltran-Velasco, A.I.; Ruisoto, P.; Diaz Arroyo, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Impact of the COVID-19 Pandemic on Mental Disorders. A Critical Review. Int. J. Environ. Res. Public Health 2021, 18, 10041. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Tornero-Aguilera, J.F.; Ruisoto, P.; Mielgo-Ayuso, J. Inflammation in COVID-19 and the Effects of Non-Pharmacological Interventions during the Pandemic: A Review. Int. J. Mol. Sci. 2022, 23, 15584. [Google Scholar] [CrossRef]
- Sahar, N.; Lee, O.; Hoffman, S.J.; Sritharan, L. Overview of Key Legal, Political, and Social Challenges Facing Global Vaccination Efforts. Imaginations 2020, 11, 127–153. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Navarro-Jiménez, E.; Simón-Sanjurjo, J.A.; Beltran-Velasco, A.I.; Laborde-Cárdenas, C.C.; Benitez-Agudelo, J.C.; Bustamante-Sánchez, Á.; Tornero-Aguilera, J.F. Mis–Dis Information in COVID-19 Health Crisis: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 5321. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Neuro-Vulnerability in Energy Metabolism Regulation: A Comprehensive Narrative Review. Nutrients 2023, 15, 3106. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef] [PubMed]
| Study Location | Vaccine(s) | Variant(s) | Effectiveness | Key Finding |
|---|---|---|---|---|
| United Kingdom | BNT162b2, ChAdOx1 | Delta (B.1.617.2) | Reduced effectiveness: BNT162b2 (−13%), ChAdOx1 (−16%). Two doses are comparable to natural infection. | Effectiveness is higher in younger adults; peak viral load is significant for infection risk. |
| Southern California | Moderna (2 doses) | Delta, Alpha | Delta: 86.7%, Alpha: 98.4%. Declined over time (from 94.1% to 80%). | Effectiveness against Delta wanes over time since vaccination. |
| Brazil | Ad26.COV2.S (Janssen) | Emerging variants | Symptoms: 50.9%, Hospitalization: 72.9%, ICU: 92.5%, Death: 90.5% | A single dose showed strong protection during variant emergence. |
| India | 2 or 3 doses (type not specified) | Alpha, Beta, Delta, Omicron | The third dose is highly effective at neutralizing variants. | Antibodies declined over 12 months; a booster dose is essential. |
| Canada | mRNA (single dose) | Alpha, Gamma, other variants | 72% (other), 67% (Alpha), 61% (Gamma) | The mRNA vaccine showed only minimal reduction in protection. |
| South Africa | NVX-CoV2373 | B.1.351 (Beta) | Overall: 49.4%, HIV-negative: 60.1%, Variant-specific: 51.0% | Reduced efficacy due to spike protein mutations in the variant. |
| Vaccine Combination | Population | Outcome | Key Finding |
|---|---|---|---|
| BCG + DTP | Infants (Bangladesh, India, Senegal) | Reduced all-cause mortality | Lower mortality with co-administration; possible nonspecific immunity via BCG |
| MMRV + Meningococcal C | Children > 12 months (Europe) | Similar immunogenicity and safety | Co-administration produced a strong immune response to all strains; safe and well-tolerated |
| Meningococcal C + Seasonal Influenza | Adults (Philippines) | Adequate safety; reduced antibody titers | Safe, but lower meningococcal antibody titers suggest possible immune interference |
| Meningococcal C + Hepatitis A/B | Adolescents | Optimal safety and efficacy | Strong antibody titers up to 7 months, regardless of co-administration |
| Meningococcal C + DTaP-HepB-IPV/Hib | Pediatric population | Similar reactogenicity and immunogenicity | No reduction in immunogenicity; acceptable safety profile |
| COVID-19 + Seasonal Influenza | Adults, the elderly, and at-risk populations | Improved compliance, mixed efficacy signals | Reduced COVID-19 risk in some studies; no major safety concerns; meta-analysis supports modest protection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente-Suárez, V.J.; Redondo-Flórez, L.; Bustamante-Sánchez, A.; Martín-Rodríguez, A.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Biometric Strategies to Improve Vaccine Immunogenicity and Effectiveness. Biomimetics 2025, 10, 439. https://doi.org/10.3390/biomimetics10070439
Clemente-Suárez VJ, Redondo-Flórez L, Bustamante-Sánchez A, Martín-Rodríguez A, Yáñez-Sepúlveda R, Tornero-Aguilera JF. Biometric Strategies to Improve Vaccine Immunogenicity and Effectiveness. Biomimetics. 2025; 10(7):439. https://doi.org/10.3390/biomimetics10070439
Chicago/Turabian StyleClemente-Suárez, Vicente Javier, Laura Redondo-Flórez, Alvaro Bustamante-Sánchez, Alexandra Martín-Rodríguez, Rodrigo Yáñez-Sepúlveda, and Jose Francisco Tornero-Aguilera. 2025. "Biometric Strategies to Improve Vaccine Immunogenicity and Effectiveness" Biomimetics 10, no. 7: 439. https://doi.org/10.3390/biomimetics10070439
APA StyleClemente-Suárez, V. J., Redondo-Flórez, L., Bustamante-Sánchez, A., Martín-Rodríguez, A., Yáñez-Sepúlveda, R., & Tornero-Aguilera, J. F. (2025). Biometric Strategies to Improve Vaccine Immunogenicity and Effectiveness. Biomimetics, 10(7), 439. https://doi.org/10.3390/biomimetics10070439









