Binder Jetting 3D Printing of Biomass–Fungi Composite Materials: A Preliminary Experimental Study
Abstract
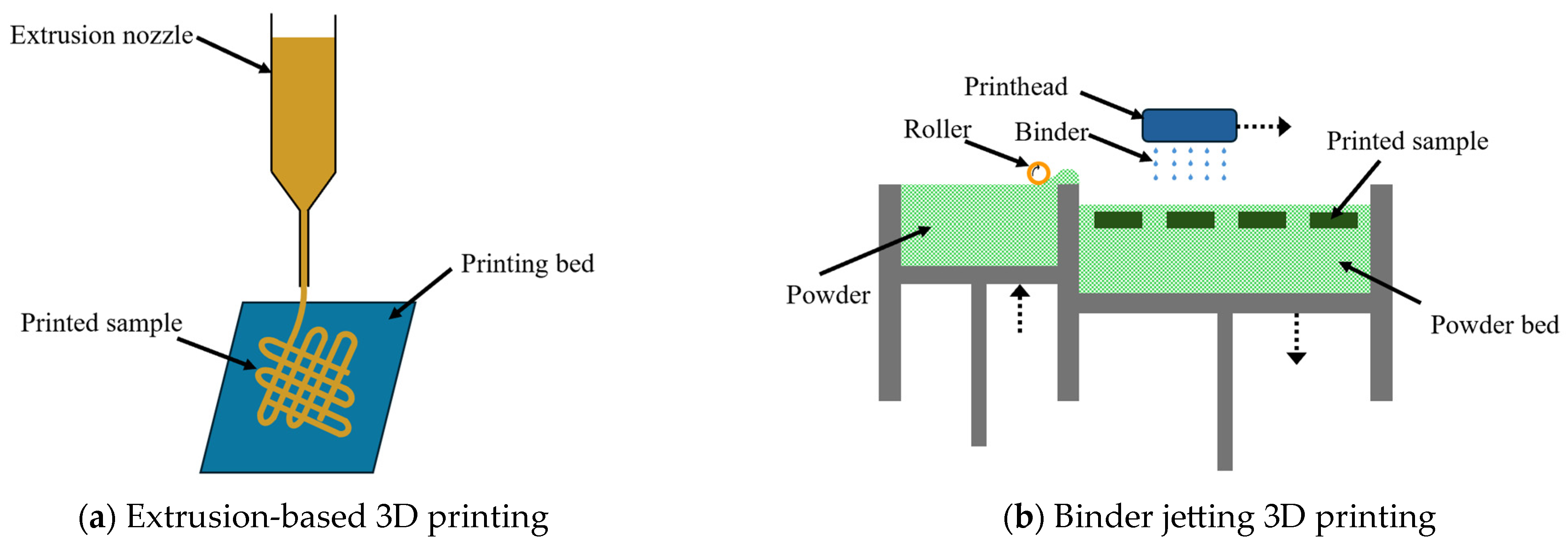
1. Introduction
| Reference | Main Contribution |
|---|---|
| [38] | Preliminary assessment of the suitability of 3D printing for biomass–fungi composite materials |
| [39] | Effects of biomass–fungi mixture composition on print quality |
| [40] | Effects of waiting time (the interval between mixture preparation and 3D printing) on mechanical and rheological properties of biomass–fungi mixtures and print quality |
| [41] | Effects of mixing and printing parameters on fungal growth |
| [42] | Effects of incorporating ionic crosslinking on the print quality and physicochemical properties of printed samples |
| [43] | Effects of sodium alginate and calcium chloride on the growth of fungi in biomass–fungi composite materials |
| [44] | Development of organic waste-based ink (containing mycelium) for printing |
| [51] | Large-scale robotic 3D printing of mycelium-based materials |
| [45] | Development of extrudable mixture (containing biomass, clay, and mycelium) for printing |
| [46] | Effects of geometrical configuration on mycelium growth in printed samples |
| [47] | Systematic review of a wide range of printable materials from different types of biomass (such as wood, timber, fruit, seeds, and herbaceous plants) |
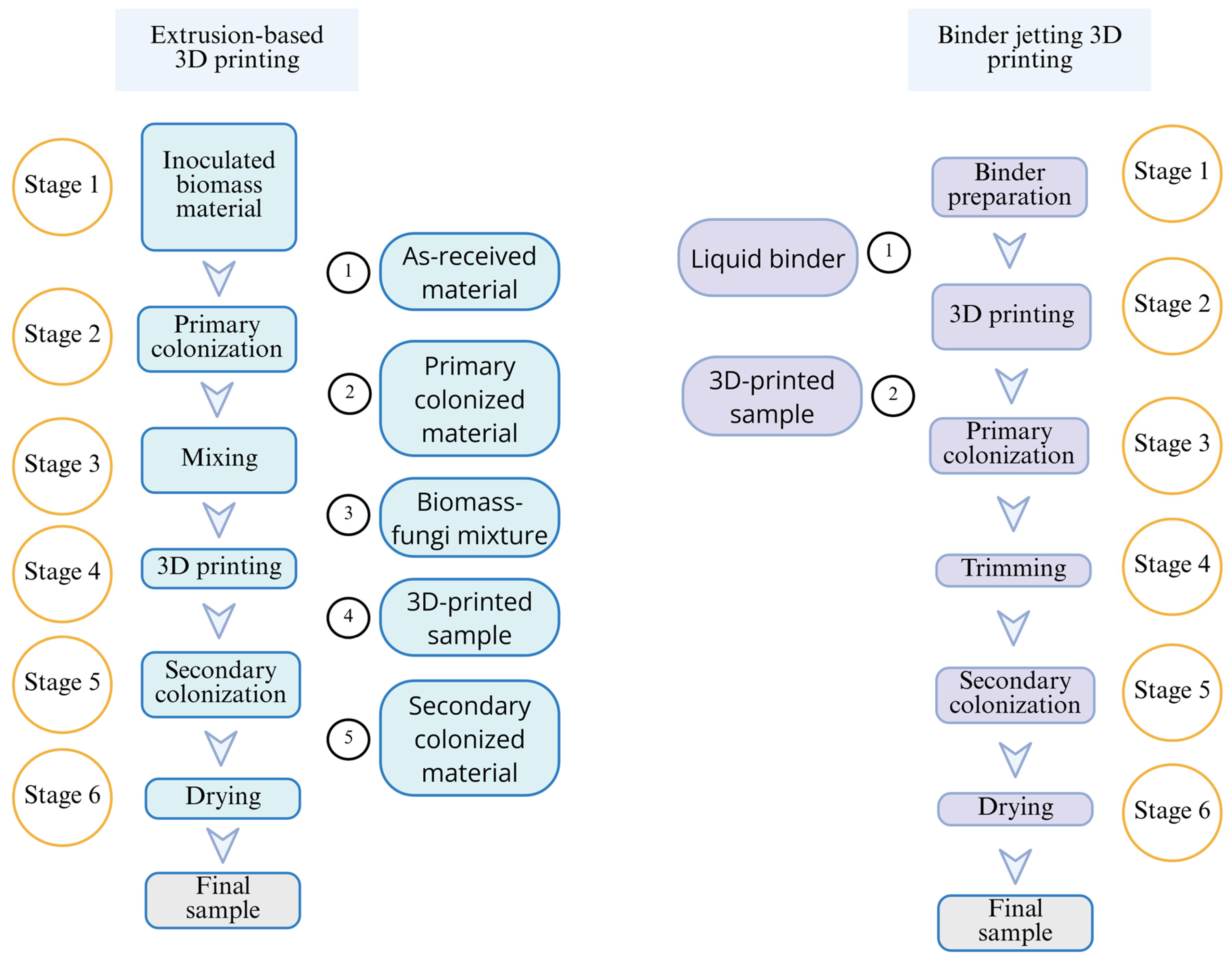
2. Materials and Methods
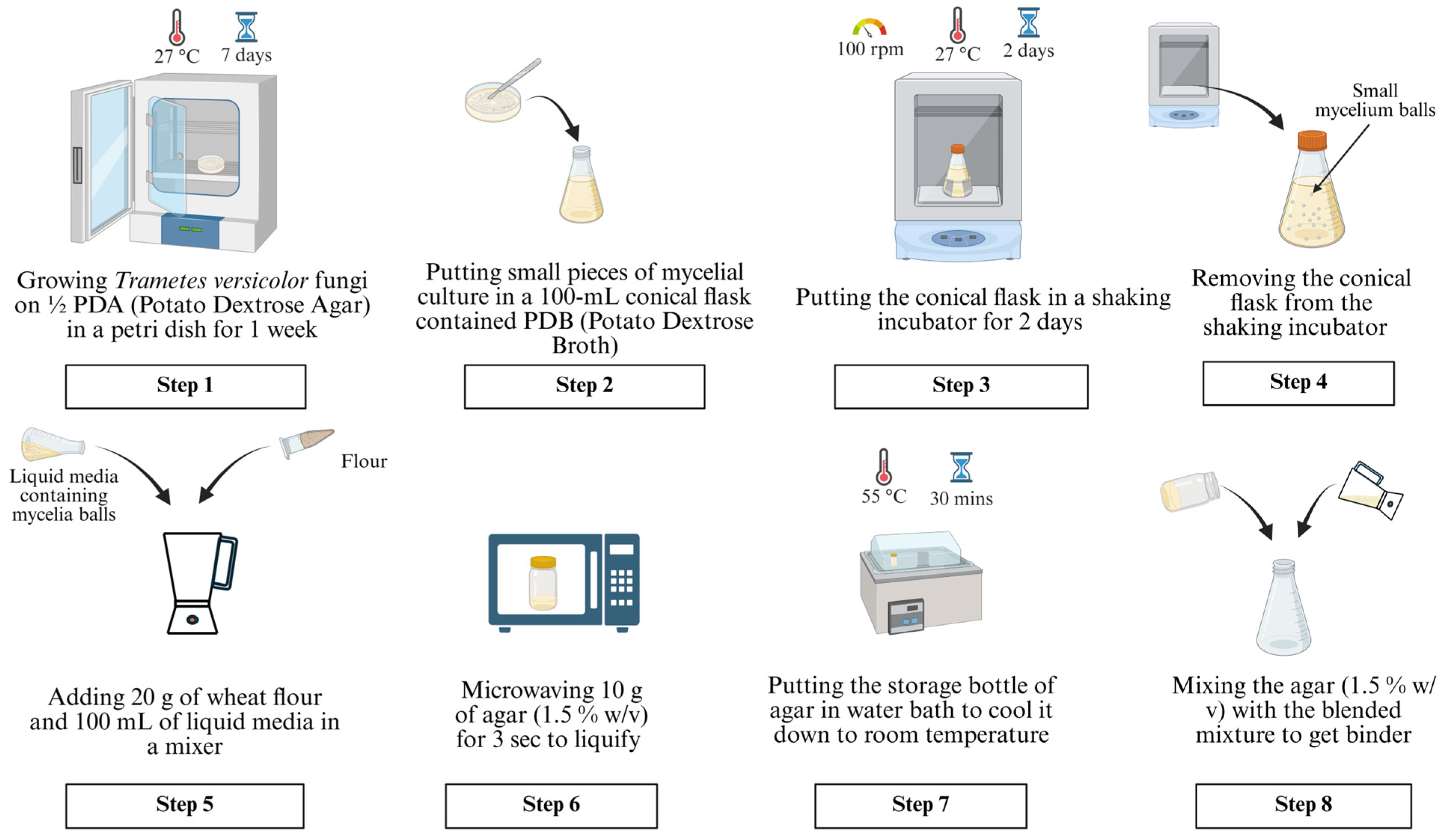
2.1. Preparation of Liquid Binder Containing Fungi Cells
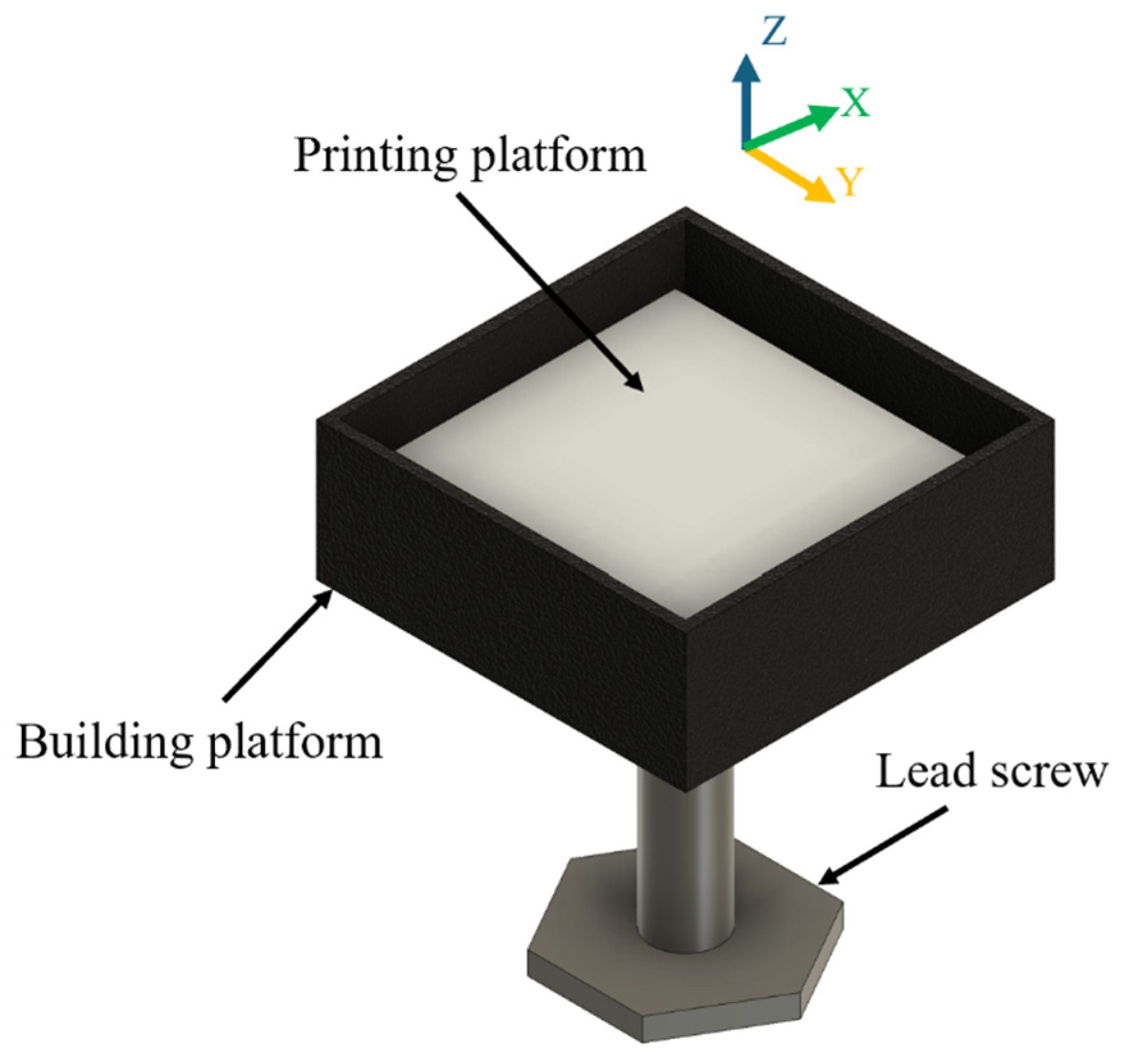
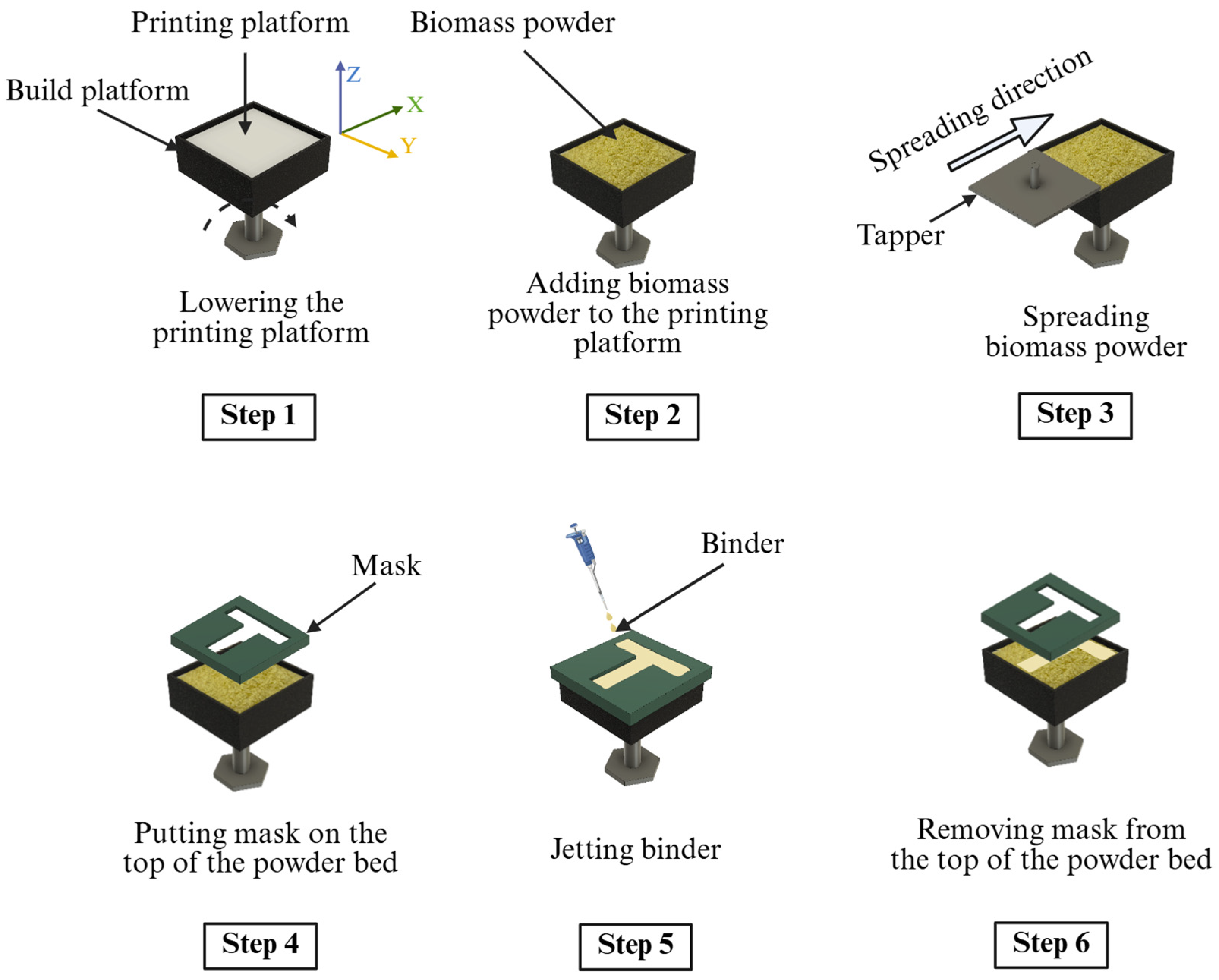
2.2. Printing of Samples Using Binder Jetting
2.3. Processing of Printed Samples to Prepare Final Samples
2.4. Taking Micrographs Using Scanning Electron Microscopy (SEM)
3. Results and Discussion
4. Conclusions and Future Research Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pilapitiya, P.N.T.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Wojnowska-Baryła, I.; Bernat, K.; Zaborowska, M. Plastic waste degradation in landfill conditions: The problem with microplastics, and their direct and indirect environmental effects. Int. J. Environ. Res. Public Health 2022, 19, 13223. [Google Scholar] [CrossRef]
- Akib, Y.M.; Bedsole, C.O.; Rahman, A.M.; Hamilton, J.; Khan, F.; Pei, Z.; Shaw, B.D.; Ufodike, C.O. A Preliminary Experimental Study on Biodegradation of 3D-Printed Samples from Biomass–Fungi Composite Materials. J. Compos. Sci. 2024, 8, 412. [Google Scholar] [CrossRef]
- Van Wylick, A.; Elsacker, E.; Yap, L.L.; Peeters, E.; De Laet, L. Mycelium composites and their biodegradability: An exploration on the disintegration of mycelium-based materials in soil. Constr. Technol. Archit. 2022, 1, 652–659. [Google Scholar] [CrossRef]
- Zimele, Z.; Irbe, I.; Grinins, J.; Bikovens, O.; Verovkins, A.; Bajare, D. Novel Mycelium-Based Biocomposites (MBB) as Building Materials. J. Renew. Mater. 2020, 8, 1067–1076. [Google Scholar] [CrossRef]
- Ly, L.; Jitjak, W. Biocomposites from agricultural wastes and mycelia of a local mushroom, Lentinus squarrosulus (Mont.) Singer. Open Agric. 2022, 7, 634–643. [Google Scholar] [CrossRef]
- Angelova, G.; Yemendzhiev, H.; Zaharieva, R.; Brazkova, M.; Koleva, R.; Stefanova, P.; Baldzhieva, R.; Vladev, V.; Krastanov, A. Mycelium-Based Composites Derived from Lignocellulosic Residual By-Products: An Insight into Their Physico-Mechanical Properties and Biodegradation Profile. Appl. Sci. 2025, 15, 6333. [Google Scholar] [CrossRef]
- Grimm, D.; Wosten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef]
- Holt, G.A.; McIntyre, G.; Flagg, D.; Bayer, E.; Wanjura, J.D.; Pelletier, M.G. Fungal Mycelium and Cotton Plant Materials in the Manufacture of Biodegradable Molded Packaging Material: Evaluation Study of Select Blends of Cotton Byproducts. J. Biobased Mater. Bioenergy 2012, 6, 431–439. [Google Scholar] [CrossRef]
- Heisel, F.; Hebel, D.E. Pioneering construction materials through prototypological research. Biomimetics 2019, 4, 56. [Google Scholar] [CrossRef]
- Abhijith, R.; Ashok, A.; Rejeesh, C. Sustainable packaging applications from mycelium to substitute polystyrene: A review. Mater. Today Proc. 2018, 5, 2139–2145. [Google Scholar] [CrossRef]
- Abrams, M. Construction materials made from ‘shrooms’. Am. Soc. Mech. Eng. 2014. [Google Scholar]
- Jiang, L.; Walczyk, D.; McIntyre, G.; Bucinell, R.; Tudryn, G. Manufacturing of biocomposite sandwich structures using mycelium-bound cores and preforms. J. Manuf. Process. 2017, 28, 50–59. [Google Scholar] [CrossRef]
- Jiang, L.; Walczyk, D.; McIntyre, G.; Bucinell, R. A new approach to manufacturing biocomposite sandwich structures: Mycelium-based cores. In Proceedings of the International Manufacturing Science and Engineering Conference, Blacksburg, VA, USA, 27 June–1 July 2016; p. V001T002A025. [Google Scholar] [CrossRef]
- Jonnala, S.N.; Gogoi, D.; Devi, S.; Kumar, M.; Kumar, C. A comprehensive study of building materials and bricks for residential construction. Constr. Build. Mater. 2024, 425, 135931. [Google Scholar] [CrossRef]
- U.S. Energy Information. Administration. Biomass Explained. Available online: https://www.eia.gov/energyexplained/biomass/ (accessed on 20 June 2024).
- Jones, M.P.; Lawrie, A.C.; Huynh, T.T.; Morrison, P.D.; Mautner, A.; Bismarck, A.; John, S. Agricultural by-product suitability for the production of chitinous composites and nanofibers utilising Trametes versicolor and Polyporus brumalis mycelial growth. Process Biochem. 2019, 80, 95–102. [Google Scholar] [CrossRef]
- Aiduang, W.; Chanthaluck, A.; Kumla, J.; Jatuwong, K.; Srinuanpan, S.; Waroonkun, T.; Oranratmanee, R.; Lumyong, S.; Suwannarach, N. Amazing Fungi for Eco-Friendly Composite Materials: A Comprehensive Review. J. Fungi 2022, 8, 842. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Sustainable Green Composites: Value Addition to Agricultural Residues and Perennial Grasses. ACS Sustain. Chem. Eng. 2013, 1, 325–333. [Google Scholar] [CrossRef]
- Sydor, M.; Cofta, G.; Doczekalska, B.; Bonenberg, A. Fungi in Mycelium-Based Composites: Usage and Recommendations. Materials 2022, 15, 6283. [Google Scholar] [CrossRef]
- Verma, N.; Eswari, J.S.; Mahapatra, C. Green sustainable biocomposites: Substitute to plastics with innovative fungal mycelium based biomaterial. J. Environ. Chem. Eng. 2023, 11, 110396. [Google Scholar] [CrossRef]
- Schmidt, B.; Freidank-Pohl, C.; Zillessen, J.; Stelzer, L.; Guitar, T.N.; Lühr, C.; Müller, H.; Zhang, F.; Hammel, J.U.; Briesen, H.; et al. Mechanical, physical and thermal properties of composite materials produced with the basidiomycete Fomes fomentarius. Fungal Biol. Biotechnol. 2023, 10, 22. [Google Scholar] [CrossRef]
- Saez, D.; Grizmann, D.; Trautz, M.; Werner, A. Exploring the Binding Capacity of Mycelium and Wood-Based Composites for Use in Construction. Biomimetics 2022, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tao, J.; Wu, Z.; Weiland, K.; Wang, Z.; Masania, K.; Wang, B. Fabrication of Living Entangled Network Composites Enabled by Mycelium. Adv. Sci. 2024, 11, 2309370. [Google Scholar] [CrossRef]
- Sun, W.; Tajvidi, M.; Howell, C.; Hunt, C.G. Functionality of Surface Mycelium Interfaces in Wood Bonding. ACS Appl. Mater. Interfaces 2020, 12, 57431–57440. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Van Wylick, A.; Ruytinx, J.; De Laet, L.; Peeters, E. A comprehensive framework for the production of mycelium-based lignocellulosic composites. Sci. Total Environ. 2020, 725, 138431. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials From Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Jones, M.; Huynh, T.; Dekiwadia, C.; Daver, F.; John, S. Mycelium composites: A review of engineering characteristics and growth kinetics. J. Bionanosci. 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Cheng, P.; Han, Z.; Chen, Y.; Ye, L. Recent progress in non-planar 3D printing of continuous fiber-reinforced composites. Compos. Part. A Appl. Sci. Manuf. 2025, 194, 108900. [Google Scholar] [CrossRef]
- Cheng, P.; Li, S.; Peng, Y.; Duigou, A.L.; Wang, K.; Ahzi, S. 3D/4D Printed Functional Continuous Fiber-reinforced Polymer Composites: Progress and Perspectives. Chin. J. Mech. Eng. Addit. Manuf. Front. 2023, 2, 100090. [Google Scholar] [CrossRef]
- Cheng, P.; Ye, Z.; Huang, Y.; Wang, D.; Peng, Y.; Wang, K.; Ahzi, S. Electrical resistance-based self-monitoring of manufacturing damage in 3D printed continuous carbon fiber reinforced composites. Compos. Commun. 2023, 43, 101749. [Google Scholar] [CrossRef]
- Mecheter, A.; Tarlochan, F. Fused Filament Fabrication Three-Dimensional Printing: Assessing the Influence of Geometric Complexity and Process Parameters on Energy and the Environment. Sustainability 2023, 15, 12319. [Google Scholar] [CrossRef]
- Fidan, I.; Naikwadi, V.; Alkunte, S.; Mishra, R.; Tantawi, K. Energy Efficiency in Additive Manufacturing: Condensed Review. Technologies 2024, 12, 21. [Google Scholar] [CrossRef]
- Tabassum, T.; Ahmad Mir, A. A review of 3d printing technology-the future of sustainable construction. Mater. Today Proc. 2023, 93, 408–414. [Google Scholar] [CrossRef]
- Muth, J.; Klunker, A.; Völlmecke, C. Putting 3D printing to good use—Additive Manufacturing and the Sustainable Development Goals. Front. Sustain. 2023, 4, 1196228. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Vasselli, J.; Lucht, M.; Pei, Z.; Shaw, B.; Grasley, Z.; Wei, X.; Zou, N. 3D Printing of Biomass-Fungi Composite Material: A Preliminary Study. Manuf. Lett. 2020, 24, 96–99. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Rahman, A.M.; Wei, X.; Pei, Z.; Truong, D.; Lucht, M.; Zou, N. 3D Printing of Biomass–Fungi Composite Material: Effects of Mixture Composition on Print Quality. J. Manuf. Mater. Process. 2021, 5, 112. [Google Scholar] [CrossRef]
- Rahman, A.M.; Bhardwaj, A.; Pei, Z.; Ufodike, C.; Castell-Perez, E. The 3D Printing of Biomass–Fungi Composites: Effects of Waiting Time after Mixture Preparation on Mechanical Properties, Rheological Properties, Minimum Extrusion Pressure, and Print Quality of the Prepared Mixture. J. Compos. Sci. 2022, 6, 237. [Google Scholar] [CrossRef]
- Rahman, A.M.; Bhardwaj, A.; Vasselli, J.G.; Pei, Z.; Shaw, B.D. Three-Dimensional Printing of Biomass–Fungi Biocomposite Materials: The Effects of Mixing and Printing Parameters on Fungal Growth. J. Manuf. Mater. Process. 2023, 8, 2. [Google Scholar] [CrossRef]
- Rahman, A.M.; Akib, Y.M.; Bedsole, C.O.; Pei, Z.; Shaw, B.D.; Ufodike, C.O.; Castell-Perez, E. Effects of Incorporating Ionic Crosslinking on 3D Printing of Biomass–Fungi Composite Materials. Biomimetics 2024, 9, 411. [Google Scholar] [CrossRef]
- Rahman, A.M.; Bedsole, C.O.; Akib, Y.M.; Hamilton, J.; Rahman, T.T.; Shaw, B.D.; Pei, Z. Effects of Sodium Alginate and Calcium Chloride on Fungal Growth and Viability in Biomass-Fungi Composite Materials Used for 3D Printing. Biomimetics 2024, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Soh, E.; Teoh, J.H.; Leong, B.; Xing, T.; Le Ferrand, H. 3D printing of mycelium engineered living materials using a waste-based ink and non-sterile conditions. Mater. Des. 2023, 236, 112481. [Google Scholar] [CrossRef]
- Ibrahim, F.; Castellano, G.; Carcassi, O.B.; Paoletti, I.M. MycoCode: Development of an Extrudable Paste for 3D Printing Mycelium-Bound Composites. In Architecture and Design for Industry 4.0: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2023; pp. 503–519. [Google Scholar]
- Gomaa, H.; Chau, W.M.; Karazi, Y.; Biala, E.; Akbar, Z.; Wortmann, T.; Ostermann, M. Influence of Geometry on Growth and Strength of 3D-Printed Mycelium Composites: A Data-Driven Study. In Design Modelling Symposium Berlin; Springer: Berlin/Heidelberg, Germany, 2024; pp. 331–345. [Google Scholar] [CrossRef]
- Romani, A.; Suriano, R.; Levi, M. Biomass waste materials through extrusion-based additive manufacturing: A systematic literature review. J. Clean. Prod. 2023, 386, 135779. [Google Scholar] [CrossRef]
- Pei, Z.; Rahman, A.M.; Shaw, B.D.; Bedsole, C.O. Three-Dimensional Printing Using Biomass–Fungi Composite Materials: Brief Retrospective and Prospective Views. Bioengineering 2024, 11, 840. [Google Scholar] [CrossRef]
- Ziaee, M.; Crane, N.B. Binder jetting: A review of process, materials, and methods. Addit. Manuf. 2019, 28, 781–801. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Elsacker, E.; Peeters, E.; De Laet, L. Large-scale robotic extrusion-based additive manufacturing with living mycelium materials. Sustain. Futures 2022, 4, 100085. [Google Scholar] [CrossRef]
- Cheny, T.; Colin, C.; Verquin, B. Experimental evaluation of binder infiltration depth and axial overlap to control properties of green parts produced by Binder Jetting. Addit. Manuf. 2024, 87, 104231. [Google Scholar] [CrossRef]
- Zhou, Z.; Mitchell, C.A.; Buchanan, F.J.; Dunne, N.J. Effects of Heat Treatment on the Mechanical and Degradation Properties of 3D-Printed Calcium-Sulphate-Based Scaffolds. Int. Sch. Res. Not. 2013, 2013, 750720. [Google Scholar] [CrossRef]
- Šnajdr, J.; Baldrian, P. Temperature affects the production, activity and stability of ligninolytic enzymes in Pleurotus ostreatus and Trametes versicolor. Folia Microbiol. 2007, 52, 498–502. [Google Scholar] [CrossRef]
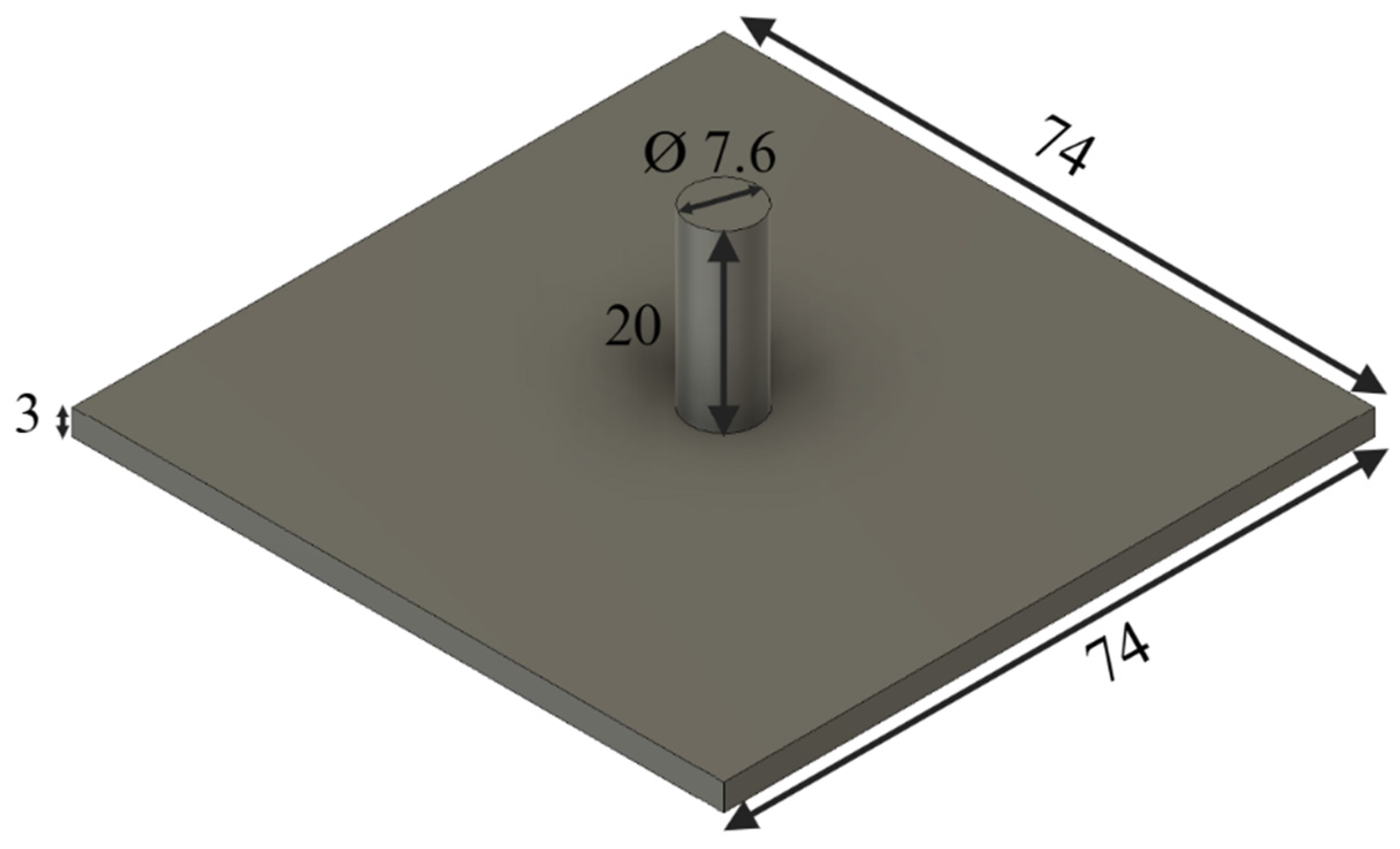
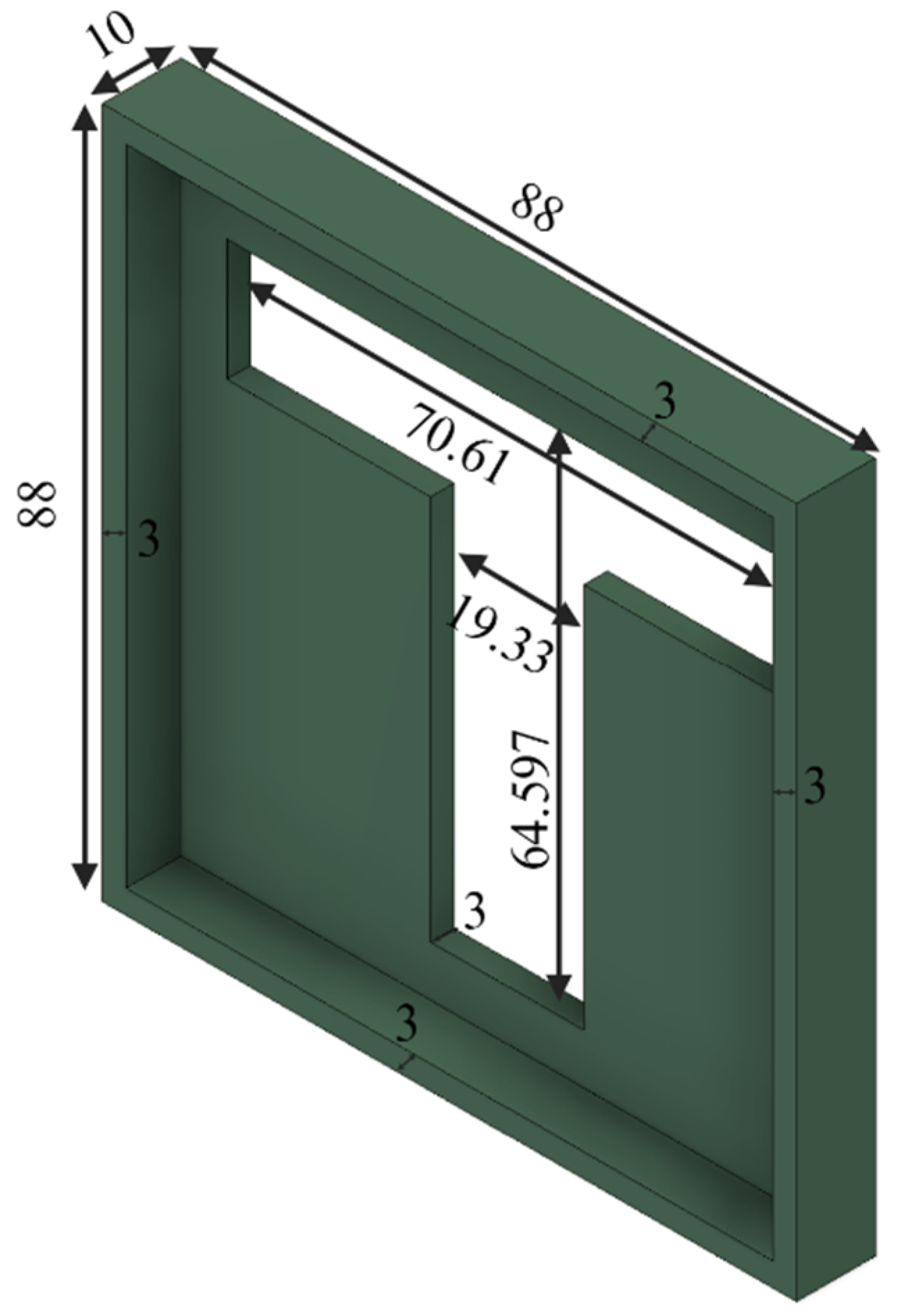
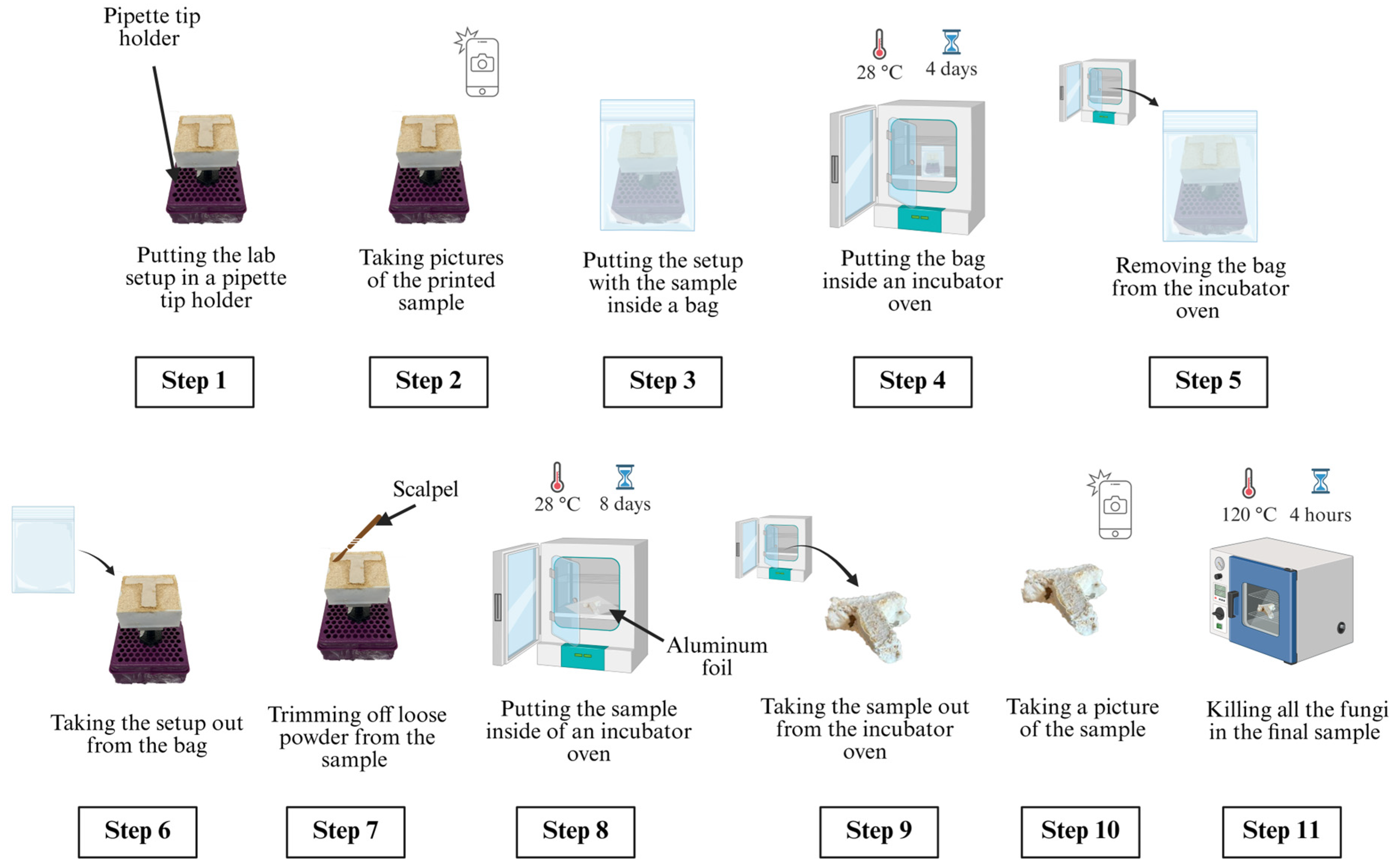

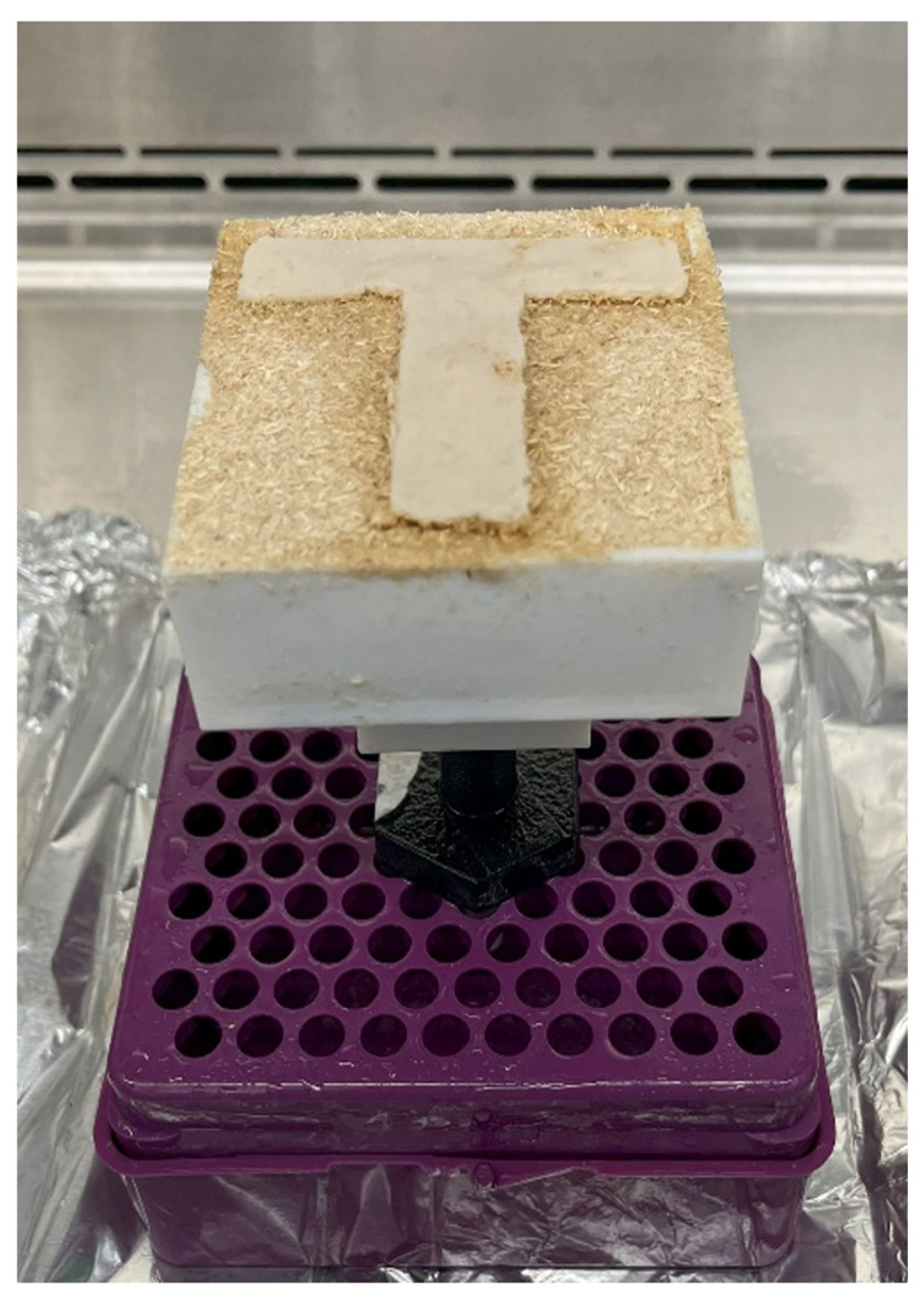
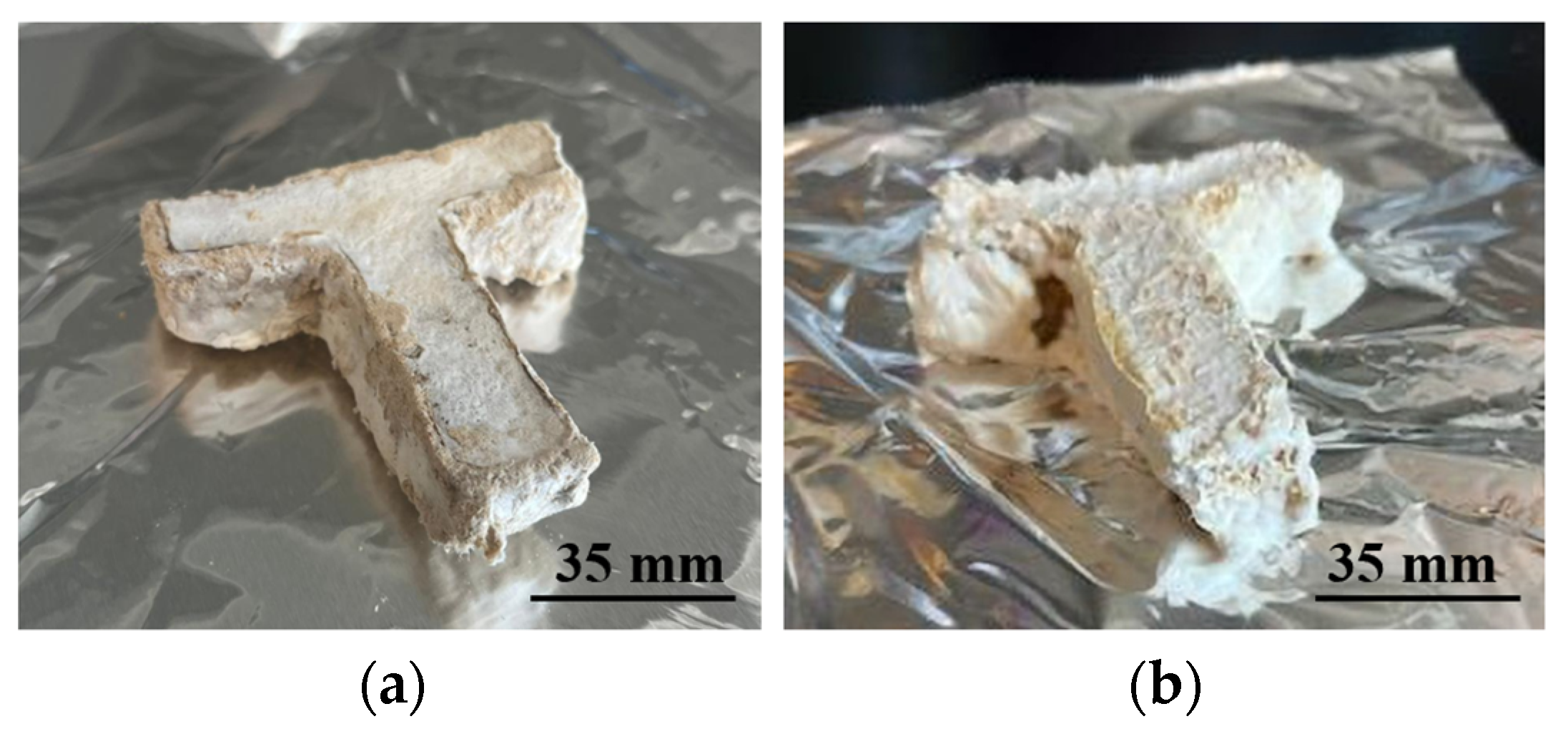

| Particle Size (mm) | Binder Amount (mL) |
|---|---|
| 0.15 | 12.75 ± 4.35 |
| 2 | 10.5 ± 1 |
| Extrusion-Based 3D Printing | Binder Jetting 3D Printing | |
|---|---|---|
| Working principle | Material is extruded through a nozzle with force or pressure | Liquid binder is deposited onto selected areas of the powder bed of biomass particles |
| Material form | Paste-like mixture containing biomass particles and fungi | Biomass powder on powder bed; and fungi embedded binder |
| Production throughput | Slower process | Faster Process |
| Suitable for single-piece or batch production | Suitable for mass production | |
| Low achievable resolution | High achievable resolution | |
| Unable to print samples with complex geometry | Able to print samples with complex geometry | |
| Cost per part | Low machine cost | High machine cost |
| Easier to scale for large parts | Difficult to print very large parts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akib, Y.M.; Bedsole, C.O.; Sanders, J.; Warren, H.; Pei, Z.; Shaw, B.D. Binder Jetting 3D Printing of Biomass–Fungi Composite Materials: A Preliminary Experimental Study. Biomimetics 2025, 10, 441. https://doi.org/10.3390/biomimetics10070441
Akib YM, Bedsole CO, Sanders J, Warren H, Pei Z, Shaw BD. Binder Jetting 3D Printing of Biomass–Fungi Composite Materials: A Preliminary Experimental Study. Biomimetics. 2025; 10(7):441. https://doi.org/10.3390/biomimetics10070441
Chicago/Turabian StyleAkib, Yeasir Mohammad, Caleb Oliver Bedsole, Jackson Sanders, Harlie Warren, Zhijian Pei, and Brian D. Shaw. 2025. "Binder Jetting 3D Printing of Biomass–Fungi Composite Materials: A Preliminary Experimental Study" Biomimetics 10, no. 7: 441. https://doi.org/10.3390/biomimetics10070441
APA StyleAkib, Y. M., Bedsole, C. O., Sanders, J., Warren, H., Pei, Z., & Shaw, B. D. (2025). Binder Jetting 3D Printing of Biomass–Fungi Composite Materials: A Preliminary Experimental Study. Biomimetics, 10(7), 441. https://doi.org/10.3390/biomimetics10070441







