Biomimetic Polyurethanes in Tissue Engineering
Abstract
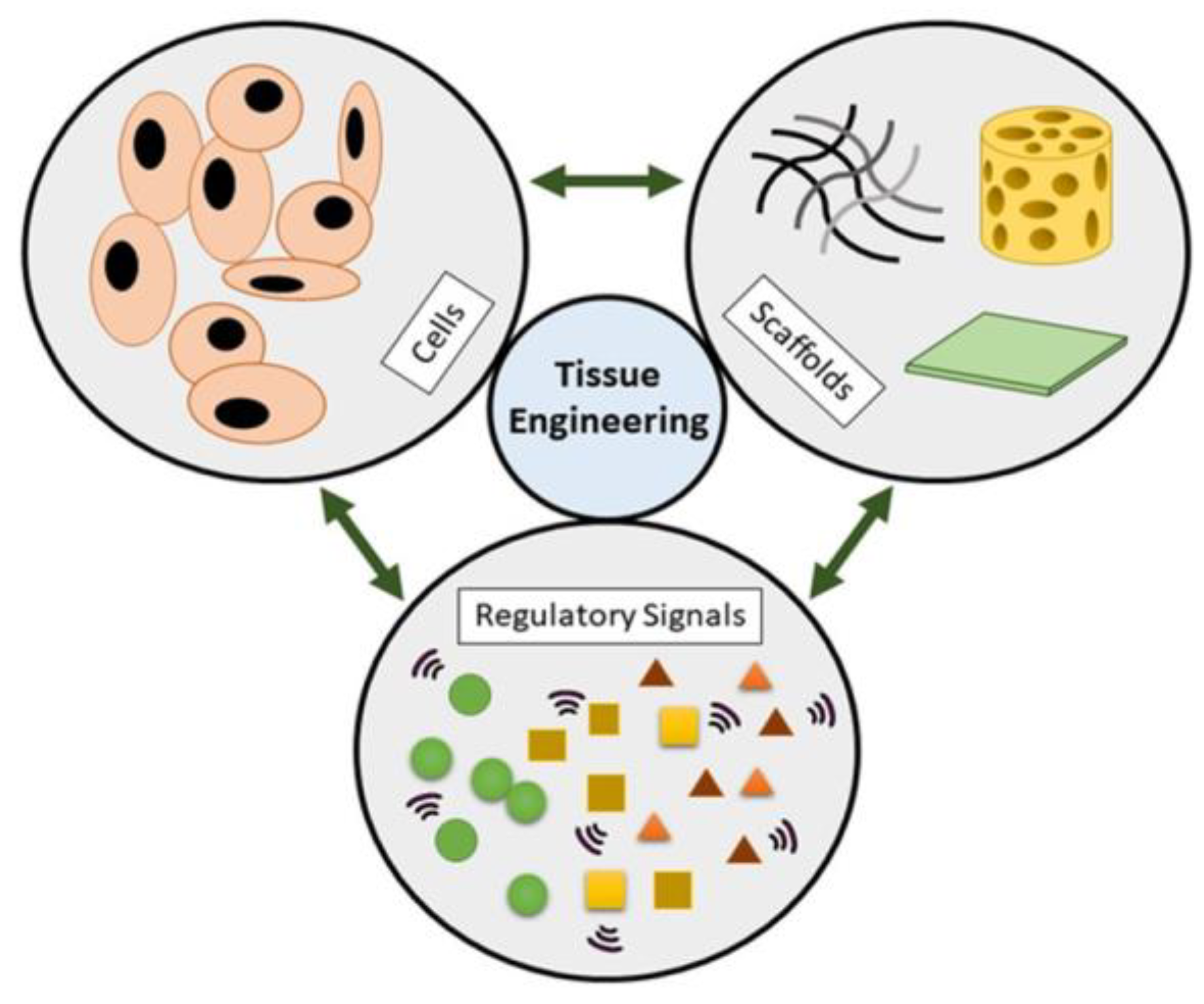
1. Introduction
2. Biomimetics in Tissue Engineering
- Natural origin;
- Bacterial origin;
- Synthetic;
- Synthetic from renewable raw materials [51].
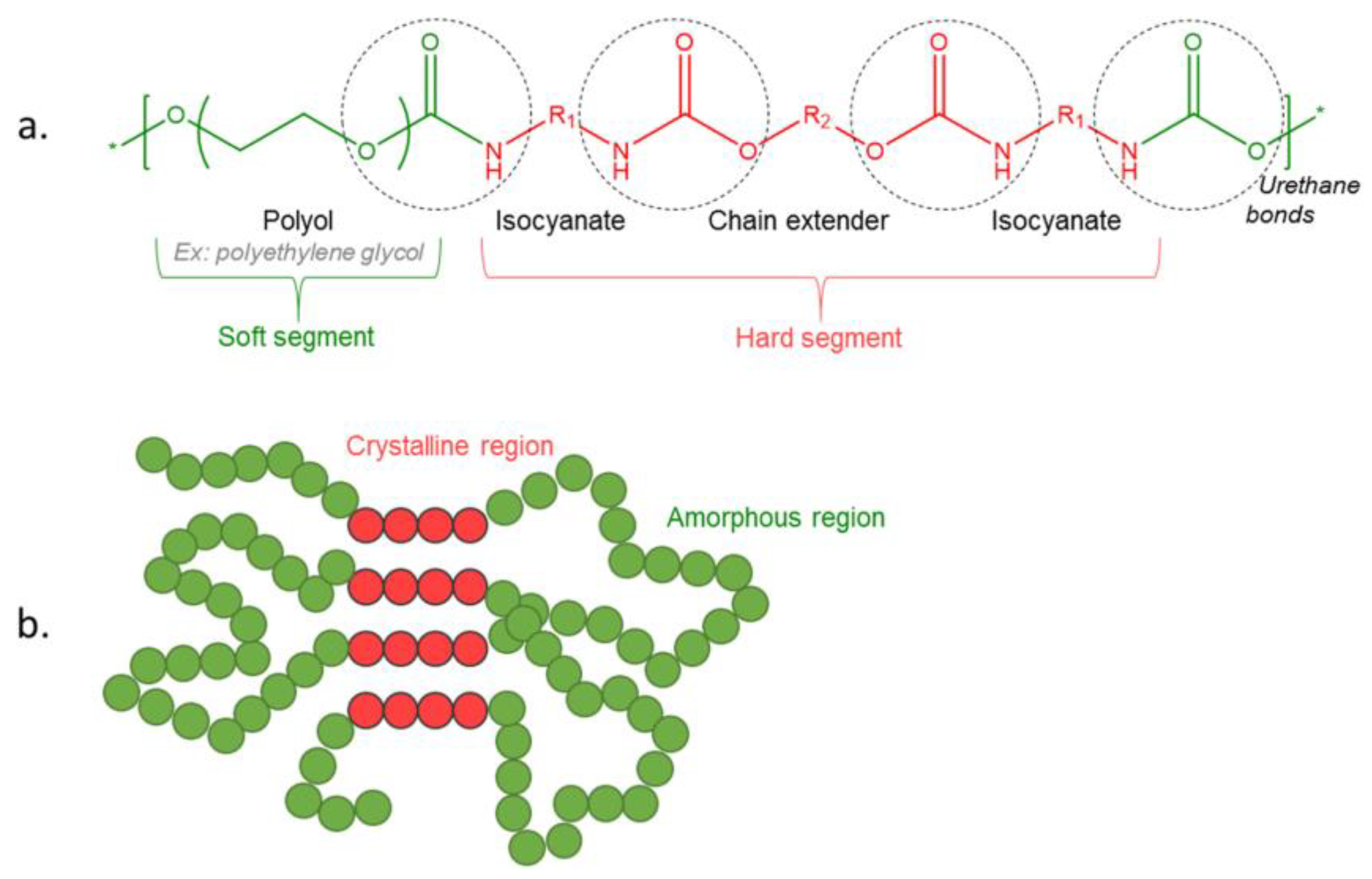
3. Polyurethane Chemistry
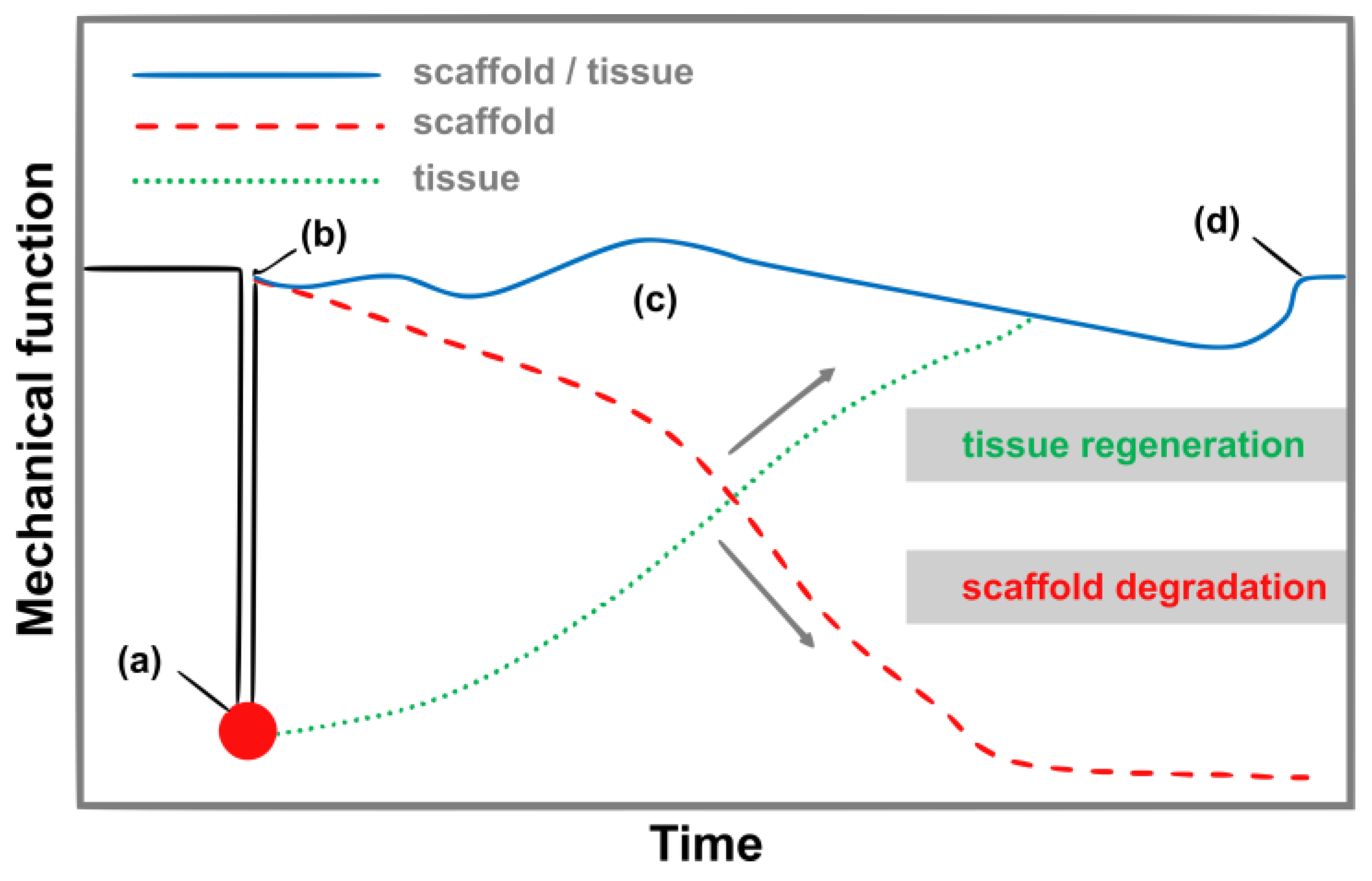
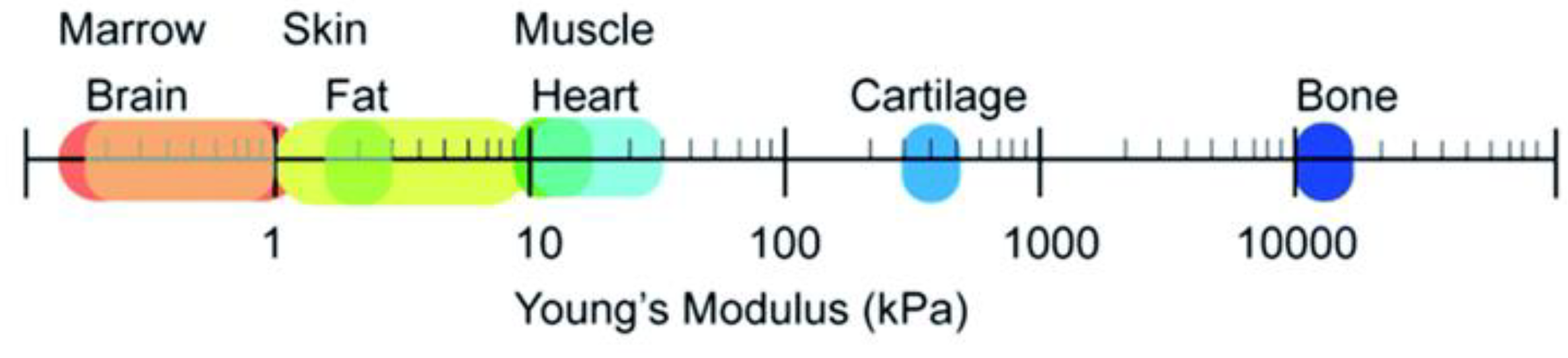
4. Soft Tissues, Hard Tissue
5. Hard-Tissue Engineering
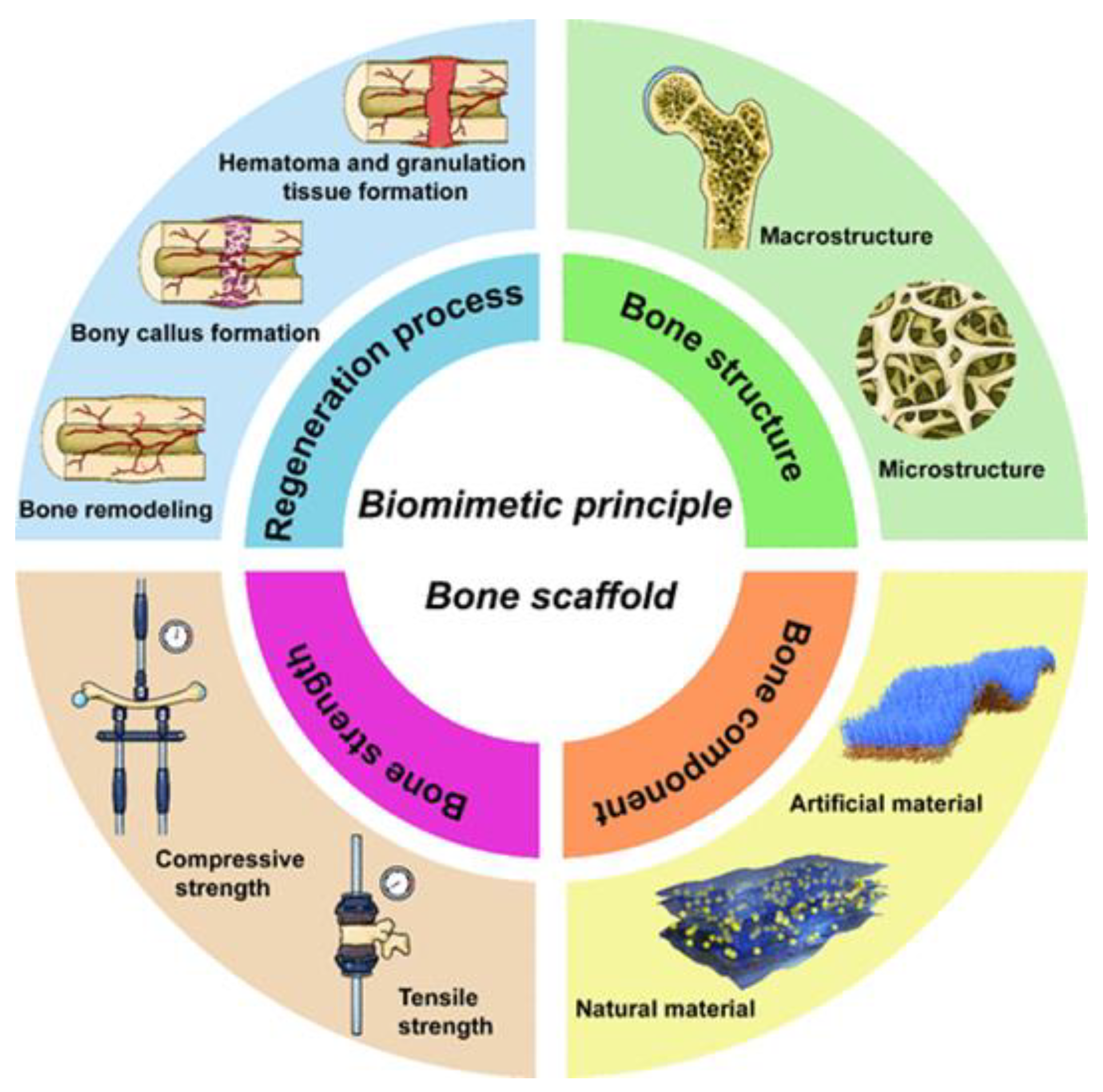
5.1. Bone Tissue Engineering
- (1)
- Compositional biomimetics: materials should mimic the natural chemical composition of bone, which can affect their biocompatibility and ability to integrate with surrounding tissues.
- (2)
- Structural biomimetics: the structure of the scaffold should mimic the hierarchical organization of bone, which can improve the mechanical properties and functionality of the grafts.
- (3)
- Mechanical strength biomimetics: materials should have adequate strength and flexibility to meet the biomechanical demands of the body.
- (4)
- Biomimetic regeneration process: the design should take into account the natural processes of bone healing and regeneration, supporting cell proliferation and new bone formation.
- -
- Osteoblasts, which are responsible for bone synthesis;
- -
- Osteoclasts, which are responsible for bone resorption;
- -
- Osteocytes and lining cells, which are permanently present in bone tissue.
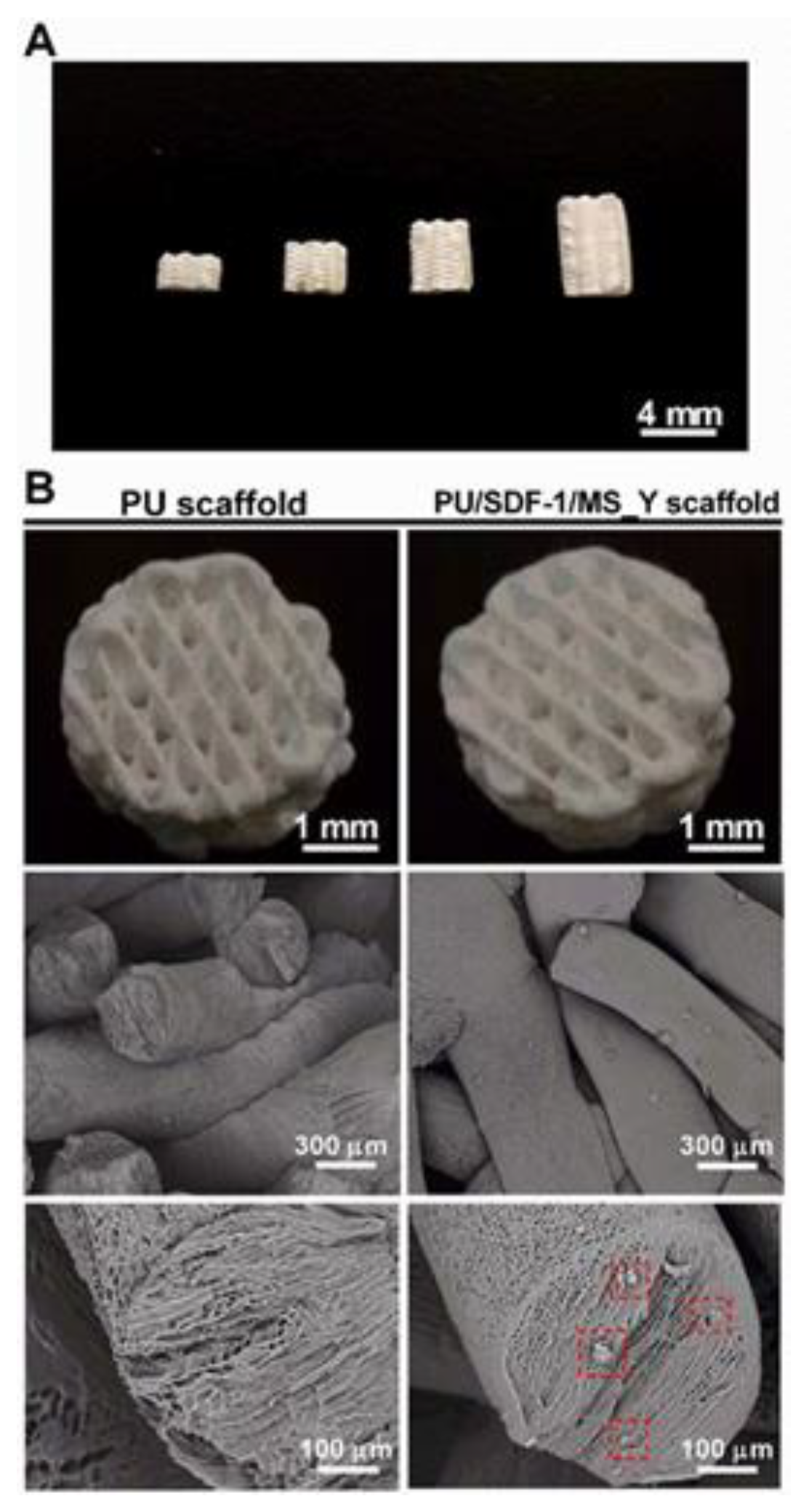
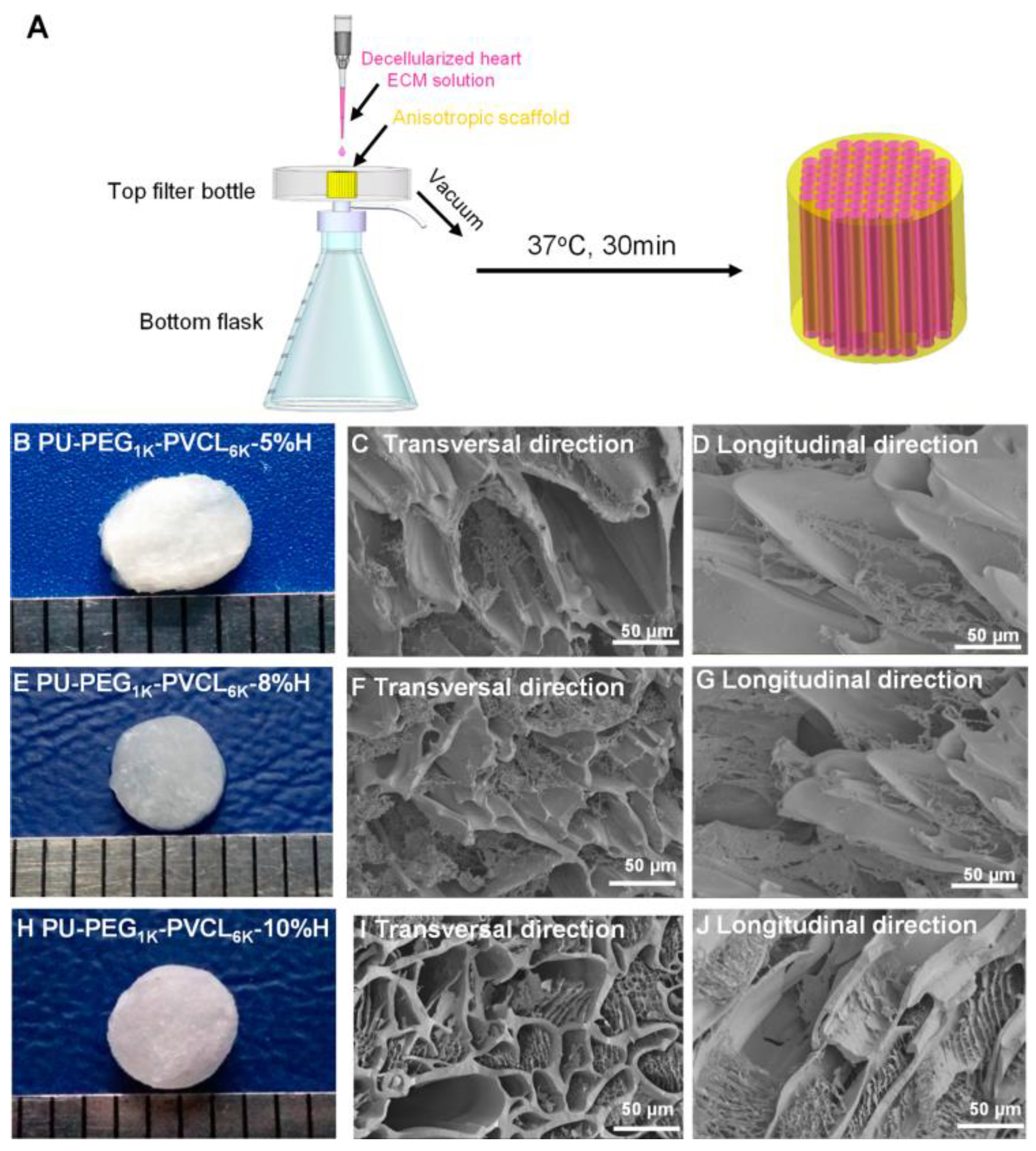
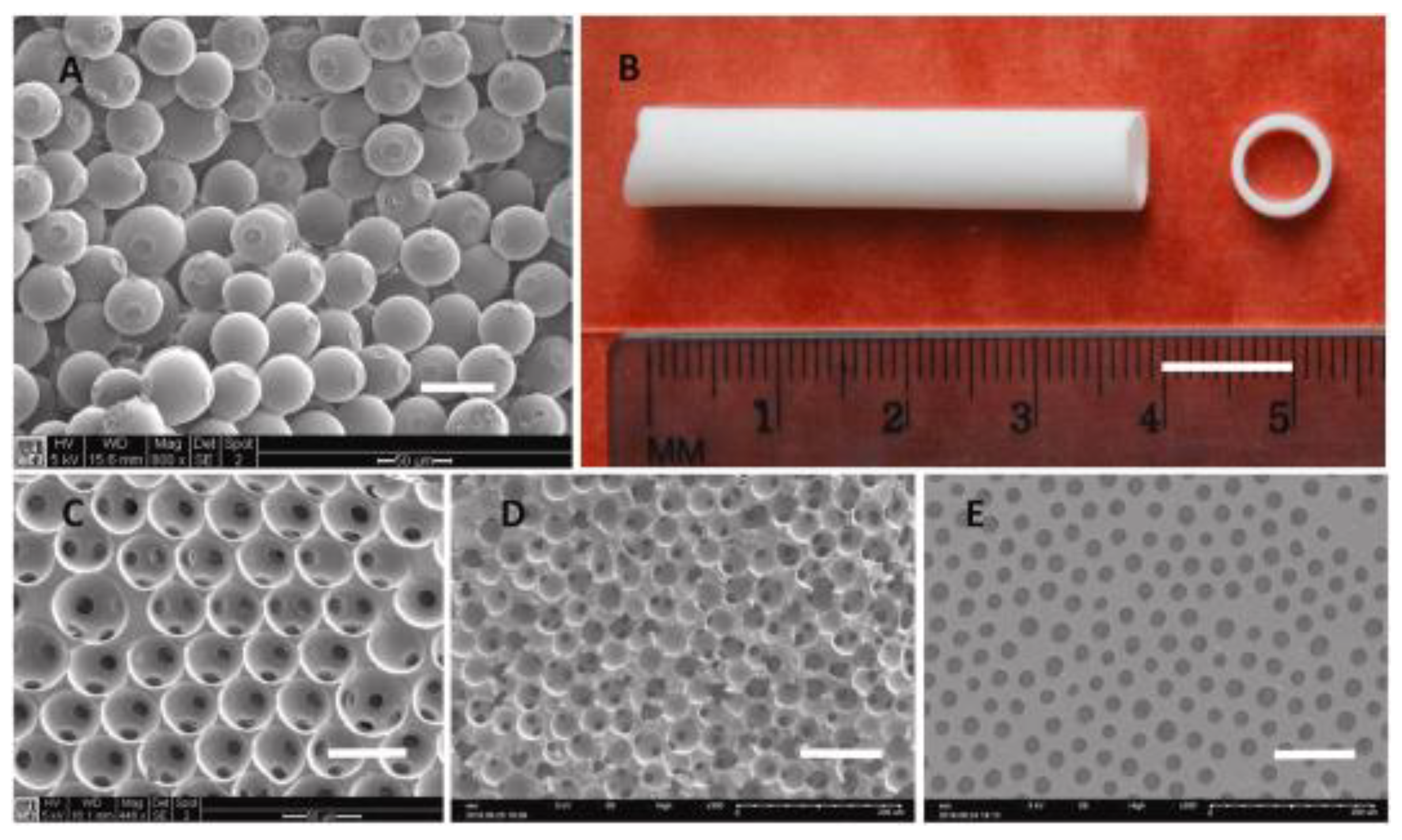
Polyurethane Scaffolds for Bone Engineering
6. Soft-Tissue Engineering
6.1. Skeletal Muscle Tissue Engineering
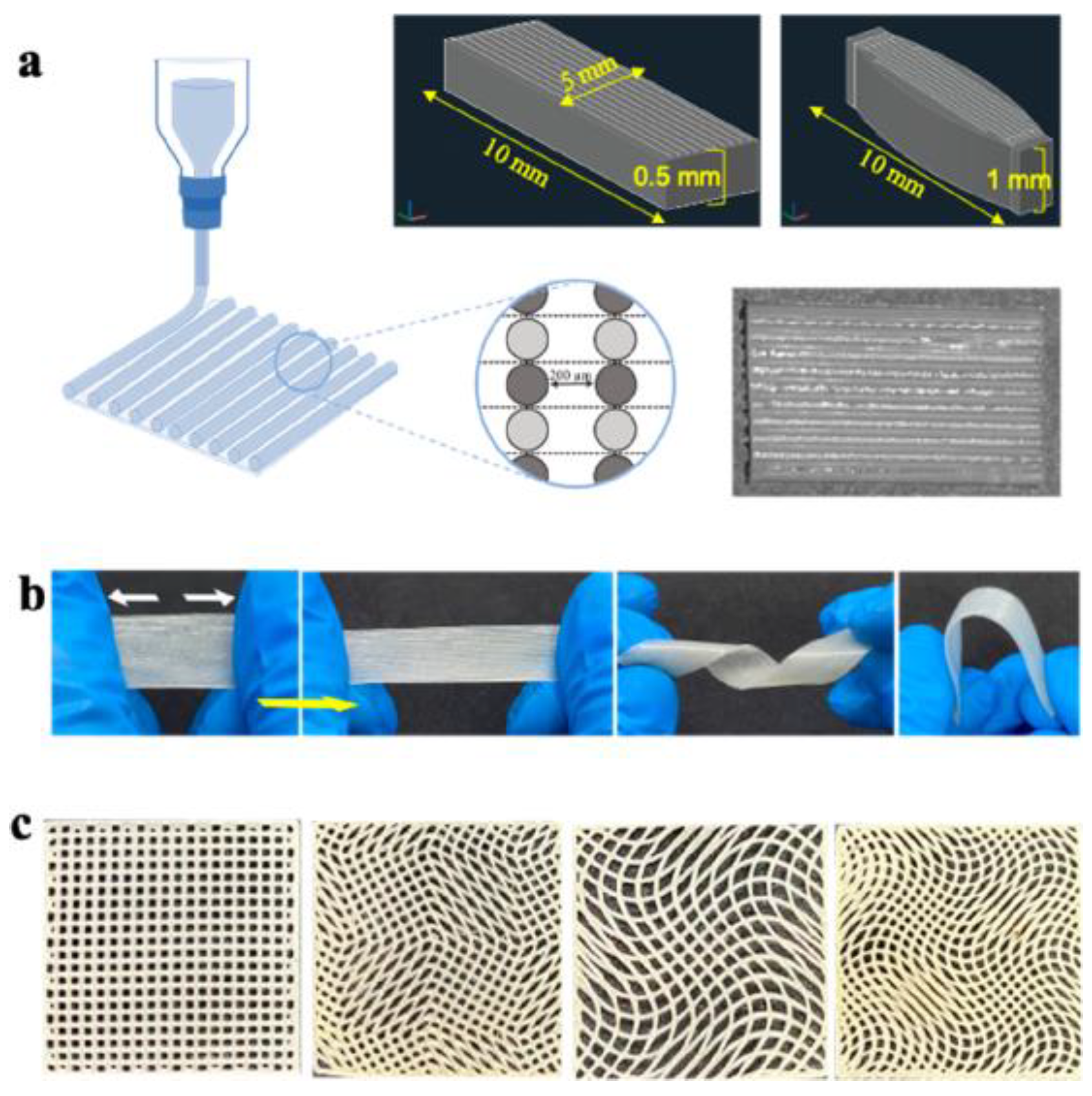
Polyurethane Scaffolds for Skeletal Muscle Engineering
6.2. Cardiovascular Tissue Engineering
- -
- Blood vessels: Creating bio-engineered blood vessels that can replace damaged or blocked vessels is crucial to restoring proper blood circulation. The use of biodegradable scaffolds and hydrogels allows for the development of a structure that not only provides physical support, but also promotes integration with existing tissue.
- -
- Heart muscle: Regeneration or replacement of damaged heart muscle after heart attacks or other diseases is an important goal. Research into stem cells and various biomaterials for tissue engineering could lead to new therapies that speed healing and improve heart function.
- -
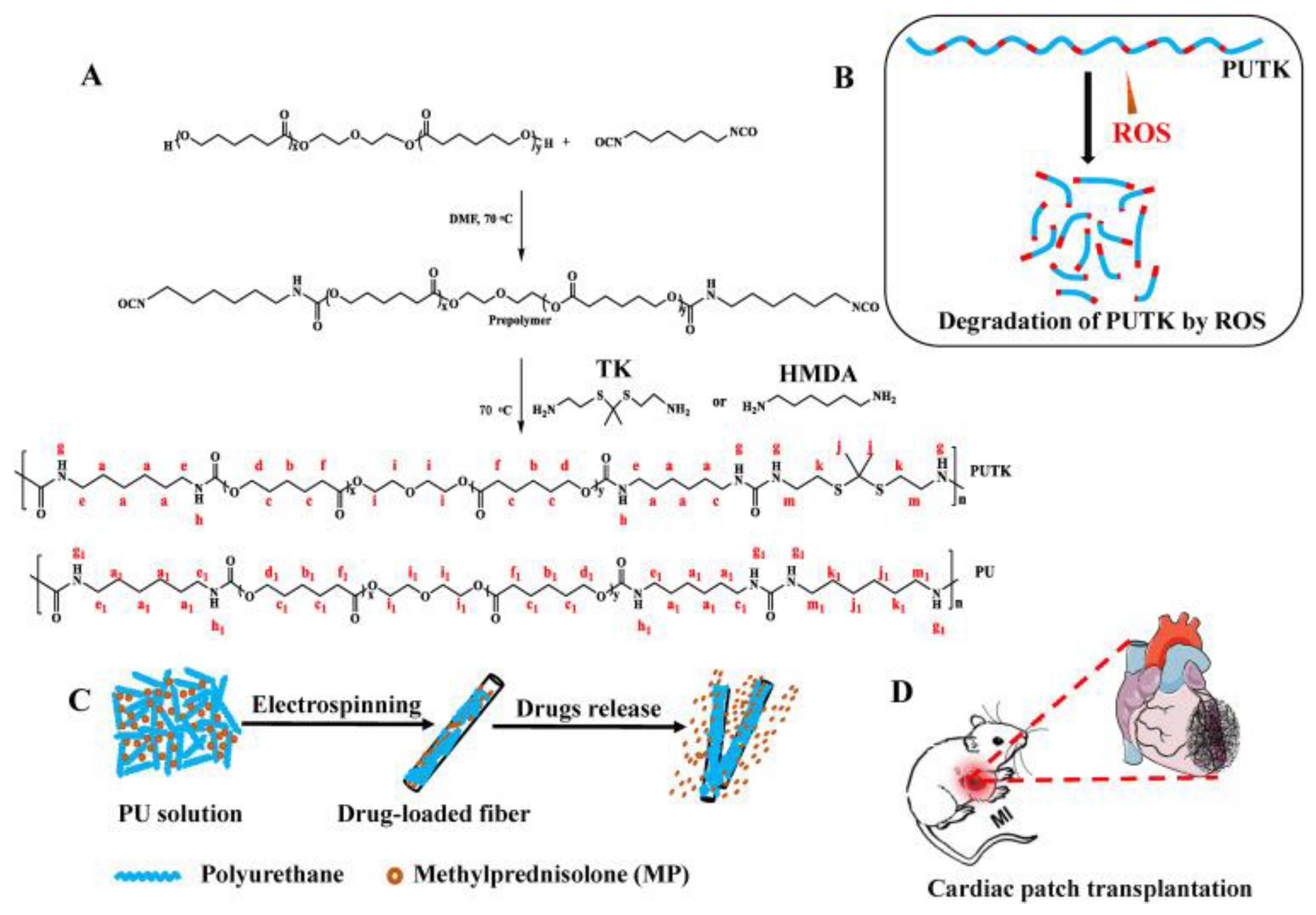
Polyurethane Scaffolds for Cardiac Muscle Engineering
6.3. Cardiac Tissue Engineering
Polyurethane Scaffolds for Cardiac Valve
6.4. Vascular Tissue Engineering
- -
- Elastic (large): in their structure, elastic fibers and membranes play the main role,
- -
- Muscular (medium and small): in their walls, there is more smooth muscle tissue.
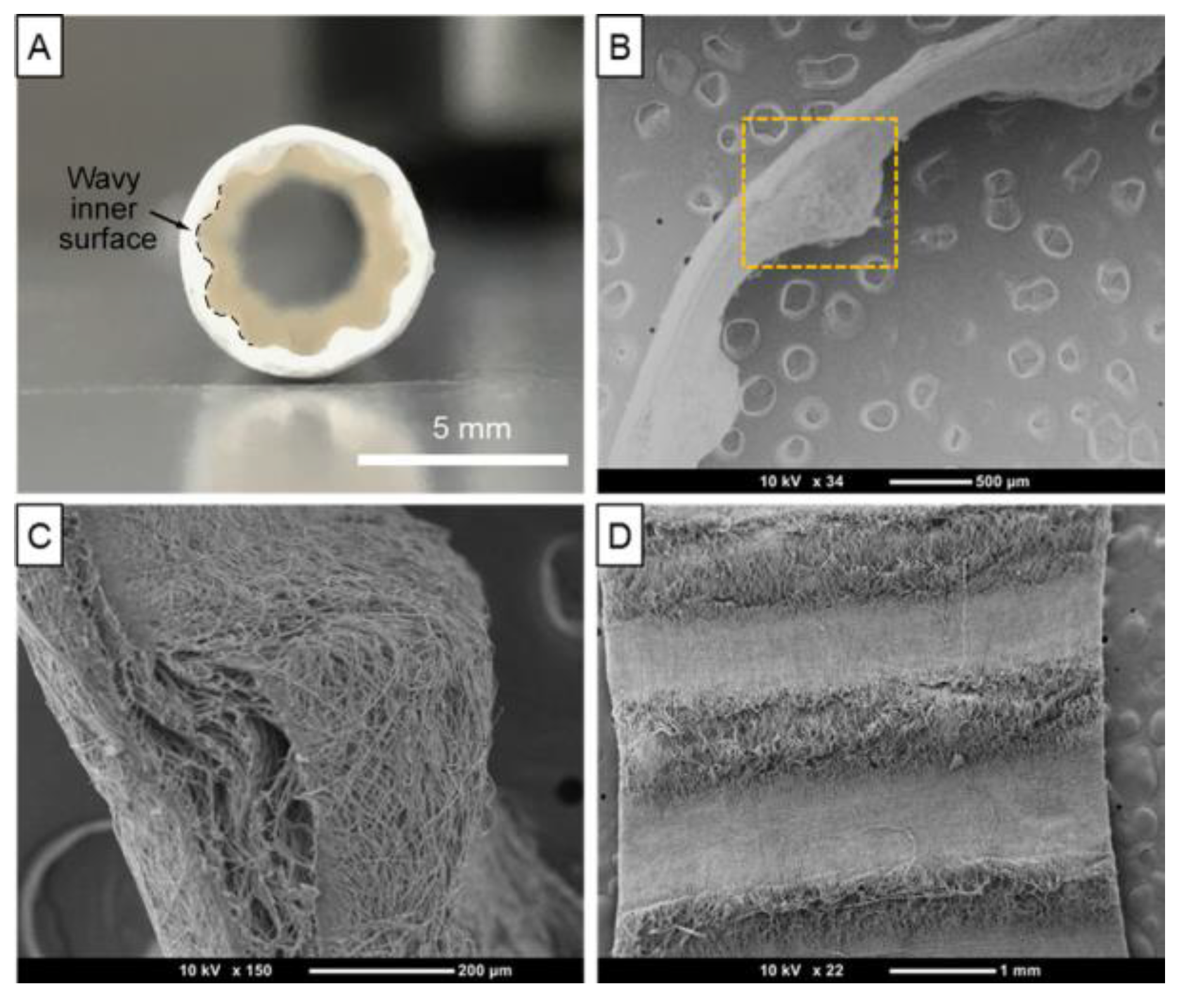
Polyurethane Scaffolds for Vascular Engineering
7. Advantages and Disadvantages of Polyurethanes Compared to Other Polymer Biomaterials
8. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Bar-Cohen, Y. Biomimetics—Biologically Inspired Technologies, 1st ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 18–40. [Google Scholar]
- Bar-Cohen, Y. Biomimetics—Using nature to inspire human innovations. Bioinsp. Biomim. 2006, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A. Biomimetism and nanotechnology—Materials and methods inspired by nature. Przegląd Mech. 2017, 1, 11–15. [Google Scholar] [CrossRef]
- Bhushan, B. Biomimetics: Lessons from nature—An overview. Phil. Trans. R. Soc. A 2009, 367, 1445–1486. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.F.V. Biomimetics—A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2009, 223, 919–939. [Google Scholar] [CrossRef]
- Bonser, R.H.C.; Vincent, J.F.V. Technology trajectories, innovation, and the growth of biomimetics. Proc. IMechE Part C J. Mech. Eng. Sci. 2007, 221, 1177–1180. [Google Scholar] [CrossRef]
- Hench, L.L.; Thompson, I. Twenty-first century challenges for biomaterials. J. R. Soc. Interface 2010, 6, 379–391. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Tathe, A.; Ghodke, M.; Nikalje, A.P. A brief review: Biomaterials and their application. Int. J. Pharm Pharm. Sci. 2010, 2, 19–23. [Google Scholar]
- Uludag, H. Grand challenges in biomaterials. Front. Bioeng. Biotechnol. 2014, 2, 43–46. [Google Scholar]
- Badylak, S. The extracellular matrix as a scaffold for tissue reconstruction. Cell Dev. Biol. 2002, 13, 377–383. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, M.L. Current concepts of extracellular matrix. J. Orthop. Sci. 2006, 11, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, R.; Mohebichamkhorami, F.; Taghipour, N.; Keshel, S.H. The effect of extracellular matrix remodeling on material-based strategies for bone regeneration: Review article. Tissue Cell 2022, 76, 101748–101767. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Kima, K.; Parkb, I.; Hoshibac, T.; Jiangd, H. Design of artificial extracellular matrices for tissue engineering. Prog. Polym. Sci. 2011, 36, 238–268. [Google Scholar] [CrossRef]
- Kim, T.; Shin, H.; Lim, D. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Koumentakou, I.; Michailidou, G.; Noordam, M.J.; Bikiaris, D.N. Biocompatible Synthetic Polymers for Tissue Engineering Purposes. Biomacromolecules 2022, 23, 1841–1863. [Google Scholar] [CrossRef]
- Rosso, F.; Giordano, A.; Barbarisi, M.; Barbarisi, A. From cell–ECM interactions to tissue engineering. J. Cell Physiol. 2004, 199, 174–180. [Google Scholar] [CrossRef]
- Chandra, P.K.; Soker, S.; Atala, A. Chapter 1—Tissue engineering: Current status and future perspectives. In Principles of Tissue Engineering, 5th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 1–35. [Google Scholar]
- Wojtowicz, A.; Szostak, D.; Malejczyk, J. Tissue engineering in oral surgery—Review of new methodology. Nowa Stomatol. 2002, 1, 41–45. [Google Scholar]
- Zulkiflee, I.; Fauzi, M.B. Gelatin-Polyvinyl Alcohol Film for Tissue Engineering: A Concise Review. Biomedicines 2021, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Akter, F. Tissue Engineering Made Easy; Academic Press: London, UK, 2016. [Google Scholar]
- Grayson, W.L.; Martens, T.P.; Eng, G.M.; Radisic, M.; Vunjak-Novakovic, G. Biomimetic approach to tissue engineering. Semin. Cell Dev. Biol. 2009, 20, 665–673. [Google Scholar] [CrossRef]
- Jakab, K.; Norotte, C.; Marga, F.; Murphy, K.; Vunjak-Novakovic, G.; Forgacs, G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2010, 2, 022001. [Google Scholar] [CrossRef]
- Tabata, Y. Significance of release technology in tissue engineering. Drug Discov. Today 2005, 10, 1639–1646. [Google Scholar] [CrossRef]
- Chan, B.; Leong, B. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef]
- Liao, S.; Chan, C.; Ramakrishna, S. Stem cells and biomimetic materials strategies for tissue engineering. Mater. Sci. Eng. C 2008, 28, 1189–1202. [Google Scholar] [CrossRef]
- Liu, C.; Xia, Z.; Czernuszka, J. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2008, 85, 1051–1064. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.; Du, Z.; Chua, C. The design of scaffolds for use in tissue engineering part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef]
- Owen, S.; Shoichet, M. Design of three-dimensional biomimetic scaffolds. J. Biomed. Mater. Res. A 2010, 94, 1321–1331. [Google Scholar] [CrossRef]
- Seidi, A.; Ramalingam, M.; Elloumi-Hannachi, I.; Ostrovidov, S. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater. 2011, 7, 1441–1451. [Google Scholar] [CrossRef]
- Ma, P. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Mooney, D. New materials for tissue engineering: Towards greater control over the biological response. Trends Biotechnol. 2008, 26, 382–392. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Vert, M.; Li, S.; Spenlehauer, G.; Guerin, P. Bioresorbability and biocompatibility of aliphatic polyesters. J. Mater. Sci. Mater. Med. 1992, 3, 432–446. [Google Scholar] [CrossRef]
- Koria, P. Delivery of Growth Factors for Tissue Regeneration and Wound Healing. BioDrugs 2012, 26, 163–175. [Google Scholar] [CrossRef]
- Fortunato, T.; De Bank, P.; Pula, G. Vascular Regenerative Surgery: Promised Land for Tissue Engineers? Int. J. Stem. Cell Res. Transplant. 2017, 5, 268–276. [Google Scholar]
- Williams, D.F. To engineer is to create: The link between engineering and regeneration. Trends Biotechnol. 2006, 24, 4–8. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Hashimoto, Y.; Negishi, J. Fabrication of a cell culture scaffold that mimics the composition and structure of bone marrow extracellular matrix. J. Biosci. Bioeng. 2024, 138, 83–88. [Google Scholar] [CrossRef]
- Butler, D.L.; Goldstein, S.A.; Guilak, F. Functional tissue engineering: The role of biomechanics. J. Biomech. Eng. 2000, 122, 570–575. [Google Scholar] [CrossRef]
- Adachia, T.; Osakoa, Y.; Tanakaa, M.; Hojoa, M.; Hollister, S.J. Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration. Biomaterials 2006, 27, 3964–3972. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Burdick, J.A. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 2008, 60, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Błażewicz, S.; Stoch, L. Biocybernetyka i Inżynieria Biomechaniczna; Akademicka Oficyna Wydawnicza EXITL: Warszawa, Poland, 1999. [Google Scholar]
- Bronzino, J.D. Tissue Engineering and Artificial Organs; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Chen, D.R.; Bei, J.Z.; Wang, S.G. Polycaprolactone microparticles and their biodegradation. Polym. Degrad. Stab. 2000, 67, 455–459. [Google Scholar] [CrossRef]
- Wyleżoł, M.; Ostrowska, B.; Wróbel, E.; Muzalewska, M.; Grabowski, M.; Wyszyński, D.; Zubrzycki, J.; Piech, P.; Klepka, T. Inżynieria Biomedyczna. Metody Przyrostowe w Technice Medycznej; Politechnika Lubelska, Agencja TOP: Lublin, Poland, 2016. [Google Scholar]
- Nair, L.; Laurencin, C. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Piozzi, A.; Francolini, I. Chapter 9: Biomimetic Polyurethanes. In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications; Muñoz-Bonilla, A., Cerrada, M., Fernández-García, M., Eds.; Royal Society of Chemistry: London, UK, 2013; pp. 224–278. [Google Scholar]
- Wu, Y.; Simonovsky, F.I.; Ratner, B.D.; Horbett, T.A. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: A comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. J. Biomed. Mater. Res. 2005, 74A, 722–738. [Google Scholar] [CrossRef]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef]
- Florjańczyk, Z.; Penczek, S. Chemia Polimerów, v.II; Oficyna Wydawnicza Politechniki Warszawskiej: Warszawa, Poland, 2002. [Google Scholar]
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Materiały Poliuretanowe; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2016. [Google Scholar]
- Randall, D.; Lee, S. The Polyurethane Book; Wiley Ltd.: New York, NY, USA, 2002. [Google Scholar]
- Szycher, M. Szycher’s Handbook of Polyurethane; CRC Press: Boca Raton, FL, USA; Taylor &Francis Group: Abingdon, UK, 2003. [Google Scholar]
- Magnina, A.; Polleta, E.; Phalipb, V.; Avérous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 2020, 39, 107457–107481. [Google Scholar] [CrossRef]
- Szycher, M. Szycher’s Handbook of Polyurethanes; Taylor & Francis INC.: Abingdon, UK, 2012. [Google Scholar]
- Prisacariu, C. Polyurethane Elastomers—From Morphology to Mechanical Aspects; Springer Wien: New York, NY, USA, 2011. [Google Scholar]
- Rueda-Larraz, L.; Fernandez d’Arlas, B.; Tercjak, A.; Ribes, A.; Mondragon, I.; Eceiza, A. Synthesis and microstructure–mechanical property relationships of segmented polyurethanes based on a PCL–PTHF–PCL block copolymer as soft segment. Eur. Polym. J. 2009, 45, 2096–2109. [Google Scholar] [CrossRef]
- Souza, F.M.; Kahol, P.K.; Gupta, R.K. Polyurethane Chemistry: Renewable Polyols and Isocyanates; American Chemical Society: Washington, DC, USA, 2021; Chapter 1; pp. 1–24. [Google Scholar]
- Jin, X.; Guo, N.; You, Z.; Tan, Y. Design and Performance of Polyurethane Elastomers Composed with Different Soft Segments. Materials 2020, 13, 4991. [Google Scholar] [CrossRef] [PubMed]
- Fernández-D’Arlas, B.; Alonso-Varona, A.; Palomares, T.; Corcuera, M.A.; Eceiza, A. Studies on the morphology, properties and biocompatibility of aliphatic diisocyanate-polycarbonate polyurethanes. Polym. Degrad. Stab. 2015, 122, 153–160. [Google Scholar] [CrossRef]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Tien, Y.; Wei, K. Hydrogen bonding and mechanical properties in segmented montmorillonite/polyurethane nanocomposites of different hard segment ratios. Polymer 2001, 42, 3213–3221. [Google Scholar] [CrossRef]
- Saha, J.K.; Rahman, M.M.; Haq, B.; Shehri, D.A.; Jang, J. Theoretical and Experimental Studies of Hydrogen Bonded Dihydroxybenzene Isomers Polyurethane Adhesive Material. Polymers 2022, 14, 1701. [Google Scholar] [CrossRef] [PubMed]
- Giubertoni, G.; Hilbers, M.; Caporaletti, F.; Laity, P.; Groen, H.; Weide, A.; Bonn, D.; Woutersen, S. Hydrogen Bonds under Stress: Strain-Induced Structural Changes in Polyurethane Revealed by Rheological Two-Dimensional Infrared Spectroscopy. J. Phys. Chem. Lett. 2023, 14, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Mikulčić, H.; Jin, Q.; Stančin, H.; Wang, X.; Li, S.; Tan, H.; Duić, N. Thermogravimetric Analysis Investigation of Polyurethane Plastic Thermal Properties Under Different Atmospheric Conditions. J. Sustain. Dev. Energy Water Environ. Syst. 2019, 7, 355–367. [Google Scholar] [CrossRef]
- Xu, C.; Hong, Y. Rational design of biodegradable thermoplastic polyurethanes for tissue repair. Bioact. Mater. 2022, 15, 250–271. [Google Scholar] [CrossRef]
- Ciardelli, G.; Sartori, S.; Silvestri, A.; Serafini, P.; Caporale, A.; Boccafoschi, F. Multiblock polyurethanes in biomedical applications: Fine tuning of degradation and biomimetic properties. AIP Conf. Proc. 2010, 1255, 8–16. [Google Scholar]
- Niu, Y.Q.; Chen, K.V.C.; He, T.; Yu, W.Y.; Huang, S.W.; Xu, K.T. Scaffolds from block polyurethanes based on poly(e-caprolactone) (PCL) and poly(ethylene glycol) (PEG) for peripheral nerve regeneration. Biomaterials 2014, 35, 4266–4277. [Google Scholar] [CrossRef]
- Yao, C.; Hedrick, M.; Pareek, G.; Renzulli, J.; Haleblian, G.; Webster, T.J. Nanostructured polyurethane-poly-lactic-co-glycolic acid scaffolds increase bladder tissue regeneration: An in vivo study. Int. J. Nanomed. 2013, 8, 3285–3296. [Google Scholar]
- Xu, Y.; Guan, J. Interaction of cells with polyurethane scaffolds. In Advances in Polyurethane Biomaterials; Cooper, S.L., Guan, J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 523–542. [Google Scholar]
- García-Estrada, P.; García-Bon, M.A.; López-Naranjo, E.J.; Basaldúa-Pérez, D.N.; Santos, A.; Navarro-Partida, J. Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics 2021, 13, 701. [Google Scholar] [CrossRef]
- Su, T.; Xu, M.; Lu, F.; Chang, Q. Adipogenesis or osteogenesis: Destiny decision made by mechanical properties of biomaterials. RSC Adv. 2022, 12, 24501–24510. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, T.C.; Woodhouse, K.A.; Hauschka, S.D.; Murry, C.E.; Stayton, P.S. Spatially organized layers of cardiomyocytes on biodegradable polyurethane films for myocardial repair. J. Biomed. Mater. Res. A 2003, 66, 586–595. [Google Scholar] [CrossRef]
- Guan, J.; Fujimoto, K.L.; Sacks, M.S.; Wagner, V.R. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials 2005, 26, 3961–3971. [Google Scholar] [CrossRef]
- Sharifpoor, S.; Labow, R.S.; Santerre, J.P. Synthesis and Characterization of Degradable Polar Hydrophobic Ionic Polyurethane Scaffolds for Vascular Tissue Engineering Applications. Biomacromolecules 2009, 10, 2729–2739. [Google Scholar] [CrossRef] [PubMed]
- Riboldi, S.A.; Sampaolesi, M.; Neuenschwanderc, P.; Cossu, G.; Mantero, S. Electrospun degradable polyesterurethane membranes: Potential scaffolds for skeletal muscle tissue engineering. Biomaterials 2005, 26, 4606–4615. [Google Scholar] [CrossRef]
- Grad, S.; Kupcsik, L.; Gorna, K.; Gogolewski, S.; Alini, M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: Potential and limitations. Biomaterials 2003, 24, 5163–5171. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Ferrone, M.L.; Raut, C.P. Modern surgical therapy: Limb salvage and the role of amputation for extremity soft-tissue sarcomas. Surg. Oncol. Clin. N. Am. 2012, 21, 201–213. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Liu, R.; Liu, Y.; Tian, F.; Gao, X.; Wang, Z.; Lu, B.; Wu, W.; Hui, H.; et al. Four-Dimensional Perspective on Biomimetic Design and Fabrication of Bone Scaffolds for Comprehensive Bone Regeneration. CS Mater. Lett. 2024, 6, 4262–4281. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Ethier, C.R.; Simmons, C.A. Skeletal biomechanics. In Introductory Biomechanics From Cells to Organisms; Ethier, C.R., Simmons, C.A., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 379–443. [Google Scholar]
- Feldmann, A.; Schweizer, M.; Stucki, S.; Nolte, L. Experimental evaluation of cortical bone substitute materials for tool development, surgical training and drill bit wear investigations. Med. Eng. Phys. 2019, 66, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.T.; Douglas, T.; Acil, Y.; Seitz, H.; Sivananthan, S.; Wiltfang, J.; Warnke, P.H. Biocompatibility of individually designed scaffolds with human periosteum for use in tissue engineering. J. Mater. Sci. Mater. Med. 2010, 21, 1255–1262. [Google Scholar] [CrossRef]
- Brunello, G.; Sivolella, S.; Meneghello, R.; Ferroni, L.; Gardin, C.; Piattelli, A.; Zavan, B.; Bressan, E. Powder-based 3D printing for bone tissue engineering. Biotechnol. Adv. 2016, 34, 740–753. [Google Scholar] [CrossRef]
- Kantaros, A.; Chatzidai, N.; Karalekas, D. 3D printing-assisted design of scaffold structures. Int. J. Adv. Manuf. Technol. 2016, 82, 559–571. [Google Scholar] [CrossRef]
- Warnke, P.H.; Seitz, H.; Warnke, F.; Becker, S.T.; Sivananthan, S.; Sherry, E.; Liu, Q.; Wiltfang, J.; Douglas, T. Ceramic scaffolds produced by computer-assisted 3D printing and sintering: Characterization and biocompatibility investigations. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 212–217. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Torres, F.G.; Nazhat, S.N.; Sheikh Md Fadzullah, S.H.; Maquet, V.; Boccaccini, A.R. Mechanical properties and bioactivity of porous PLGA/TiO2 nanoparticle-filled composites for tissue engineering scaffolds. Compos. Sci. Technol. 2007, 67, 1139–1147. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Progress in the Advancement of Porous Biopolymer Scaffold: Tissue Engineering Application. Ind. Eng. Chem. Res. 2019, 58, 6163–6194. [Google Scholar] [CrossRef]
- McEnery, M.A.P.; Lu, S.; Gupta, M.K.; Zienkiewicz, K.J.; Wenke, J.C.; Kalpakci, K.N.; Shimko, D.A.; Duvalla, C.L.; Guelcher, S.A. Oxidatively degradable poly(thioketal urethane)/ceramic composite bone cements with bone-like strength. RSC Adv. 2016, 6, 109414–109424. [Google Scholar] [CrossRef]
- McGough, M.A.P.; Shiels, S.M.; Boller, L.A.; Zienkiewicz, K.J.; Duvall, C.L.; Wenke, J.C.; Guelcher, S.A. Poly(Thioketal Urethane) Autograft Extenders in an Intertransverse Process Model of Bone Formation. Tissue Eng. Part A 2019, 25, 949–963. [Google Scholar] [CrossRef] [PubMed]
- McGough, M.A.P.; Boller, L.A.; Groff, D.M.; Schoenecker, J.G.; Nyman, J.S.; Wenke, J.C.; Rhodes, C.; Shimko, D.; Duvall, C.L.; Guelcher, S.A. Nanocrystalline Hydroxyapatite−Poly(thioketal urethane) Nanocomposites Stimulate a Combined Intramembranous and Endochondral Ossification Response in Rabbits. ACS Biomater. Sci. Eng. 2020, 6, 564–574. [Google Scholar] [CrossRef]
- Boller, L.A.; Jones, A.A.; Cochran, D.L.; Guelcher, S.A. Compression-resistant polymer/ceramic composite scaffolds augmented with rhBMP-2 promote new bone formation in a nonhuman primate mandibular ridge augmentation model. Int. J. Oral Maxillofac. Implants. 2020, 35, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Talley, A.D.; McEnery, M.A.; Kalpakci, K.N.; Zienkiewicz, K.J.; Shimko, D.A.; Guelcher, S.A. Remodeling of injectable, low-viscosity polymer/ceramic bone grafts in a sheep femoral defect model. J. Biomed. Mater. Res. Part B 2017, 105, 2333–2343. [Google Scholar] [CrossRef]
- Shiels, S.M.; Anne, D.; Talley, A.D.; McGough, M.A.P.; Zienkiewicz, K.J.; Kalpakci, K.; Shimko, D.; Guelcher, S.A.; Wenke, J.C. Injectable and compression-resistant low viscosity polymer/ceramic composite carriers for rhBMP-2 in a rabbit model of posterolateral fusion: A pilot study. J. Orthop. Surg. Res. 2017, 12, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, C.; Mascarenhas, R.; Coelho, P.; Brites, C.; Pereira, A.; Gogolewski, S. Elastomeric enriched biodegradable polyurethane sponges for critical bone defects: A successful case study reducing donor site morbidity. J. Mater. Sci. Mater. Med. 2016, 27, 61–73. [Google Scholar] [CrossRef]
- Lei, K.; Zhu, Q.; Wang, X.; Xiao, H.; Zheng, Z. In Vitro and in Vivo Characterization of a Foam-Like Polyurethane Bone Adhesive for Promoting Bone Tissue Growth. ACS Biomater. Sci. Eng. 2019, 5, 5489–5497. [Google Scholar]
- Zhu, Q.; Li, X.; Fan, Z.; Xu, Y.; Niu, H.; Lia, C.; Dang, Y.; Huang, Z.; Wang, Y.; Guan, J. Biomimetic polyurethane/TiO2 nanocomposite scaffolds capable of promoting biomineralization and mesenchymal stem cell proliferation. Mater. Sci. Eng. C 2018, 85, 79–87. [Google Scholar] [CrossRef]
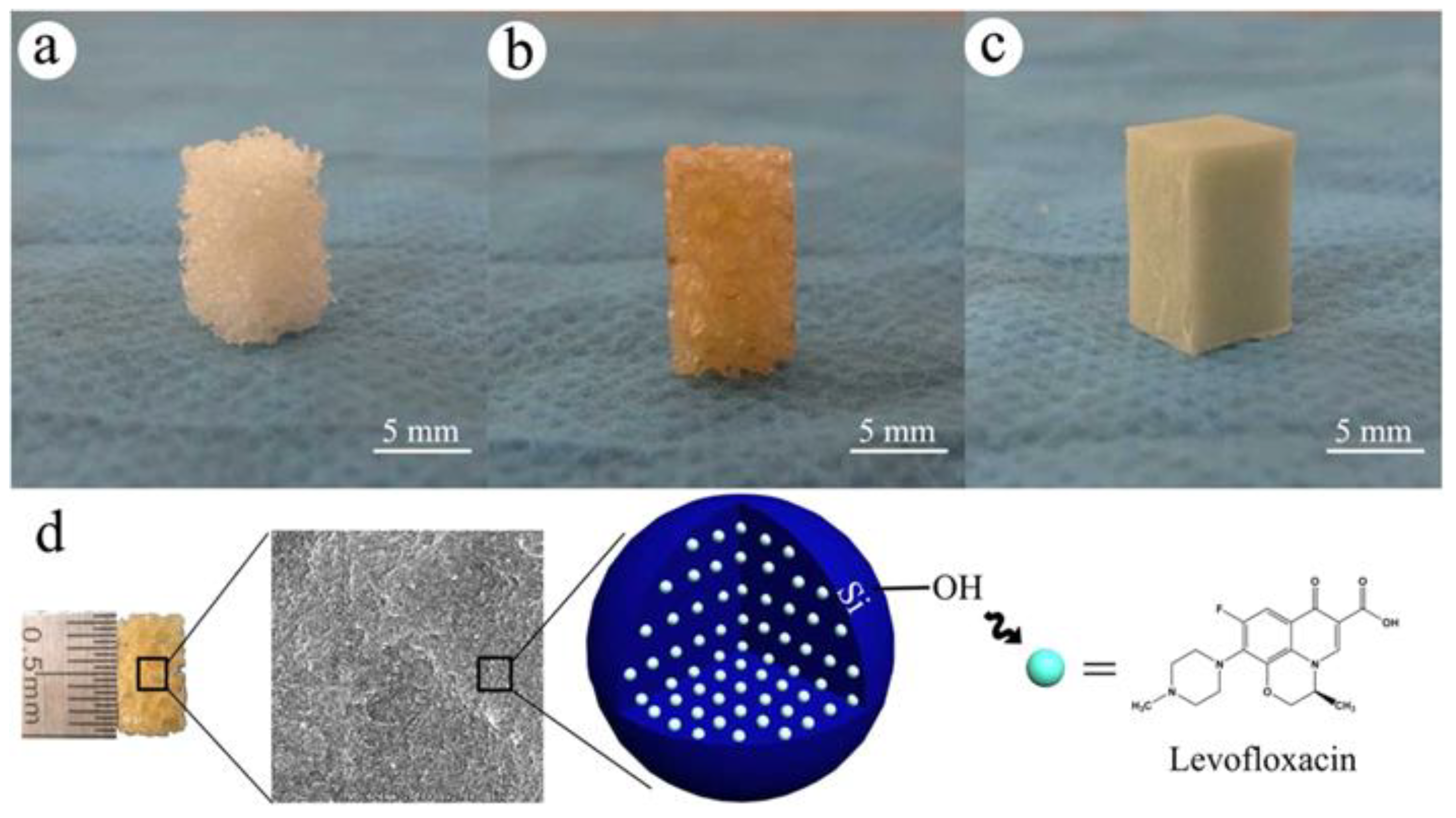
- Wang, Q.; Chen, C.; Liu, W.; He, X.; Zhou, N.; Zhang, D.; Gu, H.; Li, J.; Jiang, J.; Huang, W. Levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold for the treatment of chronic osteomyelitis with bone defects. Sci. Rep. 2017, 7, 41808–41821. [Google Scholar] [CrossRef]
- Kuang, Z.; Dai, G.; Wan, R.; Zhang, D.; Zhao, C.; Chen, C.; Li, J.; Gu, H.; Huang, W. Osteogenic and antibacterial dual functions of a novel levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold. Genes Dis. 2021, 8, 193–202. [Google Scholar] [CrossRef]
- Lin, L.; Shao, J.; Ma, J.; Zou, Q.; Li, J.; Zuo, Y.; Yang, F.; Li, Y. Development of ciprofloxacin and nano-hydroxyapatite dual-loaded polyurethane scaffolds for simultaneous treatment of bone defects and osteomyelitis. Mater. Lett. 2019, 253, 86–89. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, W.; Wu, X.D.; He, X.; Lin, X.; Wang, H.; Li, J.; Jiang, J.; Huang, W. Efficacy of novel nano-hydroxyapatite/polyurethane composite scaffolds with silver phosphate particles in chronic osteomyelitis. J. Mater. Sci. Mater. Med. 2019, 30–59. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
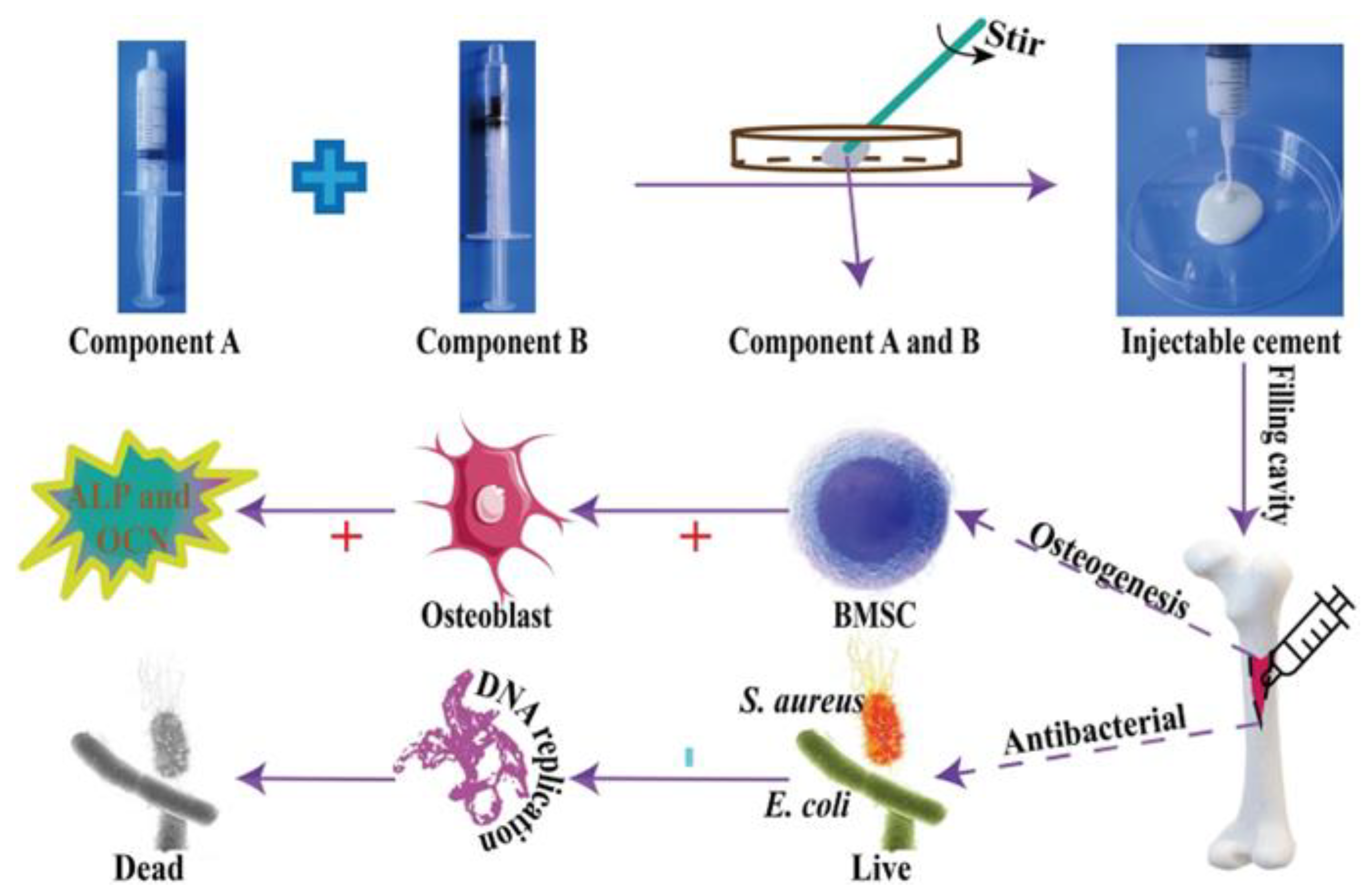
- Zhang, J.; Lei, X.; Tang, J.; Chen, J.; Zhao, Q.; Fang, W.; Zhang, Y.; Li, Y.; Zuo, Y. Efect of Antibacterial Enoxacin on the Properties of Injectable Nano-hydroxyapatite/Polyurethane Cement for Bone Repairing. J. Bionic Eng. 2022, 19, 483–496. [Google Scholar] [CrossRef]
- Anderson, J.M.; Marchant, R.E. Biomaterials: Factors favoring colonization and infection. In Infections Associated with Indwelling Medical Devices; Waldvogel, F.A., Bisno, A.L., Eds.; ASM Press: Washington, DC, USA, 2000; pp. 89–109. [Google Scholar]
- Saidin, S.; Jumat, M.A.; Mohd Amin, N.A.A.; Hammadi, A.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111382–111399. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Gao, P.; Yang, W.; Lei, X.; Wong, T.; Ismail, A.F.; Wang, L. Antibacterial polyurethane composite scaffolds for minimally invasive alveolar bone repair. Appl. Mater. Today 2023, 31, 101752–101764. [Google Scholar] [CrossRef]
- Rahmani-Moghadam, E.; Talaei-Khozani, T.; Zarrin, V.; Vojdani, Z. Thymoquinone loading into hydroxyapatite/alginate scafolds accelerated the osteogenic diferentiation of the mesenchymal stem cells. BioMed. Eng. OnLine 2021, 20, 20–76. [Google Scholar] [CrossRef]
- Klontzas, M.E.; Kenanidis, E.I.; Heliotis, M.; Tsiridis, E.; Mantalaris, A. Mesenchymal Stem Cell Therapy for Bone Regeneration. Clin Orthop Surg. 2018, 10, 271–278. [Google Scholar]
- Tahlawi, A.; Klontzas, M.E.; Allenby, M.C.; Morais, J.C.F.; Panoskaltsis, N.; Mantalaris, A. RGD-functionalized polyurethane scaffolds promote umbilical cord blood mesenchymal stem cell expansion and osteogenic differentiation. J. Tissue Eng. Regen. Med. 2018, 13, 2784–2814. [Google Scholar] [CrossRef]
- Shaabani, A.; Sedghi, R. Preparation of chitosan biguanidine/PANI-containing self-healing semi-conductive waterborne scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 264, 118045–118062. [Google Scholar] [CrossRef] [PubMed]
- Sita Shrestha, S.; Shrestha, B.K.; Ko, S.W.; Kandel, R.; Park, C.H.; Kim, C.S. Engineered cellular microenvironments from functionalized multiwalled carbon nanotubes integrating Zein/Chitosan @Polyurethane for bone cell regeneration. Carbohydr. Polym. 2021, 251, 117035–117051. [Google Scholar] [CrossRef] [PubMed]
- Wanga, C.; Huang, W.; Zhoue, Y.; Hea, L.; Hea, Z.; Chena, Z.; Hea, X.; Tian, S.; Liao, J.; Lua, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Suresh, K.S.; He, H.; Rajput, R.S.; Feng, Q.; Ramesh, S.; Wang, Y.; Krishnan, S.; Ostrovidov, S.; Camci-Unal, G.; et al. 3D Printing of Micro- and Nanoscale Bone Substitutes: A Review on Technical and Translational Perspectives. Int. J. Nanomed. 2021, 16, 4289–4319. [Google Scholar] [CrossRef]
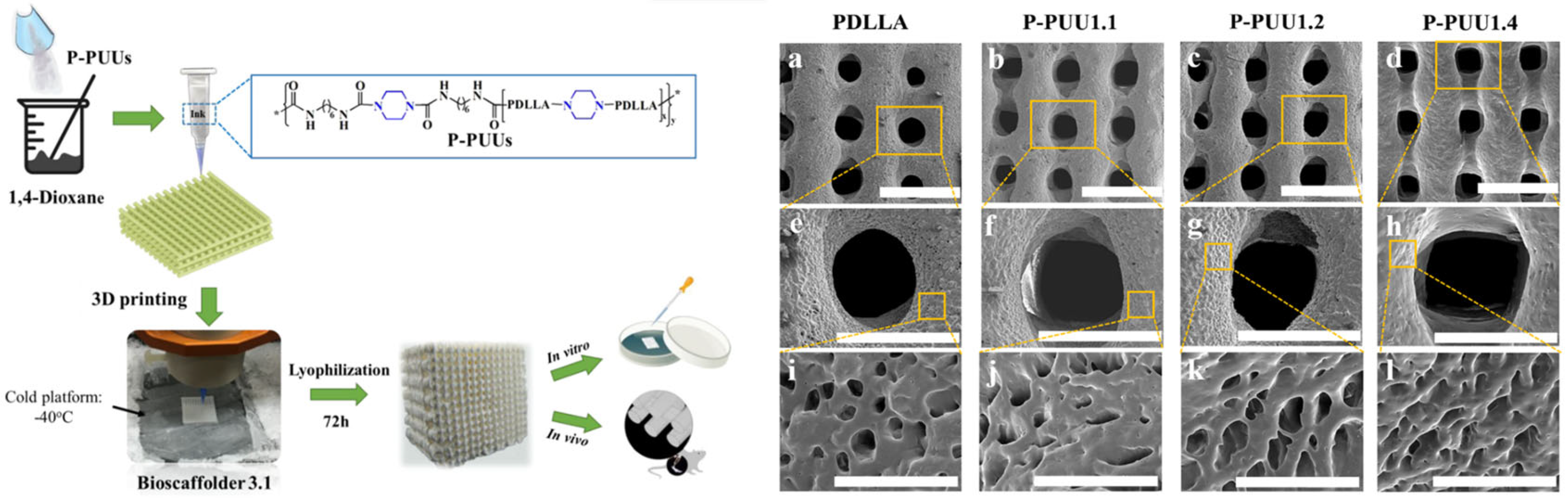
- Ma, Y.; Hu, N.; Liu, J.; Zhai, X.; Wu, M.; Hu, C.; Li, L.; Lai, Y.; Pan, H.; Lu, W.W.; et al. Three-Dimensional Printing of Biodegradable Piperazine-Based Polyurethane-Urea Scaffolds with Enhanced Osteogenesis for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 9415–9424. [Google Scholar] [CrossRef]
- Wen, Y.T.; Dai, N.T.; Hsu, S.H. Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater. 2019, 88, 301–313. [Google Scholar] [CrossRef]
- Adhami, M.; Dastidar, A.G.; Anjani, Q.K.; Detamornrat, U.; Tarrés, Q.; Delgado-Aguilar, M.; Acheson, J.G.; Manda, K.; Clarke, S.A.; Moreno-Castellanos, N.; et al. 3D-printing of dipyridamole/thermoplastic polyurethane materials for bone regeneration. Drug Deliv. Transl. Res. 2024, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Zhang, W.; Deng, J.; Nie, Y.; Du, X.; Qin, L.; Lai, Y. 3D-printed NIR-responsive shape memory polyurethane/magnesium scaffolds with tight-contact for robust bone regeneration. Bioact. Mater. 2022, 16, 218–231. [Google Scholar] [CrossRef]
- Yıldırım, A.M.; Sanli, A.; Türkoglu, N.; Denktaş, C. Fabrication of electrospun nanofibrous clinoptilolite doped thermoplastic polyurethane scaffolds for skeletal muscle tissue engineering. J. Appl. Polym. Sci. 2023, 140, 54233–54245. [Google Scholar] [CrossRef]
- Warren, G.L.; Hulderman, T.; Jensen, N.; McKinstry, M.; Mishra, M.; Luster, M.I.; Simeonova, P.P. Physiological role of tumor necrosis factor α in traumatic muscle injury. FASEB J. 2002, 16, 1630–1632. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, G.; Liu, Z.; Guo, M.; Jiang, X.; Taskin, M.B.; Zhang, Z.; Liu, J.; Tang, J.; Bai, R.; et al. Mussel inspired polynorepinephrine functionalized electrospun Polycaprolactone microfibers for muscle regeneration. Sci. Rep. 2017, 7, 8197. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, I.; Seol, Y.-J.; Ko, I.K.; Yoo, J.J.; Atala, A.; Lee, S.J. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat. Commun. 2020, 11, 1025. [Google Scholar] [CrossRef]
- Gotti, C.; Sensini, A.; Fornaia, G.; Gualandi, C.; Zucchelli, A.; Focarete, M.L. Biomimetic hierarchically arranged nanofibrous structures resembling the architecture and the passive mechanical properties of skeletal muscles: A step forward toward artificial muscle. Front. Bioeng. Biotechnol. 2020, 8, 767–789. [Google Scholar] [CrossRef]
- Chen, J.; Dong, R.; Ge, J.; Guo, B.; Ma, P.X. Biocompatible, Biodegradable, and Electroactive Polyurethane-Urea Elastomers with Tunable Hydrophilicity for Skeletal Muscle Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 28273–28285. [Google Scholar] [CrossRef]
- Chen, J.; Ge, J.; Guo, B.; Gao, K.; Ma, P.X. Nanofibrous polylactide composite scaffolds with electroactivity and sustained release capacity for tissue engineering. J. Mater. Chem. B 2016, 4, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yepez, G.; Wei, Z.; Liu, F.; Bugarin, A.; Hong, Y. Synthesis and characterization of conductive, biodegradable, elastomeric polyurethanes for biomedical applications. J. Biomed. Mater. Res. A 2016, 104, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhu, Y.; Gao, R.; Yu, W.; Li, L.; Xu, K. Synthesis, Characterizations and Biocompatibility of Novel Block Polyurethanes Based on Poly(lactic acid) (PLA) and Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P3/4HB). J. Inorg. Organomet. Polym. 2015, 25, 81–90. [Google Scholar] [CrossRef]
- Martínez, E.; Engel, E.; Planell, J.A.; Samitier, J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat.-Anat. Anz. 2009, 191, 126–135. [Google Scholar] [CrossRef]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef]
- Wolf, M.T.; Dearth, C.L.; Sonnenberg, S.B.; Loboa, E.G.; Badylak, S.F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv. Drug Deliv. Rev. 2015, 84, 208–221. [Google Scholar] [CrossRef]
- Meng, J.X.; Hou, C.Y.; Zhang, Q.H.; Li, Y.G.; Wang, H.Z. Light-driven artificial muscles based on electrospun microfiber yarns. Sci. China Tech. Sci. 2019, 62, 965–970. [Google Scholar] [CrossRef]
- Łopianiak, I.; Kawecka, A.; Civelek, M.; Wojasinski, M.; Cicha, I.; Ciach, T.; Butruk-Raszeja, B.A. Characterization of Blow-Spun Polyurethane Scaffolds−Influence of Fiber Alignment and Fiber Diameter on Pericyte Growth. ACS Biomater. Sci. Eng. 2024, 10, 4388–4399. [Google Scholar] [CrossRef] [PubMed]
- Łopianiak, I.; Wojasinski, M.; Butruk-Raszeja, B. Properties of Polyurethane Fibrous Materials Produced by Solution Blow Spinning. Chem. Process Eng. 2020, 41, 267–276. [Google Scholar]
- Blake, C.; Massey, O.; Boyd-Moss, M.; Firipis, K.; Rifai, A.; Franks, S.; Quigley, A.; Kapsa, R.; Nisbet, D.R.; Williams, R.J. Replace and repair: Biomimetic bioprinting for effective muscle engineering. APL Bioeng. 2021, 5, 031502-1–031502-16. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Naniz, M.A.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2021, 9, 535–573. [Google Scholar] [CrossRef]
- Azarmgin, S.; Torabinejad, B.; Kalantarzadeh, R.; Garcia, H.; Velazquez, C.A.; Lopez, G.; Vazquez, M.; Rosales, G.; Heidari, B.S.; Davachi, S.M. Polyurethanes and Their Biomedical Applications. ACS Biomater. Sci. Eng. 2024, 10, 6828–6859. [Google Scholar] [CrossRef]
- Gokyer, S.; Yilgor, E.; Yilgor, I.; Berber, E.; Vrana, E.; Orhan, K.; Monsef, Y.A.; Guvener, O.; Zinnuroglu, M.; Oto, C.; et al. 3D Printed Biodegradable Polyurethaneurea Elastomer Recapitulates Skeletal Muscle Structure and Function. ACS Biomater. Sci. Eng. 2021, 7, 5189–5205. [Google Scholar] [CrossRef]
- Ketabat, F.; Alcorn, J.; Kelly, M.E.; Badea, I.; Chen, X. Cardiac Tissue Engineering: A Journey from Scaffold Fabrication to In Vitro Characterization. Small Sci. 2024, 4, 2400079–2400096. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Murry, C.E. Heart regeneration. Nature 2011, 473, 326–335. [Google Scholar] [CrossRef]
- Hirt, M.N.; Hansen, A.; Eschenhagen, T. Cardiac tissue engineering: State of the art. Circ. Res. 2014, 114, 354–367. [Google Scholar] [CrossRef]
- Boroumand, S.; Asadpour, S.; Akbarzadeh, A.; Faridi-Majidi, R.; Ghanbari, H. Heart valve tissue engineering: An overview of heart valve decellularization processes. Regener. Med. 2018, 13, 41–54. [Google Scholar] [CrossRef]
- Akter, F.; Hamid, H. Chapter 6—Cardiovascular Tissue Engineering. In Tissue Engineering Made Easy; Akter, F., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 55–65. [Google Scholar]
- Sartori, S.; Chiono, V.; Tonda-Turo, C.; Mattu, C.; Gianluca, C. Biomimetic polyurethanes in nano and regenerative medicine. J. Mater. Chem. B 2014, 2, 5128–5144. [Google Scholar] [CrossRef] [PubMed]
- Jawad, H.; Lyon, A.R.; Harding, S.E.; Ali, N.N.; Boccaccini, A.R. Myocardial tissue engineering. Br. Med. Bull. 2008, 87, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Shah, G.; Wu, Y.; Torre-Amione, G.; King, N.M.; Lahmers, S.; Witt, C.C.; Becker, K.; Labeit, S.; Granzier, H.L. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation 2004, 110, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Udriste, A.S.; Niculescu, A.G.; Iliută, L.; Bajeu, T.; Georgescu, A.; Grumezescu, A.M.; Bădilă, E. Progress in Biomaterials for Cardiac Tissue Engineering and Regeneration. Polymers 2023, 15, 1177. [Google Scholar] [CrossRef]
- Asadpour, S.; Yeganeh, H.; Ai, J.; Kargozar, S.; Rashtbar, M.; Alexander Seifalian, A.; Ghanbari, H. Polyurethane-Polycaprolactone Blend Patches: Scaffold Characterization and Cardiomyoblast Adhesion, Proliferation, and Function. ACS Biomater. Sci. Eng. 2018, 4, 4299–4310. [Google Scholar] [CrossRef]
- Baheiraei, N.; Gharibi, R.; Yeganeh, H.; Miragoli, M.; Salvarani, N.; Di Pasquale, E.; Condorelli, G. Electroactive polyurethane/siloxane derived from castor oil as a versatile cardiac patch, part I: Synthesis, characterization, and myoblast proliferation and differentiation. J. Biomed. Mater. Res. Part A 2015, 104, 775–787. [Google Scholar] [CrossRef]
- Baheiraei, N.; Gharibi, R.; Yeganeh, H.; Miragoli, M.; Salvarani, N.; Di Pasquale, E.; Condorelli, G. Electroactive polyurethane/siloxane derived from castor oil as a versatile cardiac patch, part II: HL-1 cytocompatibility and electrical characterizations. J. Biomed. Mater. Res. Part A 2016, 104, 1398–1407. [Google Scholar] [CrossRef]
- Bahrami, S.; Solouk, A.; Mirzadeh, H.; Seifalian, A.M. Electroconductive polyurethane/graphene nanocomposite for biomedical applications. Compos. Part B 2019, 168, 421–431. [Google Scholar] [CrossRef]
- Mani, M.P.; Jaganathan, S.K.; Ahmad Athif Faudzi, A.A.; Sunar, M.S. Engineered Electrospun Polyurethane Composite Patch Combined with Bi-functional Components Rendering High Strength for Cardiac Tissue Engineering. Polymers 2019, 11, 705. [Google Scholar] [CrossRef]
- Tao, Z.W.; Wu, S.; Cosgriff-Hernandez, E.M.; Jacot, J.G. Evaluation of a polyurethane-reinforced hydrogel patch in a rat right ventricle wall replacement model. Acta Biomater. 2019, 101, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Liao, H.C.; Hsu, S.H.; Chen, R.S.; Wu, M.C.; Yang, Y.F.; Wu, C.C.; Chen, M.H.; Su, W.F. A novel polyurethane/cellulose fibrous scaffold for cardiac tissue engineering. RSC Adv. 2015, 5, 6932–6939. [Google Scholar] [CrossRef]
- Yao, Y.; Ding, J.; Wang, Z.; Zhang, H.; Xie, J.; Wang, Y.; Hong, L.; Mao, Z.; Gao, J.; Gao, C. ROS-responsive polyurethane fibrous patches loaded with methylprednisolone (MP) for restoring structures and functions of infarcted myocardium in vivo. Biomaterials 2020, 232, 119726–119740. [Google Scholar] [CrossRef]
- Demir, M.M.; Yilgor, I.; Yilgor, E.; Erman, B. Electrospinning of Polyurethane Fibers. Polymer 2002, 43, 3303–3309. [Google Scholar] [CrossRef]
- Boffito, M.; Sartori, S.; Ciardelli, G. Polymeric scaffolds for cardiac tissue engineering: Requirements and fabrication technologies. Polym. Int. 2013, 63, 2–11. [Google Scholar] [CrossRef]
- Xu, C.; Okpokwasili, C.; Huang, Y.; Shi, X.; Wu, J.; Liao, J.; Tang, L.; Hong, Y. Optimizing Anisotropic Polyurethane Scaffolds to Mechanically Match with Native Myocardium. ACS Biomater. Sci. Eng. 2020, 6, 2757–2769. [Google Scholar] [CrossRef]
- Shao, Z.; Tao, T.; Xu, H.; Cen Chen, C.; Lee, I.S.; Chung, S.; Dong, Z.; Li, W.; Ma, L.; Bai, H. Qianming Chen Recent progress in biomaterials for heart valve replacement: Structure, function, and biomimetic design. VIEW 2021, 2, 20200142–20200159. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Answini, G.A.; Yakubov, S.J.; Rai, B.; Smith, J.M.; Duff, S.; Shannon, F.L.; Sakwa, M.; Beith, J.; Heimansohn, D. Preliminary evaluation of a novel polymeric valve following surgical implantation for symptomatic aortic valve disease. J. Am. Coll. Cardiol. Intv. 2021, 14, 2754–2756. [Google Scholar] [CrossRef]
- Ovcharenko, E.A.; Seifalian, A.; Rezvova, M.A.; Klyshnikov, K.Y.; Glushkova, T.V.; Akenteva, T.N.; Antonova, L.V.; Velikanova, E.A.; Chernonosova, V.S.; Shevelev, G.Y. A new nanocomposite copolymer based on functionalised Graphene oxide for development of heart valves. Sci. Rep. 2020, 10, 5271–5285. [Google Scholar] [CrossRef]
- Rezvova, M.A.; Klyshnikov, K.Y.; Gritskevich, A.A.; Ovcharenko, E.A. Polymeric Heart Valves Will Displace Mechanical and Tissue Heart Valves: A New Era for the Medical Devices. Int. J. Mol. Sci. 2023, 24, 3963. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate risk patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.H.; Kodali, S.; Makkar, R.R.; Herrmann, H.C.; Williams, M.; Babaliaros, V.; Leon, M.B. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: A propensity score analysis. Lancet 2016, 387, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, B.; Tzamtzis, S.; Sheridan, R.; Mullen, M.J.; John Yap, J.; Seifalian, A.M.; Burriesci, G. In Vitro Hydrodynamic Assessment of a New Transcatheter Heart Valve Concept (the TRISKELE). J. Cardiovasc. Trans. Res. 2016, 10, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Kachel, M.; Castillero, E.; Xue, Y.; Kalfa, D.; Ferrari, G.; George, I. Polymeric prosthetic heart valves: A review of current technologies and future directions. Front. Cardiovasc. Med. 2023, 10, 1137827–1137840. [Google Scholar] [CrossRef]
- Hasan, A.; Ragaert, K.; Swieszkowski, W.; Selimović, Š.; Paul, A.; Camci-Unal, G.; Mofrad, M.R.; Khademhosseini, A. Biomechanical properties of native and tissue engineered heart valve constructs. J. Biomech. 2014, 47, 1949–1963. [Google Scholar] [CrossRef]
- Melo, S.F.; Nondonfaz, A.; Aqil, A.; Pierrard, A.; Hulin, A.; Delierneux, C.; Ditkowski, B.; Gustin, M.; Legrand, M.; Tullemans, B.M.E.; et al. Design, manufacturing and testing of a green non-isocyanate polyurethane prosthetic heart valve. Biomater. Sci. 2024, 12, 2149–2164. [Google Scholar] [CrossRef]
- Ercolani, E.; Gaudio, C.D.; Bianco, A. Vascular tissue engineering of small-diameter blood vessels: Reviewing the electrospinning approach. J. Tissue Eng. Regen. Med. 2013, 9, 861–888. [Google Scholar] [CrossRef]
- Singh, C.; Cynthia, S.; Wong, C.S.; Wang, X. Medical textiles as vascular implants and their success to mimic natural arteries. J. Funct. Biomater. 2015, 6, 500–525. [Google Scholar] [CrossRef]
- Liber-Kneć, A.; Łagan, S. Metody Badań Biomateriałów i Tkanek-Wstęp do Cwiczeń Labarotoryjnych; Politechnika Krakowska: Kraków, Poland, 2020. [Google Scholar]
- Haruguchi, H.; Teraoka, S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: A review. J. Artif. Organs. 2003, 6, 227–235. [Google Scholar] [CrossRef]
- Jirofti, N.; Mohebbi-Kalhori, D.; Samimi, A.; Hadjizadeh, A.; Kazemzadeh, G.G. Small-diameter Vascular Graft Using Co-Electrospun of Composite PCL/PU Nanofibers. Biomed. Mater. 2018, 13, 055014. [Google Scholar] [CrossRef]
- Zhen, L.; Creason, S.A.; Simonovsky, F.I.; Snyder, J.M.; Lindhartsen, S.L.; Mecwan, M.M.; Johnson, B.W.; Himmelfarb, J.; Ratner, B.D. Precision-porous polyurethane elastomers engineered for application in pro-healing vascular grafts: Synthesis, fabrication and detailed biocompatibility assessment. Biomaterials 2021, 279, 121174–121191. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, A.; Daemi, H.; Rajabi, S.; Baharvand, H. Highly tough and ultrafast self-healable dual physically crosslinked sulfated alginate-based polyurethane elastomers for vascular tissue engineering. Carbohydr. Polym. 2021, 257, 117632–117641. [Google Scholar] [CrossRef] [PubMed]
- Kianpour, G.; Bagheri, R.; Pourjavadi, A.; Ghanbari, H. Synergy of titanium dioxide nanotubes and polyurethane properties for bypass graft application: Excellent flexibility and biocompatibility. Mater. Des. 2022, 215, 11523–11533. [Google Scholar] [CrossRef]
- Yu, E.; Mi, H.Y.; Zhang, J.; Thomson, J.A.; Turng, L.S. Development of Biomimetic Thermoplastic Polyurethane/Fibroin Small-Diameter Vascular Grafts via a Novel Electrospinning Approach. J. Biomed. Mater. Res. A 2018, 106, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Wienen, D.; Gries, T.; Cooper, S.L.; Heath, D.E. An overview of polyurethane biomaterials and their use in drug delivery. J. Control. Release 2023, 363, 376–388. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hebda, E.; Pielichowski, K. Biomimetic Polyurethanes in Tissue Engineering. Biomimetics 2025, 10, 184. https://doi.org/10.3390/biomimetics10030184
Hebda E, Pielichowski K. Biomimetic Polyurethanes in Tissue Engineering. Biomimetics. 2025; 10(3):184. https://doi.org/10.3390/biomimetics10030184
Chicago/Turabian StyleHebda, Edyta, and Krzysztof Pielichowski. 2025. "Biomimetic Polyurethanes in Tissue Engineering" Biomimetics 10, no. 3: 184. https://doi.org/10.3390/biomimetics10030184
APA StyleHebda, E., & Pielichowski, K. (2025). Biomimetic Polyurethanes in Tissue Engineering. Biomimetics, 10(3), 184. https://doi.org/10.3390/biomimetics10030184








